A pancreatic pseudocyst is the collection of pancreatic secretions surrounded by fibrous tissue caused by pancreatic disease that affects the pancreatic duct. Clinical presentation is variable. Management includes percutaneous, endoscopic or surgical drainage and resection.
Material and methodsReview of a cohort of patients with pancreatic pseudocyst in a third level hospital. An analysis was performed on the demographic data, aetiology, clinical presentation, radiological and laboratory findings, type of surgical procedure, complications, recurrence and mortality. The statistical analysis was performed using Chi squared and Student's t-tests, with a p<0.05.
ResultsA total of 139 patients were included, of whom 58% were men and 42% were women, with median age of 44.5 years. Chronic pancreatitis was the most common aetiology, present in 74 patients (53%). The main complaint was abdominal pain in 73% of patients. Median size was 18cm (range 7–29) and the most frequent location was body and tail of the pancreas. Internal surgical drainage was selected in 111 (80%) patients, of whom 96 were cystojejunostomy, 20 (14%) had external surgical drainage, and 8 (6%) resection. Complications were, pancreatic fistula (12%), haemorrhage (4%), infection (4%), and other non-surgical complications (4%). Complication rate was higher if the cause was chronic pancreatitis or if the management was external surgical drainage. Recurrence rate was 6%, and a mortality rate of 1%.
ConclusionSurgical management is a viable option for the management of pancreatic pseudocyst with a low complication and recurrence rate.
El seudoquiste de páncreas es la colección de secreciones pancreáticas rodeada de una pared fibrosa secundaria a enfermedad aguda o crónica. El tratamiento incluye drenaje percutáneo, endoscópico o quirúrgico y resección.
El objetivo es presentar la experiencia quirúrgica en pacientes con seudoquiste de páncreas.
Material y métodosCohorte retrospectiva de 139 pacientes con diagnóstico de seudoquiste pancreático, durante 13 años en un hospital de tercer nivel. Se estudiaron datos demográficos, etiología, presentación clínica, datos radiológicos y de laboratorio, indicación, tipo de procedimiento quirúrgico realizado, complicaciones, recurrencia y mortalidad. Se realizó prueba de Chi cuadrada para las variables nominales y T de Student para variables continuas.
ResultadosFueron 81 hombres (58%) y 58 mujeres (42%), con una mediana de edad de 44.5 años. En 74 pacientes (53%) la etiología fue pancreatitis crónica. El síntoma más frecuente fue dolor abdominal en el 73%. La mediana de tamaño fue 18cm (7-29) y la localización más frecuente fue: cuerpo y cola en 75 pacientes (54%). El tratamiento fue: drenaje interno en 111 pacientes (80%), (96 cistoyeyunoanastomosis), en 20 (14%) drenaje externo y resección en 8 (6%). Las complicaciones fueron: fístula pancreática (12%), hemorragia postoperatoria (4%), infección (4%) y complicaciones no quirúrgicas (4%). La tasa de complicaciones fue mayor cuando el diagnóstico era pancreatitis crónica y se realizó drenaje quirúrgico externo (p<0.05). Hubo recurrencia en 7 pacientes (6%). Dos pacientes fallecieron (1%).
ConclusiónEl tratamiento quirúrgico es una opción en el manejo del seudoquiste pancreático, con baja tasa de complicaciones y recurrencia.
The pancreatic pseudocyst is defined as a localised collection of fluid rich in amylase and other enzymes surrounded by a fibrous wall or granulation tissue, and resulting from an episode of acute pancreatitis, chronic pancreatitis, pancreatic trauma or extrinsic obstruction of the pancreatic duct.1,2 It has to have persisted for a minimum of 4 weeks with or without communication to the pancreatic duct system.3
The current prevalence of pancreatic pseudocysts is 10–20% in patients with chronic pancreatitis. Alcohol consumption is the cause in 65% of cases, followed by vesicular lithiasis in 15%.1,3
Diagnosis is based on clinical, biochemical and radiological findings.1 Treatment strategies for patients with pancreatic pseudocyst have changed and continue to evolve. Management includes percutaneous drainage, internal endoscopic drainage, internal and external drainage, surgery and resection.4–6
This study reports the experience and results obtained in patients diagnosed with a pancreatic pseudocyst who underwent surgical treatment in a third level hospital over a period of 13 years.
Material and methodsA retrospective cohort was formed from all patients diagnosed with a pancreatic pseudocyst who underwent surgery in the period between 1 January 2000 and 31 December 2013 in the Gastric Surgery Department of the Hospital de Especialidades de Centro Médico Nacional Siglo XXI, Mexican Social Security Institute.
Demographic data were obtained (age and gender, aetiology (acute, chronic, idiopathic or traumatic pancreatitis), clinical presentation (pain, early satiety, jaundice, abdominal tumour), laboratory data (haemoglobin, leukocytes, serum amylase and lipase), radiological findings (location, number – single or multiple- and size), indication and type of surgical procedure undertaken (internal drainage, cystogastroanastomosis, cystoduodenoanastomosis, cystojejunoanastomosis – external drainage or resection) and complications (wound infection, postoperative bleeding, pancreatic or intestinal fistula, dehiscence of the surgical wound, pulmonary and infectious thromboembolisms). Recurrence rate and mortality were evaluated during follow-up.
The data were collected on a database and the statistical analysis was performed using SPSS software version 16 (SPSS Inc., Chicago, Ill, U.S.A.). The Chi squared test was used for comparisons for nominal variables and the Student's t-test for continuous variables. Postoperative complications and recurrence were identified as dependent variables. A p-value <0.05 was considered significant.
ResultsOne hundred and thirty-nine patients were studied, 81 males (58%) and 58 women (42%), with a median age of 44.5 (range 18–85). In 74 (53%) patients the aetiology was secondary to chronic pancreatitis, in 52 (37%) acute pancreatitis, in 11 (8%) idiopathic, and in 2 (1%) patients it was abdominal trauma.
The main symptoms were: abdominal pain in 101 cases (73%), weight loss in 53 (38%), postprandial fullness or early satiety in 48 (35%), nausea and vomiting in 47 (34%), and fever in 26 (19%). The predominant sign on physical examination was abdominal tumours in the epigastrium in 37 cases (27%); 2 cases (1%) presented with symptoms of acute abdomen secondary to rupture.
The laboratory tests revealed hyperamylasaemia in 110 patients (79%) and hyperlipasaemia in 109 (78%); anaemia in 25 cases (18%) and leukocytosis greater than 10,500/mm3 in 56 (40%).
Computed tomography scans (CT) were performed on 104 patients (79%) and abdominal ultrasound on 84 (60%). Endoscopic ultrasound and magnetic resonance were used less often and only as a diagnostic complement. The median size was 18cm (range 7–29cm) and the most common location was in the body and tail in 75 cases (54%), body in 33 (24%), head and body in 22 (16%) and head of pancreas in 9 cases (6%).
The surgical treatment chosen was internal drainage in 111 patients (80%). Cystojejunoanastomosis was performed in 96 (69%), cystogastroanastomosis in 11 (8%) and cystoduodenoanastomosis in 4 (3%). Twenty patients (14%) underwent external surgical drainage and 8 (6%) resection.
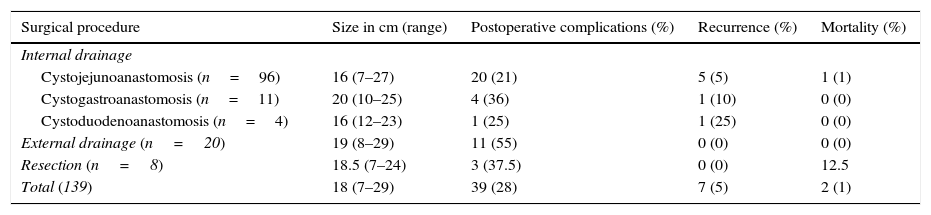
Forty-one (29%) patients presented postoperative complications. Sixteen (12%) patients developed a pancreatic fistula, 9 (6%) infection, 6 (4%) postoperative haemorrhage, and 6 (4%) non-surgical complications, of which 4 were pneumonia, one cardiac arrhythmia and one pulmonary thromboembolism. Seven patients suffered recurrence (6%). Two patients died for a mortality of 1%; one patient due to haemorrhage and the other due to septic shock. Table 1 summarises the surgical treatment associated with postoperative complications, recurrence and mortality.
Surgical treatment, preoperative and postoperative characteristics in 139 patients with pancreatic pseudocyst.
| Surgical procedure | Size in cm (range) | Postoperative complications (%) | Recurrence (%) | Mortality (%) |
|---|---|---|---|---|
| Internal drainage | ||||
| Cystojejunoanastomosis (n=96) | 16 (7–27) | 20 (21) | 5 (5) | 1 (1) |
| Cystogastroanastomosis (n=11) | 20 (10–25) | 4 (36) | 1 (10) | 0 (0) |
| Cystoduodenoanastomosis (n=4) | 16 (12–23) | 1 (25) | 1 (25) | 0 (0) |
| External drainage (n=20) | 19 (8–29) | 11 (55) | 0 (0) | 0 (0) |
| Resection (n=8) | 18.5 (7–24) | 3 (37.5) | 0 (0) | 12.5 |
| Total (139) | 18 (7–29) | 39 (28) | 7 (5) | 2 (1) |
In the analysis we performed, the factor associated with postoperative complications was a greater presence of complications when the aetiology was secondary to chronic pancreatitis (p<0.04) and with the type of surgical procedure performed – cystojejunoanastomosis had fewer postoperative complications (21%) compared with external drainage (55%, principally pancreatic fistula) (p<0.05). There were no differences in terms of recurrence and mortality, in relation to aetiology, size, location, and type of surgical procedure undertaken.
DiscussionSeveral complications can appear after an episode of pancreatitis of which the pancreatic pseudocyst is one of the most common. The pathophysiology, in most patients, is the result of the lesion or alteration to the normal anatomy of the pancreatic duct.1
The aetiology of the pancreatic pseudocyst is directly associated with the cause of the pancreatitis; the consumption of alcohol is the cause in 65% of cases, followed by vesicular lithiasis in 15%. Due to improved imaging techniques, the current prevalence is 10–20% in patients after acute pancreatitis and 20–40% in patients with chronic pancreatitis.1,3 In our study the incidence was 53% associated with chronic pancreatitis and 47% with acute pancreatitis. The prevalence of pseudocysts is higher in males, between the fourth and fifth decades of life,1,3,4 and both of these results are compatible with our study.
Diagnosis is made based on clinical, biochemical and radiological findings.1 The clinical presentation is variable, from asymptomatic patients to symptoms of abdominal emergency due to complications.1,3 The predominant symptoms reported in the literature are abdominal pain, which presents in up to 90% of patients, early satiety, nausea and vomiting (50–70%), weight loss (20–50%), jaundice (10%) and fever (10%).1 On physical examination, only 25–50% presented a palpable abdominal mass.3 There can also be sepsis secondary to infection, hypovolemic shock due to bleeding7 and acute abdomen after spontaneous rupture of the pseudocyst.7 The clinical findings in our study are similar to those reported in the literature reviewed and are important in patient assessment.
There are currently no specific laboratory studies to establish a diagnosis of pancreatic pseudocyst; however, a persistently elevated concentration of amylase and lipase can be observed in up to 50% of patients. Other findings include mild leukocytosis and alterations in liver function tests.1,4 There were elevated serum levels of the abovementioned pancreatic enzymes in most of our patients. Measurement of these is an important part of the diagnostic protocol and therefore is recommended in our study. The other biochemical findings in our study were non-specific.
With regard to radiological studies, CT is the scan of choice for patients with a suspected pseudocyst. It provides important information about the relationship of the lesion with adjacent structures, the characteristics of the biliary and pancreatic system, the presence of calcifications, the size of the pseudocyst, its extension and location; all of these are important factors in planning treatment. The sensitivity and specificity reported for CT scanning is 90–100% and 98–100%, respectively.1,4 Another widely-used study is ultrasound which has a sensitivity of 75–90% and specificity of 92–98%. Magnetic resonance and cholangiopancreatography are viable options for study and treatment1,2,4; however, they were not used very much in our population. Due to the sensitivity and specificity of CT scanning reported in the literature and its availability in our hospital, tomography was our radiological study of choice. In some cases other radiological tools were used, to complement the diagnostic protocol.
Management strategies have changed and continue to evolve.2 The American College of Gastroenterology's guidelines for the management of pancreatitis of 20138 state that an asymptomatic pancreatic pseudocyst can be managed conservatively irrespective of its size, location or extension to neighbouring structures. This is contrary to what was reported previously, when it was recommended that the lesion should be drained if it was greater than 6cm in size or had persisted longer than 6 weeks.9 These guidelines recommend invasive management to treat pancreatic pseudocysts only if there are symptoms due to the lesion itself or because it has extended to neighbouring structures and is compromising normal gastrointestinal physiology (infected pseudocyst, bleeding, biliary obstruction or delayed gastric emptying).9–12
At present management includes percutaneous, endoscopic or surgical drainage, each of which has its different advantages and disadvantages.13 We did not find any controlled prospective studies that directly compare one with another. It is not easy to establish which therapeutic drainage method is better than the rest, however, the management chosen will depend on the patient's clinical characteristics and, ideally, on the anatomy of the pancreatic duct. According to Park and Heniford,14 Nealon and Walser described a classification where they took into account the presence of stenosis or obstruction of the pancreatic duct and the communication of the pseudocyst to it. Therefore, appropriate patient selection, the underlying cause of the pancreatitis, the location of the pseudocyst, and the presence or absence of obstruction of the pancreatic duct are important factors which will determine the outcome of the drainage method chosen.
The treatment of choice in our study was surgery; this is still considered the gold standard, and is divided between internal, external drainage and resection. Internal drainage can be performed by communication between the pseudocyst and the stomach (cystogastroanastomosis), jejunum (cystojejunoanastomosis) or duodenum (cystoduodenoanastomosis).8,14,15 The choice of any of these techniques will depend on the location of the pseudocyst, the adjacent structures and the surgeon's preference. If resection is chosen, this will depend on the location of the pseudocyst, and a distal pancreatectomy or even pancreaticoduodenectomy can be performed.8
The surgical drainage chosen in our study was cystojejunoanastomosis in almost 7 out of 10 of our patients. A lower rate of complications is reported in the literature when this approach is chosen compared to other types of internal drainage.16 Nevertheless, our study did not demonstrate a difference in terms of the rate of complications compared with other internal drainage approaches. However, we did find these differences when compared with external drainage, where we found a high prevalence of pancreatic fistula; therefore this is not a procedure that we perform frequently in our hospital.
Surgical drainage has a mortality of 5–9%, with a mean of complications of around 11–24% and 5–8% recurrence.2,15 The recurrence reported in our study was 6% with 1% mortality, this coincides with that reported in the literature.
ConclusionThe rate of complications and recurrence in our study coincides with that reported in the literature. Open or laparoscopic surgical management with internal drainage (mainly towards the jejunum) is a viable and very important option in patients with a diagnosis of pancreatic pseudocyst, has a high resolution rate and low prevalence of postoperative complications.
Conflict of interestThe authors have no conflict of interests to declare.
Please cite this article as: Martínez-Ordaz JL, Toledo-Toral C, Franco-Guerrero N, Tun-Abraham M, Souza-Gallardo LM. Tratamiento quirúrgico del seudoquiste de páncreas. Cir Cir. 2016;84:288–292.