Infection by Helicobacter pylori (H. pylori) affects 50% of the world population. Simple methods for its detection are now available.
ObjectivesTo identify H. pylori by using a monoclonal coproantigen technique in paediatric patients, and to determine its association with gastrointestinal diseases.
Materials and methodsThe study included a total of 110 subjects aged 1 to 18 years. The study variables included: Family history of gastrointestinal disease, age, gender, gastrointestinal symptoms, as well as apparently healthy (asymptomatic) subjects. The monoclonal coproantigen test was performed on stool samples. Two groups, I symptomatic (n=29), and II asymptomatic (n=81) were compared using parametric and non-parametric statistics.
ResultsOf the 110 patients, 59 (54%) were male. The relationship between a family history of gastritis and a positive for H. pylori, was significant for mothers (p<0.0005), fathers (p<0.0001), and paternal grandfathers (p<0.0001). It was significant for gastric cancer in maternal grandparents (p<0.0178) and paternal grandparents (p<0.0092). The monoclonal coproantigen test was positive in 31 (28.2%) of the subjects. All were positive in group I, and only 2 in group II. A significant positive association was observed between H. pylori and various signs and symptoms, such as epigastric pain (p<0.001), recurrent peri-umbilical pain (p<0.001), bloating (p=0.016), heartburn (p=0.0007), nausea (p=0.0061), diarrhoea (p=0.0389), and constipation (p=0.0019).
ConclusionsH. pylori detection, was positive in 28% of both groups, and showed significant relationships with family gastrointestinal diseases and gastrointestinal symptoms.
La infección por Helicobacter pylori (H. pilory) afecta al 50% de la población mundial. Se dispone actualmente de métodos menos complejos para su detección.
ObjetivosIdentificar H. pylori mediante coproantígeno monoclonal y adicionalmente, su relación con gastropatías familiares.
Material y métodosEn 110 pacientes de edades entre 1 y 18 años, consideramos: antecedentes familiares de gastropatía, edad, género, síntomas gastrointestinales; también en sujetos aparentemente sanos. La prueba de coproantígeno monoclonal se realizó en muestras de materia fecal. Se compararon 2 grupos: I) sintomáticos (n=29), y II) asintomáticos (n=81), mediante estadística paramétrica y no paramétrica.
ResultadosDe la muestra, 59 (54%) fueron pacientes masculinos. La asociación entre antecedentes familiares de gastritis y positividad por H. pylori fue significativa, para: madres (p<0.0005), padres (p<0.0001), y abuelos paternos (p<0.0001); para cáncer gástrico fue significativa para abuelos maternos (p=0.0178), y para abuelos paternos (p=0.0092). La prueba de coproantígeno monoclonal fue positiva en 31 (28.2%) de los sujetos, en el grupo I todos resultaron positivos y en el grupo II, solo 2. Se observaron asociaciones significativas entre la positividad a H. pylori y diversos signos y síntomas, como: dolor epigástrico (p<0.001), dolor periumbilical recurrente (p<0.001), distensión abdominal (p=0.016), pirosis (p=0.0007), náuseas (p=0.0061), diarrea (p=0.0389), y estreñimiento (p=0.0019).
ConclusionesLa prueba de coproantígeno monoclonal resultó positiva para H. pylori en el 28% de los sujetos examinados y mostró asociaciones significativas con gastropatías familiares y sintomatología digestiva.
Helicobacter pylori (H. pylori) is estimated to affect 50% of the world population1; in underdeveloped and developing countries 80% of adults and 50% of children are colonised. In México 30% of one year-old children and even younger ones are known to be colonised. This figure rises with age until it reaches 50% before the age of 10 years old. The prevalence of infection by H. pylori is currently unknown in many countries.1–3
The degree to which the infection is aggressive and the damage it causes to gastric mucus membranes are determined by a range of factors, including: the virulence of the H. pylori strain, its associated cytotoxic gene A (cagA), inflammatory response, bacterial characteristics, host conditions and environmental factors. In paediatric ages, especially in children under the age of 5 years old, clinical patterns vary and some are distinguished more precisely at older ages. These run from asymptomatic states to epigastric pain, abdominal swelling, diarrhoea alternating with periods of constipation, pyrosis or recurring peri-umbilical pain.2,4–7
There is no gold standard test for detecting H. pylori in children; nevertheless, the 13C Urea Breath Test (13C-UBT), has been described as such, given that to date it is the most sensitive and specific. Nevertheless, it is expensive and its use in children under the age of 3 years old is not problem-free. Several methods are currently available for diagnostic support, including invasive ones such as gastric mucus membrane biopsy for histology and culture, the determination of antibodies in serum, polymerase chain reaction and the urease test. The non-invasive tests include the detection of H. pylori antigens and antibodies in saliva, urine and faeces (coproantigen) as well as H. pylori culture in faecal material. The detection of monoclonal coproantigen for H. pylori has recently been said to be a suitable test for clinical and epidemiological studies in children.1,4,8–13
It is recommended that tests which are easier to apply be used for children, as well as ones that are more economical and do not require them to collaborate, especially those who are between babyhood and preschool age.5,8–10 Two such tests are available: one is the culture of H. pylori which is viable or in cocoid form, and the other is the detection of H. pylori antigen in faecal material using mono- and polyclonal antibodies. Evidence has recently been found that the test using monoclonal coproantigen (MCA) is a valid method for the diagnosis of infections by H. pylori in children.1,2,4,5,8,13–19 Nevertheless, there is no recent evidence of its use or utility in Mexican children, at least in the north-east region.
ObjectiveThe aim of this study was to detect the presence of H. pylori using MCA in patients with definite symptoms as well as in other apparently healthy individuals in Sonora State Children's Hospital, together with its association with gastric pathologies in other members of their family.
Material and methodsPatients aged from 1 to 18 years old were selected who visited the outpatient department where the study took place from January 2013 to June 2015, and those who showed specific signs and symptoms suggestive of dyspeptic states were included, such as epigastric or recurring pain, gastritis and duodenitis. Healthy children who visited for check-ups (i.e., seemingly healthy ones) were also included. Patients with symptoms of chronic disease, those treated with steroids or immunosuppressive drugs, those with parasitosis and those who did not wish to continue in the study were excluded.
This study was approved bioethically by Sonora State Children's Hospital and the Department of Medicine and Health Sciences of Sonora University, with informed consent.
Study variables were age, sex, family background of parents and grandparents (a history of gastritis and proven infection by H. pylori, gastric ulcer, duodenal ulcer and gastric cancer). Two groups were formed: group I was composed of symptomatic subjects, while group II was composed of subjects who had visited as healthy children for check-ups and were apparently free of symptoms at the time of the study.
The subject in group I were identified by means of a structured questionnaire on gastrointestinal symptoms such as epigastric pain, vomiting, nausea, abdominal swelling, pyrosis, heartburn in the stomach or duodenum, diarrhoea, hyporexia, anorexia, upper digestive tract bleeding, recurring abdominal pain, with the following characteristics: diffuse or peri-umbilical, several times a day, in the morning or during school time, lasting for a short time, without interfering with their appetite or normal activity, and if they had lost weight. The subjects in group II were asked the same questions.
The subjects were stratified according to their age as older babies, preschool, school age and adolescents. 3 coproparasitoscopic concentration studies were performed for each one of the subjects in the study using Stoll's procedure, and in one sample coproantigen for H. pylori was determined using Amplified IDEIATM Hp StAR reagent, 2013. The faecal samples (0.1g) were stored at −70°C or were processed immediately. 500μl of faeces were used, homogenised by 15s in a vortex and centrifuged at 2500rpm for 5min, and 50μl of floating material was placed in a microcell where positive and negative control was added to it with 50μl of conjugated enzyme, after which the micro-slide was covered and incubated at from 18°C to 27°C during 1h. The complex conjugated antibody reagent determined the presence of H. pylori antigens (sandwich complex). After the automatic washing process to remove unadhered antibodies, 100μl of substrate was added then incubated at 20°C to 30°C for 10min. They were read in a spectrophotometer at 450nm, and an optical density of ≥0.190U was considered to be positive.4,8,9,14–19 The asymptomatic patients who were found to be MCA positive or had gastrointestinal symptoms associated with H. pylori were monitored as outpatients, and if symptoms appeared or if they had a family history of gastric cancer they were evaluated again.
The symptomatic children and those with positive coproantigen were prescribed treatment with proton pump inhibitors (secnidazole, amoxicillin and clarithromycin), and they were given an appointment for a follow-up test using MCA one month later.1,4,9,18–23 Likewise, those patients with very definite symptoms for H. pylori infection but coproantigen negative were also monitored; this was also performed for cases with a history of repeated episodes of gastritis or a gastric ulcer in members of the direct family. According to the clinical criterion they received eradication treatment.19–24 The patients with parasites were treated and sent to the outpatient department of the hospital for monitoring.
The differences between the groups in the numbers of positive cases were examined using Pearson's chi-squared test. To explore the relationship between a positive H. pylori case and a family history of gastric pathologies, such as gastritis, gastric ulcer and cancer Fisher's exact test and Wald's test for difference in proportions were used. The 2 tail hypothesis was used and values of p<0.05 were considered to be statistically significant. The analysis was complemented by calculating the odds ratio (OR), with confidence intervals of 95%, to examine the potential association between infection by H. pylori and the signs and symptoms of the subjects. Data analysis was carried out using JMP Pro 11.0 SAS Institute Inc software.
Results110 patients were studied, of which 51 (46%) were female and 59 (54%) were male; the majority of the patients were recorded in spring and summer. In terms of the paediatric stages considered, it was found that 71 (64%) were older babies and preschool infants, 22 (20%) were school pupils and 17 (15%) were adolescents. Among the hereditary family background of group I subjects, composed of 29 patients, 18 (62%) referred gastritis in their mother and 29 (100%) in their maternal grandfather; there were also 6 (20%) with a history of gastric cancer in their maternal grandfather. In the paternal line 19 (65%) cases were found of gastritis in the father and 19 cases (65%) in the paternal grandfather and 14 (48%) in the paternal grandmother, and, of these, 7 (24%) had a history of gastric cancer.
Regarding the backgrounds of the 81 patients in the apparently healthy group, 22 (27%) of the mothers of the children mentioned gastritis, as well as 41 (51%) of the maternal grandfathers. Gastric cancer was identified in 5 (6%) and gastric ulcer in 2 maternal grandfathers. 35 fathers (44%) and 35 paternal grandfathers had suffered gastritis; 7 (8%) had gastric cancer and one had a duodenal ulcer.
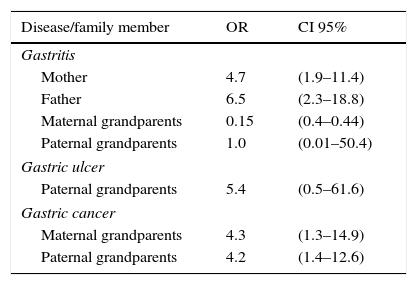
Significant associations were found between positive and symptomatic cases and a family history of gastric complaints. This was so for gastritis in mothers and fathers, as well as for gastric cancer in maternal and paternal grandfathers (Table 1).
The relationship between family members with gastric pathologies connected with HP and positive cases.
| Disease/family member | OR | CI 95% |
|---|---|---|
| Gastritis | ||
| Mother | 4.7 | (1.9–11.4) |
| Father | 6.5 | (2.3–18.8) |
| Maternal grandparents | 0.15 | (0.4–0.44) |
| Paternal grandparents | 1.0 | (0.01–50.4) |
| Gastric ulcer | ||
| Paternal grandparents | 5.4 | (0.5–61.6) |
| Gastric cancer | ||
| Maternal grandparents | 4.3 | (1.3–14.9) |
| Paternal grandparents | 4.2 | (1.4–12.6) |
Level of significance p=0.05.
HP: Helicobacter pylori; CI 95%: 95% confidence interval; OR: odds ratio.
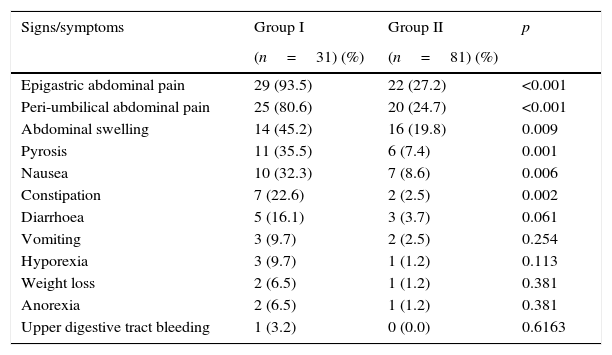
On the other hand, when both study groups were compared significant differences emerged in the distribution of clinical symptoms including abdominal epigastric pain, peri-umbilical pain, abdominal swelling, pyrosis, nausea and constipation. In all cases these were more common in the group of symptomatic patients. There were no significant differences in the other symptoms examined (Table 2).
Distribution of clinical symptoms in the study groups.
| Signs/symptoms | Group I | Group II | p |
|---|---|---|---|
| (n=31) (%) | (n=81) (%) | ||
| Epigastric abdominal pain | 29 (93.5) | 22 (27.2) | <0.001 |
| Peri-umbilical abdominal pain | 25 (80.6) | 20 (24.7) | <0.001 |
| Abdominal swelling | 14 (45.2) | 16 (19.8) | 0.009 |
| Pyrosis | 11 (35.5) | 6 (7.4) | 0.001 |
| Nausea | 10 (32.3) | 7 (8.6) | 0.006 |
| Constipation | 7 (22.6) | 2 (2.5) | 0.002 |
| Diarrhoea | 5 (16.1) | 3 (3.7) | 0.061 |
| Vomiting | 3 (9.7) | 2 (2.5) | 0.254 |
| Hyporexia | 3 (9.7) | 1 (1.2) | 0.113 |
| Weight loss | 2 (6.5) | 1 (1.2) | 0.381 |
| Anorexia | 2 (6.5) | 1 (1.2) | 0.381 |
| Upper digestive tract bleeding | 1 (3.2) | 0 (0.0) | 0.6163 |
Based on Fisher's exact test or Yates’ chi-squared test.
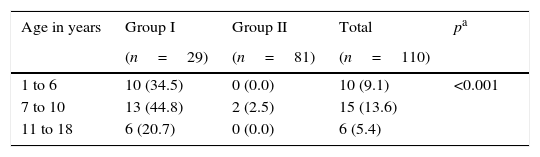
When the MCA test was performed, 29 of the 31 (93.5%) subjects in group I were found to be positive, while only 2 of the 81 patients (2.5%) were positive in group II, in those attending healthy children check-ups. Nevertheless, when they were given the questionnaire about clinical symptoms it was found that 27% of them had gastrointestinal signs associated with a possible infection by H. pylori. The difference in distribution according to age group was significant (Table 3).
Distribution of positive cases for HP infection detected by MCA, according to the age group of subjects included in the study.
| Age in years | Group I | Group II | Total | pa |
|---|---|---|---|---|
| (n=29) | (n=81) | (n=110) | ||
| 1 to 6 | 10 (34.5) | 0 (0.0) | 10 (9.1) | <0.001 |
| 7 to 10 | 13 (44.8) | 2 (2.5) | 15 (13.6) | |
| 11 to 18 | 6 (20.7) | 0 (0.0) | 6 (5.4) |
MCA: monoclonal coproantigen; HP: Helicobacter pylori.
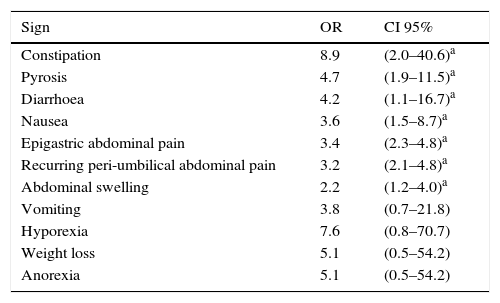
When the relationship between a positive MCA test and clinical symptoms was examined, it was found that the greatest associations arose for constipation (OR=8.9, CI 95%: 2.0–40.6), pyrosis (OR=4.7, CI 95%: 1.9–11.5) and diarrhoea (OR=4.2, CI 95%: 1.1–16.7), although nausea, abdominal pain and abdominal swelling were also associated with a positive test for H. pylori (Table 4).
The association between HP infection and study subject signs or symptoms.
| Sign | OR | CI 95% |
|---|---|---|
| Constipation | 8.9 | (2.0–40.6)a |
| Pyrosis | 4.7 | (1.9–11.5)a |
| Diarrhoea | 4.2 | (1.1–16.7)a |
| Nausea | 3.6 | (1.5–8.7)a |
| Epigastric abdominal pain | 3.4 | (2.3–4.8)a |
| Recurring peri-umbilical abdominal pain | 3.2 | (2.1–4.8)a |
| Abdominal swelling | 2.2 | (1.2–4.0)a |
| Vomiting | 3.8 | (0.7–21.8) |
| Hyporexia | 7.6 | (0.8–70.7) |
| Weight loss | 5.1 | (0.5–54.2) |
| Anorexia | 5.1 | (0.5–54.2) |
HP: Helicobacter pylori; CI 95%: 95% confidence interval; OR: odds ratio.
Finally, 87% of the 31 patients who were considered for therapeutic intervention responded well. When the MCA test was applied to them one month later it was negative; on the other hand, 2 patients in group II were treated because they had a direct family history of gastric cancer, even though they were apparently healthy. The patients who were found to be positive were offered the therapeutic procedure again.
DiscussionThis study shows that the detection of H. pylori infection by MCA is of value in children with clinical gastrointestinal symptoms. The test detected H. pylori in 94% of symptomatic subjects, although it was less able to identify H. pylori in apparently healthy subjects. The findings of this study are consistent and confirm the results of previous reports, which have shown that the technique is useful.5,8–10,13–16
Family precedents involving gastritis and gastric cancer were notably more common. In connection with the odds ratios obtained, the results should be taken with care due to the nature of the distribution of the sample of patients studied, as the test used (maximum verisimilitude) requires a larger sample.
The signs and symptoms of H. pylori in paediatric ages require proper characterisation, particularly in children younger than 3 years old, who find it harder to express themselves clearly. This is why it is known that clearer data are obtained from schoolchildren and adolescents. On the other hand, of the signs and symptoms associated with H. pylori, and even though controversies still exist, have been said to play an important role in recurring abdominal pain.8,11,16,20–26 In this report, when the relationship between H. pylori and gastrointestinal symptoms is analysed, this type of abdominal pain is the second sign in order of frequency.
A controversial question is whether H. pylori should be routinely investigated and treated, even in asymptomatic patients, due to its close association with benign and malign gastrointestinal diseases in adults and children. While screening is considered to be hardly beneficial in Canada and the United States,3–5,9,11–16,19–22 in the Latin American and Mexican paediatric population, H. pylori bacteria are a major source of morbidity; its prevalence stands at from 30% to 80%, and it is associated with chronic gastritis and peptic ulcers.2,7,14–16,18,20,22,25,26 In the Latin American Consensus on H. pylori Infection, it was concluded that studies of prevalence and associated factors in each population group should be performed, and that in Latin America H. pylori is a public health problems that requires action plans.3,14,15
Although the serological test to identify H. pylori is useful, prior to using it suitable cut-off points have to be set to define positive results in each population. In spite of the fact that it has been shown to be useful in prevalence studies, its routine use for diagnosis has not been clearly defined, given that controversies still exist; this test also has the disadvantage of being invasive.14,26–29 In children the use of the serological test is not fully defined, as it depends on variations in the immune response at different ages; it is therefore necessary to have a low cost and easy-to-perform test for routine research into H. pylori.5,8,9,19
The use of MCA in our institution made it possible to learn that at least 28% of the patients who visit the outpatient department have an infection by H. pylori. As well as its clinical value this procedure is easy to use. It is not invasive and may be of use in diagnosis and follow-up, especially in symptomatic patients who are found to be positive. Nevertheless, its cost is currently a disadvantage in our field. It does of course have the advantage that it can be used in prevalence studies, although without excluding the need to test all asymptomatic positive patients using the 13 carbon urea breath test or 13C-UBT.
On the other hand, we consider that it is prudent to continue studies in our country that make it possible, for symptomatic as well as asymptomatic patients, to discover the sensitivity and specificity of this test, so that it can be used and recommended for wider use in diagnosis as a simple and non-invasive method.1,2,5,9,12
Given that in México H. pylori infection is a public health problem, and that it is also linked to the subsequent development of gastric carcinoma, it is prudent on the one hand to follow-up the group of children with a family history of gastric cancer, and to attempt as far as is possible to discover the type of neoplasia in question. On the other hand, it is also prudent to offer the health authorities evidence for the implementation of research projects and additional accessible diagnostic procedures so they can be repeated in the general population. This would permit the creation of suitable control measures and health care actions.1,2,6,30–32
ConclusionsThe detection rate of H. pylori using MCA was similar to those recorded in the literature for this procedure. There was a statistically significant relationship for gastric pathologies in related adults with H. pylori infection and symptomatic cases with a positive MCA test. Apparently healthy patients stated they had gastrointestinal symptoms, and 2.5% were MCA positive. Respecting gastrointestinal symptoms, epigastric abdominal pain was found to be the most frequent, followed by peri-umbilical pain. When each sign and symptom was compared individually with the MCA test, the significant variables were found to be epigastric pain, recurring peri-umbilical pain, abdominal swelling, pyrosis, nausea and constipation.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestsThe authors have no conflict of interests to declare.
The authors would like to thank Dr. Ignacio Fonseca Chon, of the Industrial Engineering Department of Sonora University, for his advice on statistics.
Please cite this article as: Castillo-Montoya V, Ruiz-Bustos E, Valencia-Juillerat ME, Álvarez-Hernández G, Sotelo-Cruz N. Detección de Helicobacter pylori en niños y adolescentes mediante coproantígeno monoclonal y su asociación con gastropatías. Cir Cir. 2017;85:27–33.