Rhabdomyolysis is defined as skeletal muscle necrosis. Ultrasound assessment has recently become a useful tool for the diagnosis and monitoring of muscle diseases, including rhabdomyolysis. A case is presented on the ultrasound findings in a patient with rhabdomyolysis.
ObjectiveTo highlight the importance of ultrasound as an essential part in the diagnosis in rhabdomyolysis, to describe the ultrasound findings, and review the literature.
Clinical caseA 30 year-old with post-traumatic rhabdomyolysis of both thighs. Ultrasound was performed using a Philips Sparq model with a high-frequency linear transducer (5–10MHz), in low-dimensional scanning mode (2D), in longitudinal and transverse sections at the level of both thighs. The images obtained showed disorganisation of the orientation of the muscle fibres, ground glass image, thickening of the muscular fascia, and the presence of anechoic areas.
ConclusionsUltrasound is a useful tool in the evaluation of rhabdomyolysis.
La rabdomiólisis se define como la necrosis del músculo esquelético. Recientemente la evaluación ultrasonográfica se ha posicionado como una herramienta de gran utilidad para el diagnóstico y seguimiento de enfermedades musculares, entre ellas la rabdomiólisis. Se presenta el caso de un paciente en el que se realizó evaluación ultrasonográfica de rabdomiólisis.
ObjetivoResaltar la importancia de la ultrasonografía como parte fundamental en el diagnóstico en rabdomiólisis, describir los hallazgos ultrasonográficos y revisar la literatura disponible.
Caso clínicoPaciente de 30 años con rabdomiólisis por inmovilización prolongada de ambos muslos. Se le practicó insonación con ultrasonido modelo (Philips Sparq), empleando un transductor lineal de alta frecuencia (5-10MHz), bajo modo de escaneo bidimensional (2D), en cortes longitudinales y transversales al nivel de ambos muslos. Las imágenes obtenidas fueron: desorganización de la orientación de las fibras musculares, imagen de vidrio despulido, engrosamiento de la fascia muscular y la presencia de zonas anecoicas.
ConclusionesLa ultrasonografía es una herramienta útil en la evaluación de la rabdomiólisis.
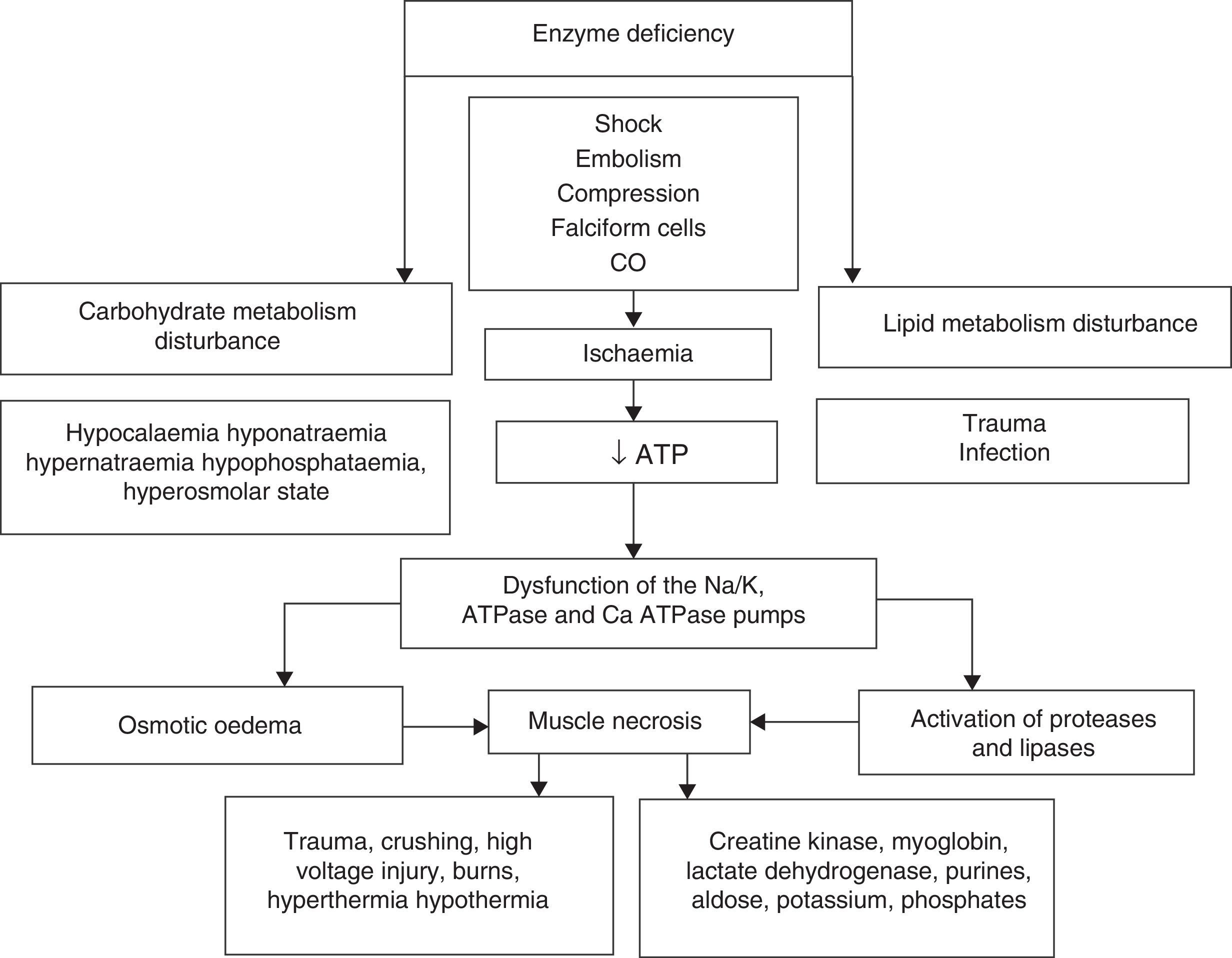
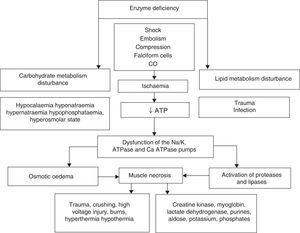
Rhabdomyolysis is secondary to necrosis of the skeletal muscle, and the resulting release of its structural components into the circulation. These include electrolytes, myoglobin and sarcolemma proteins (creatine kynase, aldolase, lactate dehydrogenase, alanine amino transferase and aspartate aminotransferase). Simultaneously there is major depletion of ATP created by dysfunction of the ionic interchange pumps, which leads to a persistent increase in calcium levels at sarcoplasmic level, continuous contraction of the muscle fibres, activation of protease and phospholipases, destruction of myofibrillar proteins of the cytoskeleton disintegrating the myocyte1–3 (Fig. 1).
The upper part summarises hereditary and acquired causes of rhabdomyolysis. Of acquired causes, ischaemia causes dysfunction of the energy-dependent pumps resulting in increased intracellular sodium (Na), activation of the 2Na/Ca2+ exchange pump and increased cytoplasmatic calcium (Ca2+). Elevated concentrations of cytoplasmatic Ca2+ cause osmotic oedema and activate the enzymatic cascade that leads to cell death with the consequent release of skeletal muscle components into the bloodstream (ATP: adenosin-triphosphate; CO: carbon monoxide).
Massive muscle necrosis manifests clinically as myalgia, muscle weakness and pigmentation of urine with no haematuria. Acute renal injury is the most serious potential complication of rhabdomyolysis, and is considered a marker of poor prognosis.4
Rhabdomyolysis is a complex entity, for which an appropriate initial approach is essential, as is follow-up monitoring of its progression in order to make correct and timely treatment decisions and avoid the serious associated complications. Early diagnosis requires high clinical suspicion and the relevant laboratory tests. Magnetic resonance is the best imaging method for diagnosing rhabdomyolysis, due to its high sensitivity and specificity in assessing the muscle. Its disadvantage is the cost, the inherent risks in transferring critically ill patients to the imaging room and time usage.5 Ultrasound has been widely used in assessing musculoskeletal disease because it is easily accessible, it is a non-invasive procedure, it can be performed at the patient's bedside, has a low learning curve and it does not use ionising radiation. Diagnosis is facilitated because the ultrasound findings, such as muscle disorganisation, are correlated with clinical symptoms and muscle insonation is used to evaluate the day-to-day progress of the rhabdomyolysis patient for purposes of comparison. Brockmann assessed the usefulness of muscle ultrasound, and reported its sensitivity to be above 81% and specificity 96% in the detection of abnormal changes in muscle tissue. It is also useful in detecting neurogenic changes, with sensitivity above 77%, and even greater specificity (98%), with lower precision in detecting myopathic changes (79%) and clearly lower precision for non-specific changes in tissue (70%).6 However, we know of no studies that assess the use of ultrasound as a diagnostic tool in rhabdomyolysis.
The objective of this study is to describe the advantages of ultrasound and its principle findings in the diagnosis and evaluation of rhabdomyolysis.7–9
Clinical caseWe present the case of a 30-year-old male patient, with no chronic degenerative diseases relevant to his current disease. The disorder started during an abseiling activity, when he was left hanging and only attached at the waist by one harness for approximately 6h, in an arched position, and his lower limbs were immobilised. After rescue, he presented pain in his spine, with induration and loss of sensitivity in the pelvic limbs and pigmented urine. He was transferred to the Fundación Clínica Médica Sur for integral care. The laboratory results were: CPK>41,000U/l, CK-MB 21.6U/l, myoglobin 44,171ng/ml, ALT 295U/I, AST 812U/I, FA 35UI/l, GGT 41UI/l, DHL 3866IU/l, BUN 104mg/dl, Cr 9.07mg/dl, uric acid 10.8mg/dl, Na 138mmol/l, K5.53mmol/l, Cl100mmol/l, corrected Ca 6.8mg/dl, phosphorus 10.1mg/dl, Mg 2.56mg/dl, albumin 1.8mg/dl.
A presumptive diagnosis was made of rhabdomyolysis and compartment syndrome of the pelvic limbs, due to the presence of induration of the limbs with loss of sensitivity, with levels up to 5 times higher than the reference CPK level, and with pigmented urine and acute renal function disturbance. The patient underwent dermofasciectomy of both thighs and was admitted to the intensive care unit.
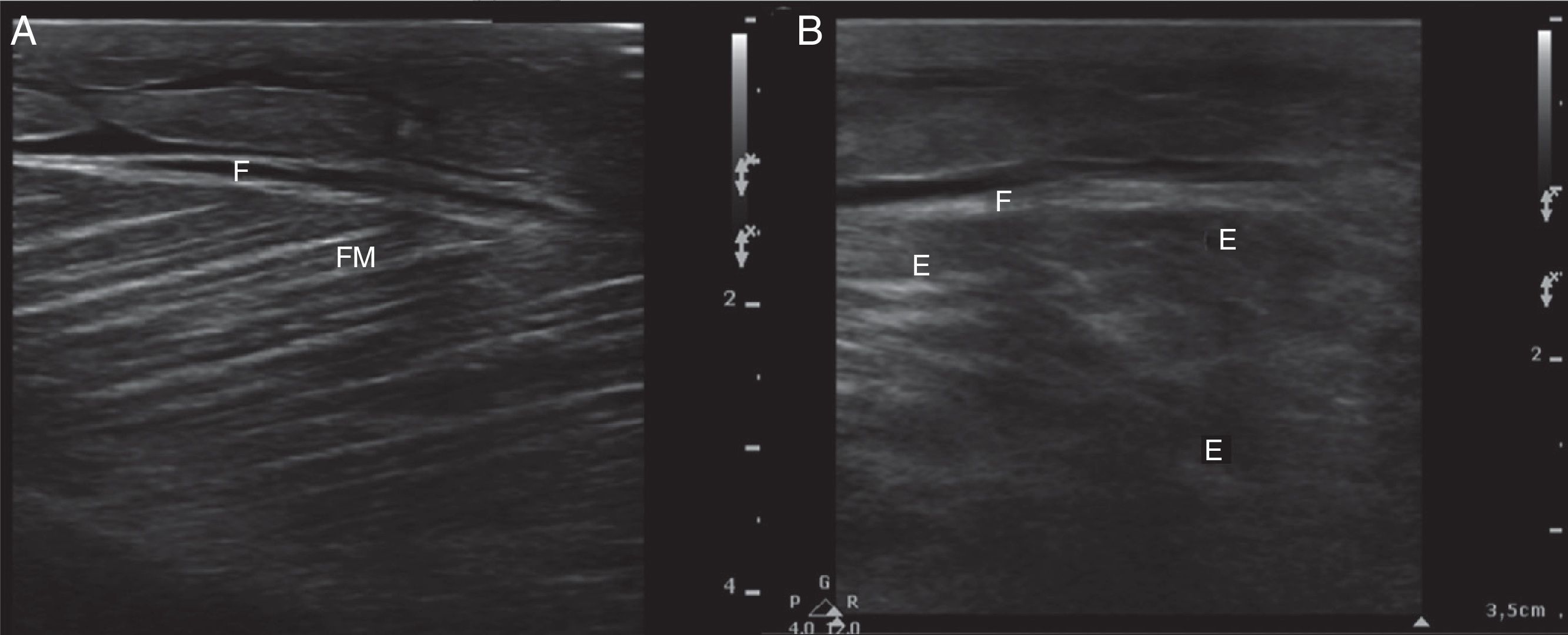
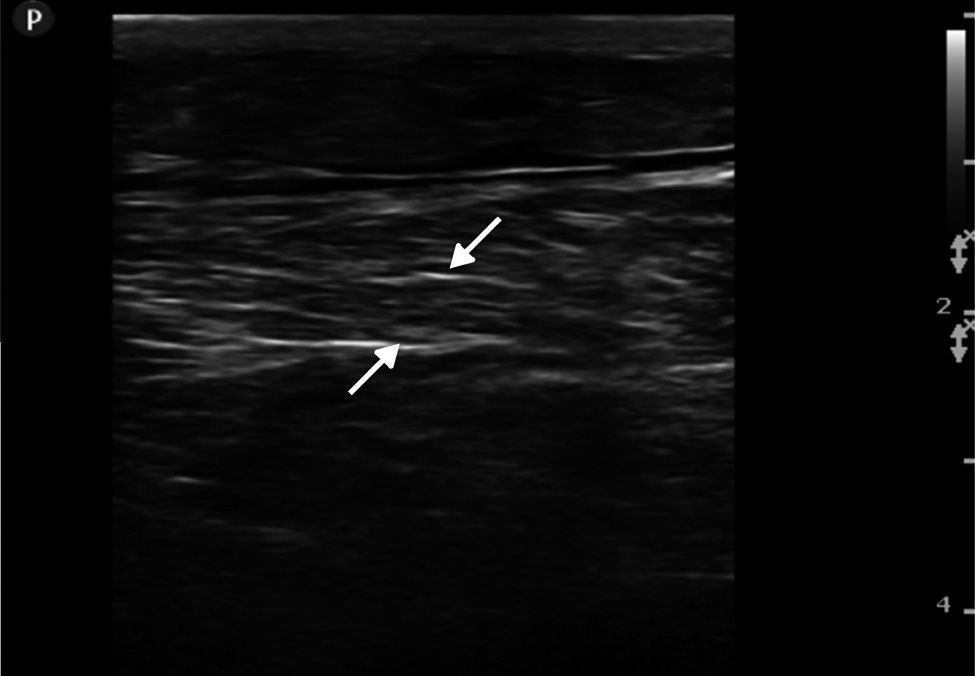
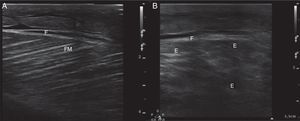
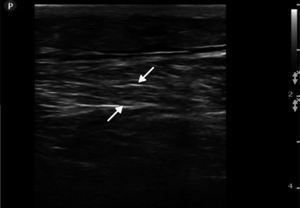
Ultrasound insonation was performed with a Philips Sparq model with a high-frequency linear transducer (5–10MHz), in low-dimensional scanning mode (2D), in longitudinal and transverse sections at the level of both thighs. The images obtained were as follows: ground glass-like or cloudy image (reduced echogenicity), thickening of muscular fascia (Fig. 2A), hyperechoic intramuscular areas in both rectus femoris muscles (Fig. 2B), irregular anechoic areas in the muscular and intramuscular periphery with no blood flow signals compatible with fluid, irregular and heterogeneous muscle fibres (muscular disorganisation) (Fig. 3). Vascularisation was preserved in the periphery depicted by the external circumflex artery with preserved flow velocities.
(A) Ultrasound image in 2D mode, longitudinal section of the left rectus femoris muscle of a healthy patient, showing muscle fascia (F) of preserved diameter and muscle fascicles (MF) distributed obliquely and evenly, characteristic image in “bundles of straw”. (B) Ultrasound image of the left rectus femoris muscle with rhabdomyolysis, showing muscle fascia thickening (F), loss of orientation of the muscle fascicles with reduction of echogenicity and anechoic areas (E).
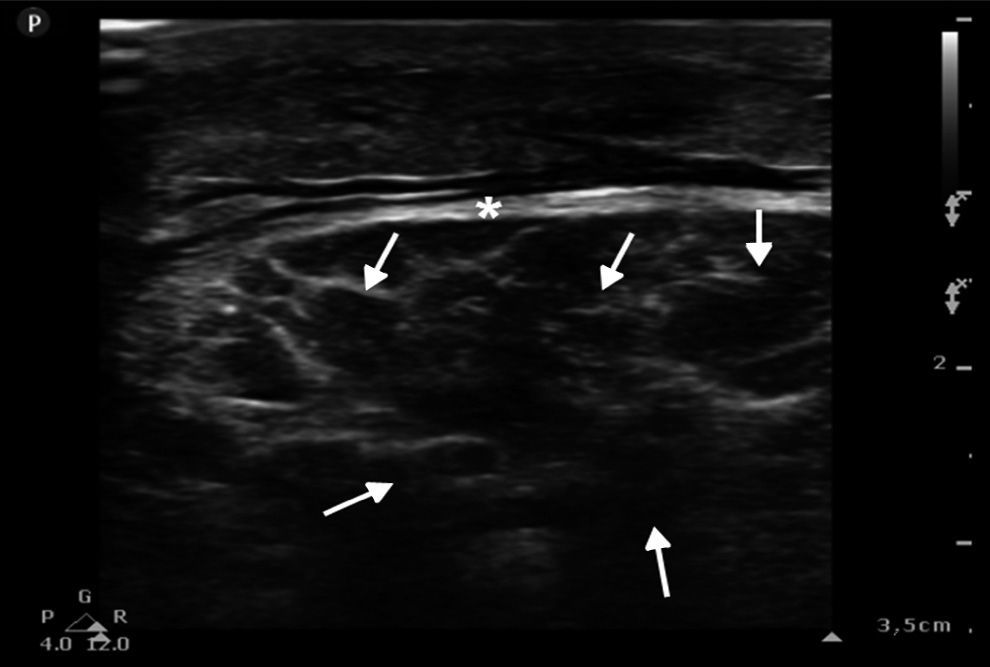
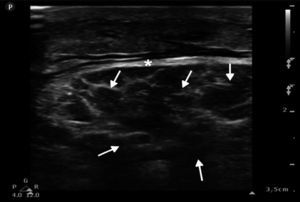
The femoral, popliteal and pedal veins and arteries were assessed and flow was not seen to be compromised, which is a relevant finding in this case, since compartment syndrome was ruled out (Fig. 4).
DiscussionRhabdomyolysis is a syndrome caused by necrosis of the skeletal muscle and the resulting release of muscle cell content. Various factors have caused this disorder to present more frequently recently in the hospital environment, 9 with the increasing incidence of severe trauma, medications, and strenuous exercise in patients lacking in physical fitness.10–13
Ultrasound methods in rhabdomyolysisSteeds et al.8 described the ultrasonic features in a patient with rhabdomyolysis secondary to heroin abuse, showing multiple hyperechoic areas at the level of the gastrocnemius muscle sheaths, with disorganised fascicular architecture, revealing similar changes in the thenar eminence and in the lumbar region of the erector muscle of the spine. Shan et al.14 described the disorder at the level of the masseter muscle in a patient with rhabdomyolysis caused by alcohol abuse, showing hyperechoic areas and disorganised muscle fibres. Su et al.15 published a case series of 19 patients diagnosed with rhabdomyolysis and 7 patients with rhabdomyolysis plus compartment syndrome, both were the result of crush injuries. The clinical features observed in these patients were ground glass-like or cloudy image, uneven anechoic areas in the muscular and intramuscular periphery, irregular and heterogeneous muscle fibres; in patients with rhabdomyolysis and compartment syndrome, the striated muscle volume increased and the flow velocity in the distal arteries decreased. Finally, Chiu et al.16 published the case of a 17-year-old patient who presented with rhabdomyolysis after running. The ultrasound scan showed diminished echogenicity, increased muscle thickness and disorganisation of the muscle fibres of the external abductor.
In line with the documented evidence, various ultrasound patterns are seen in patients with rhabdomyolysis. These include, a reverse image where the muscle septa are shown as distended and hypoechoic and the muscle fibres appear relatively hyperechoic with a ground glass-like image, irregular anechoic areas in the muscular and intramuscular periphery, with no signals of blood flow compatible with fluid and loss of muscle integrity in the affected muscle; the latter being the most representative echographic feature of rhabdomyolysis. Reduced echogenicity can be associated with local inflammation, oedema and bleeding.16 Hyperechoic intramuscular areas have been observed in several reports. They are thought to originate from muscle fibre hypercontractility, in the acute phase of the injury.17 The increased thickness of the muscle sheath is caused in the main by trauma or excessive muscle activity, which develops through sustained muscle distension and inflammatory changes in the muscle.1 Intramuscular hypoechoic areas seen on ultrasound usually present when there is rupture to the muscle fibres depicting oedema.17 Likewise, intramuscular hypoechoic areas seen on ultrasound with relative hyperechogenicity of the muscle fibres can be present in inflammatory or infectious myositis and this should be considered a differential diagnosis and a correlation made with the clinical findings.18
In our case we found the following ultrasound images: (1) reduction in echogenicity (ground glass-like or cloudy image), (2) muscular disorganisation; (3) increased diameter of the muscle fascia, (4) intramuscular hyperechoic areas, (5) uneven anechoic areas in the muscular and intramuscular periphery, with no signals of blood flow compatible with oedema, and (6) normal vascularisation with preservation of waves and flow velocities. The ultrasound findings observed in this report are similar to those reported in the international literature.
ConclusionsUltrasound in rhabdomyolysis and compartment syndrome provides vitally important information for the diagnosis, treatment and follow-up of patients with this disorder. It is an easy-to-use, technological tool that can be used at the patient's bedside.
To date, there are no studies that assess ultrasound as a diagnostic tool for rhabdomyolysis. However, given the scientific evidence, it is positioning itself as a non-invasive procedure to employ in emergencies where early diagnosis is of great importance. It is worth mentioning that this document constitutes the first evidence reported in our environment, highlighting the importance of ultrasound and its findings in rhabdomyolysis.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Carrillo-Esper R, Galván-Talamantes Y, Meza-Ayala CM, Cruz-Santana JA, Bonilla-Reséndiz LI. Manifestaciones ultrasonográficas en rabdomiólisis. Cir Cir. 2016;84:518–522.