Hypoplastic left heart syndrome (HLHS) continues to be a challenge in terms of morbimortality. Norwood-Sano surgery has been classically the correction of choice, the introduction of new techniques like biventricular repair, in favorable cases, and hybrid procedures as a step prior to cardiac transplantation have not improved the univentricular results. Technological advances and improvements in anticoagulation therapies have made possible to implant ventricular assist devices (VAD) during the neonatal period. This has motivated us to start a left VAD program in an animal model of HLHS. Our objective is to use long term VAD in a biventricular condition in patients with HLHS as a bridge to cardiac transplantation. Although the conditions have not been met to start a program for patients, the initial results show that the technique is feasible in pigs. We believe that this line of research is worth exploring as cardiac transplant in conditions of biventricular physiology could be less risky and offer better results than the classical univentricular path.
El síndrome del corazón izquierdo hipoplásico (SCIH) continúa siendo un reto en términos de morbimortalidad. La cirugía clásica de Norwood-Sano continúa siendo de elección y la introducción de nuevas técnicas como la reparación biventricular en los casos más favorables y los procedimientos híbridos para poder optar a un trasplante cardiaco posterior, no han conseguido una mejora en los resultados de la cirugía univentricular clásica. El avance de la tecnología y la terapia anticoagulante en los últimos años ha hecho realidad la asistencia ventricular mecánica (AVM) de larga duración en el periodo neonatal. Por estos motivos hemos iniciado un programa de implante de AVM izquierda en un modelo animal de hipoplasia de SCIH. Con el objetivo de que en un futuro la SCIH se pueda trasplantar en condiciones de biventricularidad, con el implante de una AVM izquierda de larga evolución. Los resultados iniciales muestran que técnicamente es posible en cerdos y, aunque actualmente no se dan las condiciones para iniciar un programa de este tipo en pacientes, pensamos que es una vía para seguir investigando, porque creemos que, el trasplante cardiaco en condiciones de biventricularidad, puede ser una opción con menos riesgos y mejores resultados que la vía univentricular.
Hypoplastic left heart syndrome (HLHS) is a congenital heart disease defined as limited development of structures in the left side of the heart, like the: mitral valve, left ventricle, aortic valve and aortic arch. It is the congenital heart disease which most limits survival, representing 25% of all the deaths in pediatric patients with congenital heart disease.1
The etiology of HLHS is unknown. There are associated genetic alterations, however, it is not known which gene or group of genes determine the disease. The probability of being born with HLHS in the general population is of 0.16 cases/1000 births, this increases to 0.36 cases/1000 births if there is a family history.2
During the 70s, various techniques were described to improve the survival of neonates with hypoplastic left heart chambers, but in vain. It was not till 1983, when William I. Norwood and his team in Boston Children's Hospital, managed to connect the pulmonary artery to the aorta and perform a systemic to pulmonary shunt. This allowed the single ventricle, in this case the right ventricle, to work as a pump for both pulmonary and systemic circulation.3 Later in 2003, the technique was refined with the modification of Shunji Sano, who optimized the Qp/Qs relation (pulmonary flow/systemic flow) using a conduit which connected the right ventricle to the pulmonary artery.4
Currently the surgical technique consists of three stages performed during infancy. The first surgery is performed in the neonatal period during the first week of life (Norwood-Sano), the second surgery is performed at 3 months of age (Glenn) and the third surgery is performed at 3rd–4th year of life (Fontan).3 This allows patients to reach adulthood, however, an important percentage (20%) will need a transplant. One, which is considered of great risk, with a mortality of around 30%.5 The percentage of patients who reach adulthood with an acceptable quality of life is around 15%.6
Although this data has been improving gradually, in great part due to advances in perioperative care, the surgical treatment of HLHS continues to be a challenge in terms of morbimortality. Due to this, various groups have investigated alternatives to the univentricular surgery of Norwood. The alternatives range from a cardiac transplant, knowing the lack of organs in this age range. To treatments which force a biventricular route, through a fetal intervention or postnatal surgery with the intention to recuperate the hypoplastic left ventricle. The team in Boston, the same who pioneered the univentricular surgery of Norwood, have recently started the biventricular repair program, using various palliative surgical techniques during infancy they try to force a biventricular route. Although this program of biventricular repair has shed new knowledge about this pathology, their results are no better than the classical Norwood-Sano.7–9
In the last 20 years there have been important technological achievements in society and especially in medicine. The improvements in ventricular assist device programs have given a second opportunity to many patients. The use of an ECMO in emergent scenarios and later the support with long term ventricular assist devices have enabled us to buy time, understand the current patient scenario, recuperate hemodynamically unstable patients and even support them till a cardiac transplant. The current assist devices allow to support patients for more than 1 year.10
Another great leap in the field of assists devices during the last years has been the improvement in drug therapy, especially with the use of new anticoagulants. Bivalirudine has made possible the use of long term assist devices in children less than 5kg, as it has dramatically reduced severe neurological events.11
In Spain there are centers with ample experience in univentricular surgery, like the hospital Gregorio Marañon. With the advent of technological improvements and ABO incompatible heart transplants12 they are trying alternative approaches. After an initial hybrid procedure perpetuating a univentricular physiology they offer a cardiac transplant,13 patient inclusion to the waiting list is considered even before birth.14,15
Another route yet to be explored is the treatment of HLHS through the early use of ventricular assist device till cardiac transplantation. The objective is to use an assist device as surrogate of the hypoplastic left ventricular to obtain a pulsatile ventricular physiology16 and thus reaching cardiac transplantation in optimal conditions. The major breakthrough in pediatric cardiac surgery in the last decade has been due to improved survival in ventricular assist device and transplantation, this, has been the inspiration for this new route for HLHS. Although a difficult route, the improvements in immunosuppressor therapies in cardiac transplantation, have made it a better option than the frequent surgeries in the univentricular route.
In HLHS the main cardiac pump, the left ventricle, is not developed. All current strategies use the single ventricle, a right ventricle, which is embryologically designed for a pulmonary circulation to support a systemic circulation which has more pressure. Meanwhile, the pulmonary circulation will develop without a pump. The flow will rely on pressure gradients and thanks to the hydraulic properties of the pulmonary and venous circulation. As survival in univentricular physiology has improved thanks to a better perioperative management, the short and long term complications have become more evident. To plan a cardiac transplant in this situation is not feasible, due to technical difficulties and poor hemodynamics, even when the patient is clinically stable and of older age.5
Due to these nuances, in the hospital La Paz, we developed an experimental model of HLHS in pigs, in order to study the feasibility of long term left ventricular assist devices like Berlin Heart. The objective is to study a biventricular route using an assist devices as bridge to cardiac transplantation in a more stable manner. With the hope of reducing morbid and mortality factors prior transplantation and thus increasing candidacy opportunities.
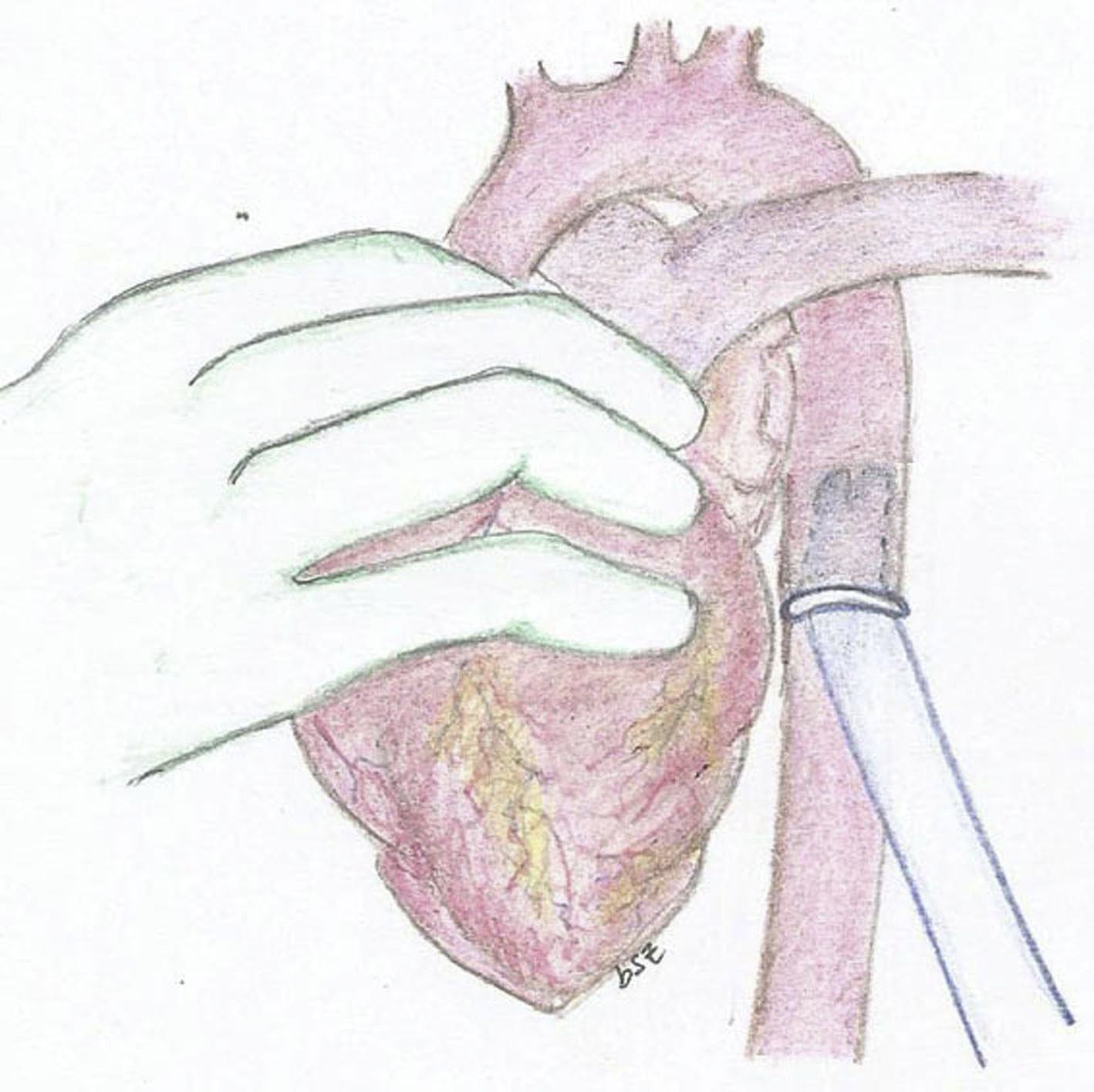
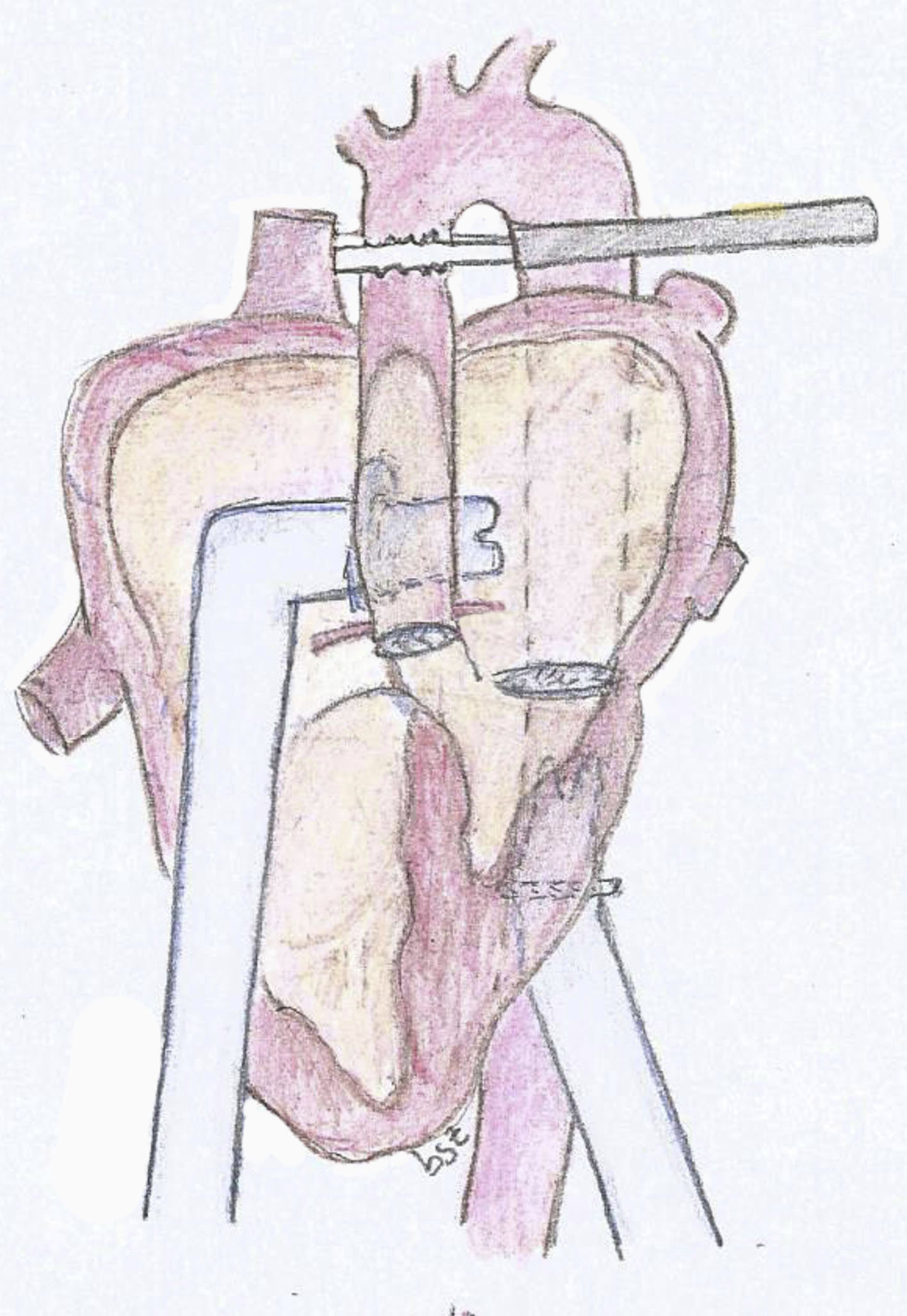
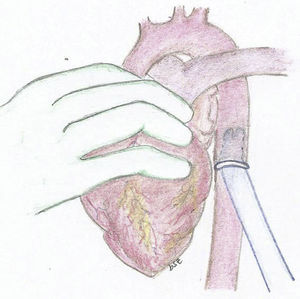
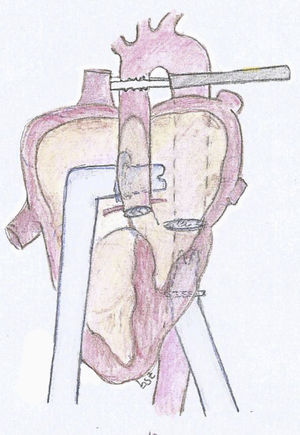
The experimental model consists of using a 25kg pig. After general anesthesia and mechanical ventilation, a median sternotomy is performed with cannulation of the ascending aorta and both vena cava. Bypass is initiated with the heart lung machine and cardiac arrest with crystalloid Celsior cardioplegia administered in an anterograde fashion. The mitral and aortic valve are closed using a bovine pericardium patch, it is imperative to maintain an adequate coronary blood flow. Once the model is established and still in bypass, we implant the cannulas of the assist device. For the arterial cannula we use an aortic 9mm Berlin Heart cannula with 60° tip. We suture a 12mm Dacron conduit in one extreme and then suture the conduit in the descending aorta in a retrocardiac fashion (Fig. 1). We then used a 9mm atrial Berlin Heart cannula with a 23mm tip as the venous cannula. This was sutured to the left atrium through the septum of the atria, the suture was reinforced in the septum with a pericardium patch. The cannula exits the heart through the right atriotomy as seen in the diagram (Fig. 2).
This model has allowed us to study, first its feasibility and in parallel the hemodynamic difficulties associated with the fact of not having a functional left ventricle.
We analyzed this model in two pigs, in both cases it was possible to wean from bypass and maintain a biventricular support for two hours, using the right ventricle and the left ventricular assist device. We measured biomarkers such as NT-proBNP and serum lactate, which were in normal levels at the end of the procedure. This shows adequate systemic perfusion of the model.
As conclusion, we are aware of the important limitations of this initial experimental study. We can affirm that the procedure is technically and hemodynamically feasible with the adequate materials. We believe in the future, an initial stage of ventricular assistance to maintain a biventricular circulation, with all its benefits and limitations of the assist device, would allow to perform a cardiac transplantation at a second stage after a few months of the initial surgery. This program cannot be initiated in patients yet, however, we believe it is worth exploring. As a cardiac transplantation in a biventricular condition could be an option with less complications and risks compared to the univentricular route. Furthermore, it would allow the patient to be more time in the transplant waiting list and thus improving the chances of receiving the organ in a better hemodynamic and neurological condition.
Conflict of interestThe authors declare that they have no conflict of interest.