Acute colonic pseudo-obstruction, or Ogilvie syndrome, is a motility abnormality characterised by rapid and progressive dilation of the large intestine. To achieve a diagnosis it is fundamental to exclude mechanical obstruction with imaging studies such as computer axial tomography. The combined incidence of Ogilvie and dysmorphic syndrome has not been described.
Clinical caseFemale patient of 28 years old with a history of infant cerebral palsy came to emergency room with 4 days of intestinal obstruction. She had hypokalaemia that was reverted, but persisted with obstruction. Later after 72h with recovery of fluids and electrolytes and administration of prokinetics, the obstruction reversed. She was discharged with no complications.
ConclusionsNon-invasive medical treatment solves most cases. Promising results have been achieved with neostigmine. In the event of no response to drug therapy, the next step is endoscopic treatment. Even with high recurrence this is preferred due to its lower level of complications in contrast to surgical decompression. Neonatal dysmorphic syndrome is often associated with disorders of the central nervous system. So far, there have been no reports on the incidence of this disease with Ogilvie syndrome, although 9% of cases have been described as associated with neurological events. Conservative management in this disease is the initial approach. Interventions should be reserved for when conservative treatment fails.
La pseudoobstrucción colónica aguda o síndrome de Ogilvie es un trastorno de la motilidad caracterizado por la dilatación del intestino grueso de inicio rápido y progresivo. Para su diagnóstico, es fundamental excluir una oclusión mecánica mediante estudios de imagen como la tomografía computada. Actualmente no contamos con la incidencia asociada entre síndrome de Ogilvie y el síndrome dismórfico.
Caso clínicoPaciente femenina de 28 años de edad, con antecedente de parálisis cerebral infantil, ingresó a urgencias por presentar síndrome de oclusión intestinal de 4 días de evolución. Con hipokalemia leve, la cual es corregida sin remitir el cuadro clínico por lo que, 72 h posteriores al tratamiento conservador con procinéticos, remite el cuadro clínico y es dada de alta de urgencias, sin complicaciones.
ConclusiónEl tratamiento médico no invasivo con la neostigmina soluciona la mayor parte de los casos de pseudoobstrucción colónica aguda o síndrome de Ogilvie y, cuando no se soluciona con el tratamiento farmacológico, la siguiente opción es el tratamiento endoscópico que, a pesar de su alta recurrencia, es preferido debido a su menor grado de complicaciones, en contraste con la descompresión quirúrgica. El síndrome dismórfico neonatal se asocia en el 9% a trastornos del sistema nervioso central. Hasta el momento no se ha descrito la incidencia de esta enfermedad con el síndrome de Ogilvie. Por otro lado, se ha descrito que el 9% se asocia a eventos neurológicos.
Acute colonic pseudo-obstruction or Ogilvie's syndrome is a motility disorder characterised by dilation of the large intestine of rapid and progressive onset. It was first described in 1948 by Ogilvie in 2 patients with no mechanical obstruction.1,2 Predisposing factors have been identified: multiple trauma, heart surgery, major surgery, metabolic disorders and narcotic administration (the latter is associated in up to 50% of cases).1 Other studies report that 19% of cases are associated with pelvic surgery, obstetric and gynaecological procedures, and 18% with orthopaedic procedures, 10% with infections, another 10% are associated with cardiac events and 9% are associated with neurological events.3,4
Clinical caseA 28-year-old female patient, diagnosed with infantile cerebral palsy at age 2, attended the Emergency Department with a 10-day history of inability to pass a stool, accompanied by slow and progressive abdominal distension, with no anorexia, hyporexia or cytophobia. Her mother reported that she did not notice any pain facies at first and that the patient continued her usual diet and, 4 days prior to her admission, had stopped passing wind. Her mother noticed her reduced stool evacuation, and started the usual management with senna leaves, linseed and psyllium, with no result.
Physical examination: The patient was younger than her chronological age, in decubitus position with dysmorphic facies, and retropulsion of the lips. Elongated thorax with well-developed breasts, no nodules or tumours, symmetrical nipples, no nipple discharge or secretion. Cardiopulmonary examination unremarkable; distended abdomen under tension with an incoercible, reducible umbilical hernia; no changes in colour or periumbilical temperature. No peristalsis; the patient awoke with pain on medium palpation, with involuntary abdominal resistance, negative rebound. Major muscular atrophy of the limbs, with upper motor neuron syndrome in the 4 limbs.
Laboratory studiesOn admission: anaemic, haemoglobin 10.9g/dl and haematocrit 31.5%, low density hypolipoproteinaemia 34. With hypokalaemia 2.5mmol/l, no electrocardiographic manifestations and hypomagnesaemia of 1.5mg/dl; the remaining laboratory results were unremarkable.
Twenty-four hours after admission, the patient presented improved hypokalaemia 3.3mmol/l, but magnesium persisted at 1.3mmol/l.
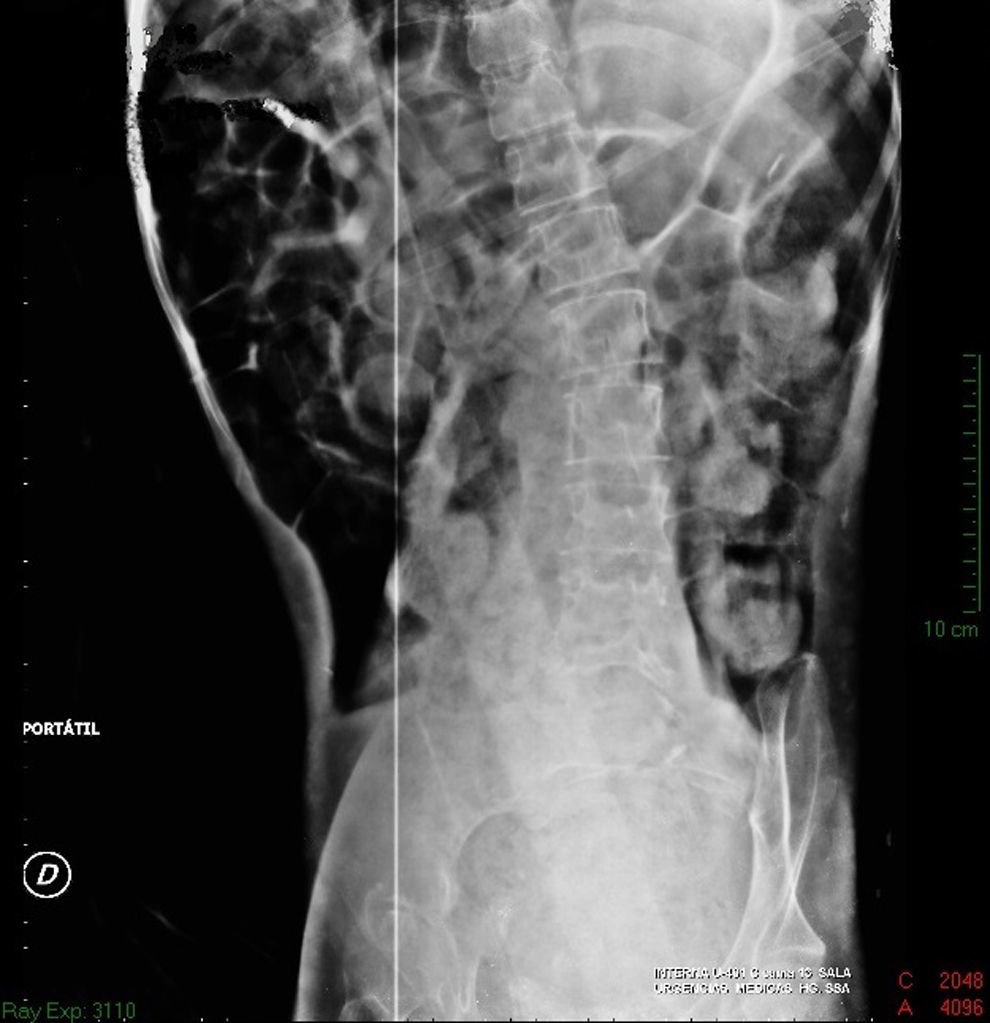
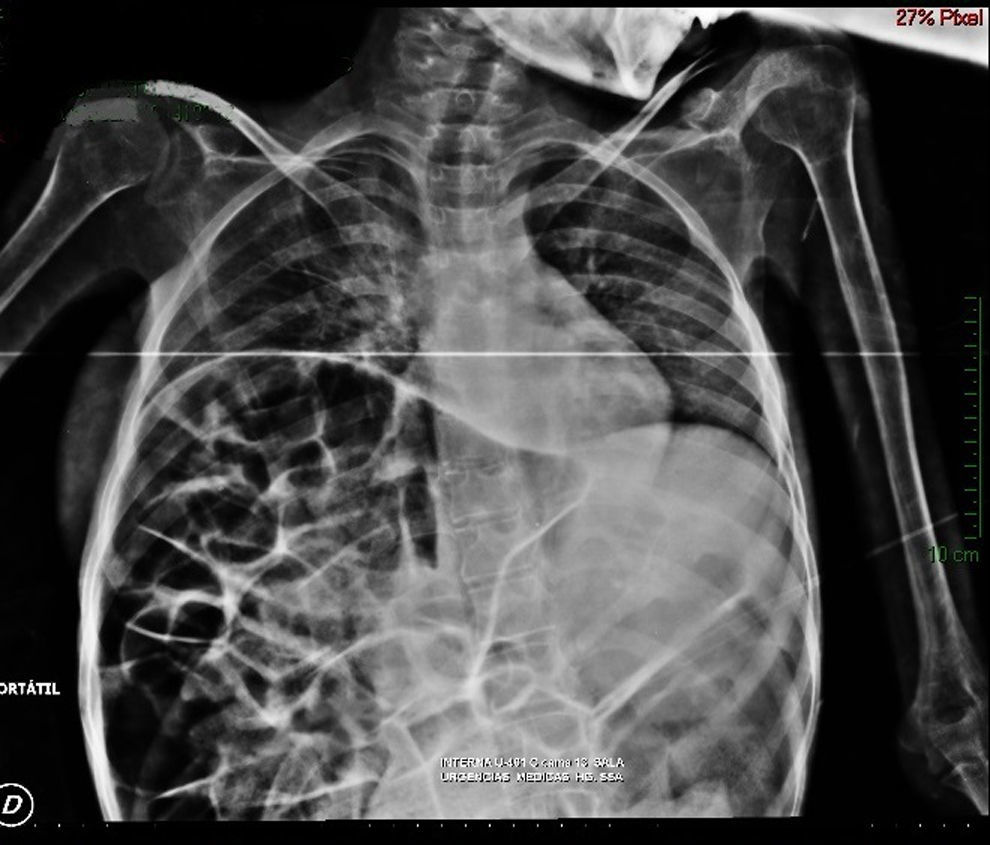
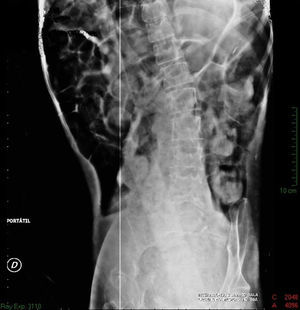
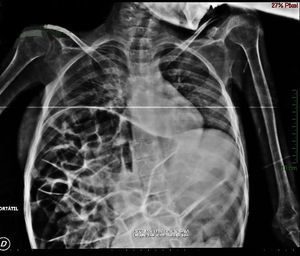
Imaging studiesMajor dilation of the large bowel loops was observed on plain X-ray of the abdomen, with an absence of gas in the rectal ampulla and interloop oedema (Fig. 1). Chest X-ray showed elevation of the diaphragm to the 4–5 intercostal space, with dilation of the intestinal loops and hepatic shadow on the left hemidiaphragm (Fig. 2).
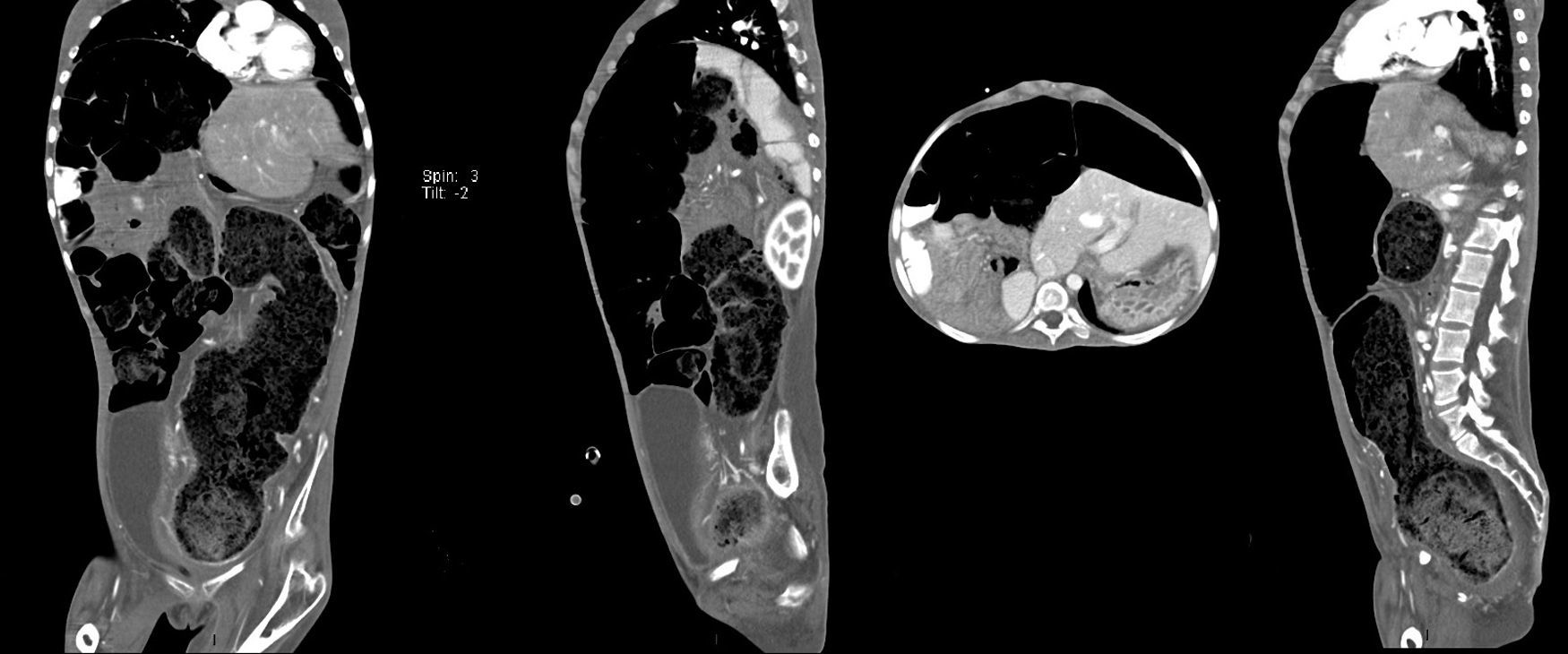
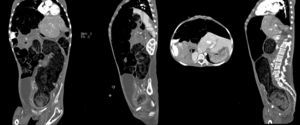
Computed axial tomography with double contrast showed sparse left posterior basal lamina pleural effusion and significant dilation of the small bowel loops, with the presence of fluid and contrast medium inside, and which were displaced towards the upper right quadrant, with significant elongation of the mesenteric vessels, indicating malrotation of the small bowel loops. There was hepatodiaphragmatic colonic interposition with major displacement of the liver to the left, inducing displacement of the spleen and stomach to the rear. The colonic frame was enlarged with the presence of abundant faecal material; the diameter of the colon had reached 53mm, and at the level of the sigmoid and rectum was 98mm in diameter. The bladder was part full, with fluid and contrast medium inside, Foley catheter status, seen to be displaced towards the right iliac fossa. It was difficult to assess the uterus, partially visible, and the adnexa could not be assessed (Fig. 3).
TreatmentDuring her stay in the Emergency department, the patient was managed with a nasogastric tube which had an initial output of 1220ml of material with intestinal characteristics. The patient was treated with crystalloids and baseline electrolyte requirements. She was also given metoclopramide 10mg IV every 8h, throughout her hospital stay. After 3 days of conservative treatment, the clinical symptoms abated and the patient passed abundant, pasty stools, with no mucous or blood. Surgical treatment was not necessary, and the patient was discharged the following day.
DiscussionThe patient presented with symptoms of intestinal occlusion and hypokalaemia which was corrected 24h later; the occlusive symptoms persisted. Tomography showed distension of the bowel loops that exceeded 9cm. The outcome was stationary and the clinical symptoms abated with support measures, in line with 70% of the cases described in the literature.
Furthermore, because the patient had low-intensity lipoprotein levels at low ranges, Bassen–Kornzweig syndrome (abetalipoproteinaemia) was suspected.5,6 This could not be documented because it was not possible to follow-up the patient and family members.
Pseudo-obstruction is characterised by intestinal obstruction symptoms, with absence of mechanical occlusion of the small or large intestine.9 The incidence of Ogilvie syndrome is uncertain and there is an estimated 30% hospital mortality, which increases according to caecal diameter. In other words, the greater the caecal diameter, the greater the rate of perforation.8 Ogilvie initially postulated that the wall of the colon and the rectum might relax in response to physiological and pharmacological stimuli, causing an automatic imbalance of intrinsic control as the basis of the clinical picture. This hypothesis has been confirmed, since symptoms are relieved by adrenergic block followed by cholinergic stimulation or by neostigmine alone.8,10,11 Clinical symptoms tend to develop progressively after dilation, ischaemia (due to vascular congestion of the wall), perforation and death. In the event that the caecum dilates more than 9–10cm (measured on the abdominal X-ray), there is imminent risk of perforation.8,12,13 The perforation threshold was considered to be 9cm.3,14 By contrast, Vanek and Al-Salti15 found that a diameter of 12cm was rarely associated with perforation; while a caecal diameter greater than 14cm has a 23% perforation incidence.3 It can be said that the perforation threshold range remains at 9–12cm.3 Saunders and Kimmey16 found that symptoms lasting more than 6 days have a high risk of perforation.3
Its pathophysiology has not been clearly defined, despite the fact that the enteric nerves contain a variety of neurotransmitters responsible for contracting and relaxing smooth muscle. The main neurotransmitters are acetylcholine, neurokinin A and substance P; while the contractility inhibitors are vasoactive intestinal polypeptide and nitric oxide. The external influence is provided by the sympathetic nerves at lumbar level: these tend to reduce intestinal motility. The parasympathetic nerves of the tenth cranial nerve, the vagus nerve and the sacral segments increase motility.3,17 Initially, Ogilvie suggested that there was an imbalance in the activity of the autonomic nervous system, of parasympathetic predominance, which resulted in excessive dilation of the colon.2,3 Furthermore, it has been indicated that there is an increase in the sympathetic tone or reduction of parasympathetic activity, which results in a pseudo-obstruction of the distal colon and dilation of the proximal colon, known as adynamic colon.3,18 Unfortunately, to date there are no animal models to exemplify and guide the mechanisms of this disorder.3 Interstitial cells of Cajal were found to be missing in patients with chronic pseudo-obstruction, in a study by Jain et al.3,19 Finally, cytokines appear to alter intestinal motility, particularly in an inflammatory state.20
It is difficult to diagnose this disease because it is impossible to differentiate it from a metabolic ileus,7 where it is crucial to rule out mechanical occlusion, therefore diagnosis by imaging is essential. Barium enema and computed axial tomography can be useful in differentiating between mechanical occlusion and metabolic ileus.
Barium enema has 80% sensitivity and 100% specificity for diagnosing mechanical occlusion of the large bowel.3,21 The preferred contrast is hydrosoluble, because of the risk of extravasation and due to the therapeutic effect that it might have in this study in facilitating decompression of the colon.3,22 However, computed axial tomography has replaced barium enema.3,23 A retrospective study of a seven year period demonstrated that tomography has 96% sensitivity and 93% specificity in diagnosing large bowel occlusion.3,21 The most common findings were proximal dilation with an intermediate transition zone in the splenic flexure or adjacent to it, and no occlusive images or abrupt changes in the calibre, to indicate mechanical occlusion, were observed.3,24
Treatment is divided into medical and surgical, and medical treatment should be started as soon as possible once the condition has been identified.
Deterioration or no improvement in 48–72h indicates that it is essential to change therapeutic strategy.3,20
Non-invasive treatment resolves most cases of pseudo-obstruction. However, it is important to bear in mind that laxatives increase the production of intestinal gas, which perpetuates distension, with a worsening of clinical symptoms, therefore they should be avoided.3,20
Position can help, especially the prone position elevating the hips with a pillow or lifting the knees to the chest. This often encourages spontaneous passing of flatus or even stools.3,25 There is evidence that 70% of patients can respond to supportive measures alone, with a complication rate of 6%, and 10% mortality.3,26 Drug therapy is another option, with prokinetic agents such as erythromycin, metoclopramide and cisapride.3 Erythromycin has a recurrence rate of 50%, and cisapride was removed from the market in 2000.3,27 Neostigmine and guanethidine have been used in some studies; promising results have been achieved with neostigmine, with 91% response after a single dose of 2mg IV over 3–5min.3,28 Furthermore, neostigmine has adverse effects such as bradycardia, arterial hypertension, asystole, convulsions, anxiety, tremors, myosis, bronchoconstriction, hyperperistalsis, nausea, vomiting, sialorrhoea, diarrhoea, diaphoresis and abdominal cramps.3 Therefore patients treated with neostigmine should be strictly monitored and an atropine syringe kept prepared and available in the event of arrhythmia.3,18
In the event of a failure to respond to drug treatment, the next step is endoscopic treatment, which should be performed without administering oral laxatives or preparing the colon.3 It is not always necessary to reach the ileocaecal valve, reaching the splenic flexure is generally sufficient.3,25,26
There is up to 40% recurrence, which poses a problem for endoscopic treatment,3,29 yet, even with this rate of recurrence, it is preferable because it has fewer complications. A perforation rate of up to 2% has been described and mortality of up to 1%, compared to surgical decompression.3,4,28
A percutaneous approach with colostomy is another endoscopic treatment option, with a 42% complication rate which includes: wound infection, haemorrhage, haematoma, perforation, resulting in peritonitis, granuloma, retraction of the stoma and umbilicated stoma.3,30
The surgical options described are open or laparoscopic colostomy and colectomy. Laparotomy is indicated if intestinal ischaemia or perforation is suspected, and when the diagnosis is not clear.3 There are reports of 30% mortality and 6% morbidity.3,14
ConclusionMost cases are resolved by non-invasive medical treatment. Promising results have been achieved with neostigmine.
If there is no response to drug treatment, the next step is endoscopic treatment. Even though it has a high recurrence rate it is preferable because of its lower rate of complications compared to surgical decompression.
Dysmorphic neonatal syndrome is usually associated with disorders of the central nervous system.
The incidence of this disease with Ogilvie syndrome has not been described to date; 9% have been described associated with neurological events.
Conservative management of this disease is the initial approach; surgical intervention should be reserved for when this fails.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestsThe authors have no conflict of interest to declare.
Please cite this article as: Rendón-Medina MA. Síndrome dismórfico neonatal y síndrome de Ogilvie. Cir Cir. 2017;85:148–153.