Training devices for percutaneous renal access are expensive, have hazardous biological materials, or radiation. Two devices were designed that eliminate some or all of these characteristics (ManiPERC and iPERC).
ObjectiveTo compare the improvement in access time to the posterior lower calix with 2 inanimate models in a group of urology residents.
Material and methodsQuasi-experimental clinical trial with 16 urology residents to compare the improvement over time of percutaneous renal access by training in 2 inanimate models (iPERC: simulated fluoroscopy and ManiPERC: real fluoroscopy).
ResultsSubjects were assigned to one of 2 groups (iPERC and ManiPERC) and a video analysis of all of them was performed before and after 20 training sessions. Both groups improved their access time; with iPERC from 133.88±41.40 to 76±12.62s (p=0.006) and from 176.5±85.81 to 68.75–18.40s (p=0.007) with ManiPERC. Comparing iPERC versus ManiPERC there was no difference between them in improving access time (ANCOVA: Model F (1.13)=1.598, p=0.228).
ConclusionsBoth models are equivalent in improving skills; however, even though none of them generated bio-waste, the absence of radioactive emissions makes iPERC the more advantageous model.
Los dispositivos de entrenamiento en punción percutánea renal son costosos, usan residuos biológicos infecciosos o emiten radiación. Diseñamos 2 dispositivos que eliminan algunas o todas estas características (ManiPERC e iPERC).
ObjetivoComparar la mejoría en el tiempo de acceso al colector posterior e inferior al practicar en los dispositivos.
Material y métodosEnsayo clínico cuasiexperimental con 16 residentes de urología. Se asignaron los sujetos a uno de dos modelos de dispositivo de entrenamiento para realizar 20 sesiones de punción y se analizaron los videos del entrenamiento antes y después de 20 sesiones.
ResultadosAmbos grupos mejoraron su tiempo de acceso; con iPERC pasó de 133.88±41.40 a 76±12.62s (p=0.006), y con ManiPERC, de 176.5±85.81 a 68.75±18.40s (p=0.007). Al comparar iPERC versus ManiPERC, no hay diferencia entre ellos en la mejoría del tiempo de acceso (ANCOVA: F Modelo (1.13=1.598, p=0.228).
ConclusionesAmbos modelos son equivalentes en la mejoría de las destrezas; sin embargo, aun cuando ninguno de ellos genera residuos biológicos, la ausencia de emisiones radiactivas hace del iPERC el modelo con mayor ventaja.
The probability of developing urinary lithiasis during a lifetime has increased in parallel to obesity and type 2 diabetes, at 12% for men and 4.8% for women, with a recurrence of 30–40%. This represents healthcare expenditure on lithiasis calculated at 2 billion dollars for the year 2000 in the United States.1
Percutaneous nephrolithotomy is the technique of choice for most renal calculi larger than 2cm, and its use has increased by 50.4% over the past 15 years as it is a minimally invasive procedure.2
The complication rate of this surgical procedure is not negligible, and it is estimated that 7.8% of patients present significant bleeding, 5.7% requiring transfusion, 3.4% present major perforation of the pyelocalyceal system, and up to 1.8% present hydrothorax. Deaths associated with the procedure have also been described,3 and perforation of the abdominal viscera: the duodenum,4,5 the intra and extrahepatic bile duct,6 spleen7–9 and, most commonly, the colon.10–12 Certain lesions can endanger the patient's life by damaging structures such as the vena cava.13
The puncture technique for percutaneous access is the procedure which is most associated with complications, and the time and number of punctures made for access are determining factors.14,1586.3% of percutaneous renal access procedures worldwide are fluoroscopy guided to enable better three-dimensional orientation of the pyelocalyceal system, and thus improve the precision of access between the complex vascular and calyceal anatomy of the kidney.16–19
There are other factors which make percutaneous access a procedure which requires a high degree of skill: the external rotation of the kidney on the coronal plane, the posterior rotation on the transversal plane and the great variability of the distance of the kidney from the skin due to each patient's body fat levels, and the presence of a duplex collecting system in the lower pole in more than half of cases.20–22
Furthermore, through procedures in vivo, on average doctors receive radiation dosages of 0.28mSv (6.04min), and the dosage would be even greater for tutors if they were present at all training sessions.23 Doctors undergoing training can receive dosages of up to 5.2mSv to the hands, 7.5mSV to the fingers and 1.6mSv to the eyes over up to 21.9min per event.24 According to the International Commission on Radiological Protection, the maximum recommended occupational exposure limit is 20mSv per year,25 therefore models where fluoroscopy is used to perform indefinite repetition sequences would appear not to be the best option.
The time to access the pyelocalyceal system during a fluoroscopy-guided nephrolithotomy is directly proportional to the time of exposure to radiation, and it has been estimated that one in 1000 people exposed to at least 10mSv throughout their lives will develop cancer.26
From 36 to 60 cases are required for the learning curve to perform percutaneous renal surgery,27 but a doctor in training will feel comfortable making access after 21 procedures; This curve is directly completed on patients as there is no appropriate model for ex vivo practice.28–31
When formal training is given on percutaneous access it is more likely that after training, the doctor will suggest the option of percutaneous nephrolithotomy (27% vs. 11%) to their patients, and those who do not suggest this option argue that it is an access procedure which requires a great deal of skill.31
There are few models for guided percutaneous renal access, and they are generally biological, which require training through repetitions on pigs’ kidneys27,32,33 and exposure at a significant accumulated dosage of radiation during these repetitions.
Given their characteristics, biological models also require appropriate facilities for surgical procedures on animals and appropriate handling of the biological waste that is generated, and also have major ethical considerations.34
Training models should improve the key points in percutaneous fluoroscopy-guided renal access, which are, the total access time and imaging time,35,36 the latter directly relates to the accumulated radiation of the patient and the surgical team, but the number of C-arm repositionings and the number of needle adjustments with the C-arm at 0° and 30° can be associated with renal trauma.
There is no model in our environment which combines the ideal characteristics for training on percutaneous renal access. Models expose the user to the risks involved in handling biological materials and the use of radiation, and those which are free from exposure are of high cost due to the software and hardware that they require.
The use of an inanimate model which simulates the clinical scenario, which enables appropriate spatial orientation of the access sites and avoids the use of biological material and the exposure to radiation of pupils and teachers, would be ideal.
We present a comparative study of 2 inanimate models developed in our hospital to improve the access time to the posterior, lower collecting system with practice sessions in a group of Urology interns.
Materials and methodsA quasi-experimental study was performed in the Speciality Hospital of the Hospital de Especialidades Centro Médico Nacional Siglo XXI del Instituto Mexicano del Seguro Social during the period between 3rd September 2013 and 1 st March 2014, with 16 interns, who practised on one of the 2 inanimate models designed by ourselves (patent pending), for training on percutaneous fluoroscopy-guided renal access.
Interns studying all or part of their urology speciality in the Instituto Mexicano del Seguro Social were included in the study, with no prior practice in percutaneous renal surgery. The exclusion criteria were: experience in percutaneous nephrolithotomy as a surgeon with more than 5 cases and having taken drugs affecting the central nervous system.
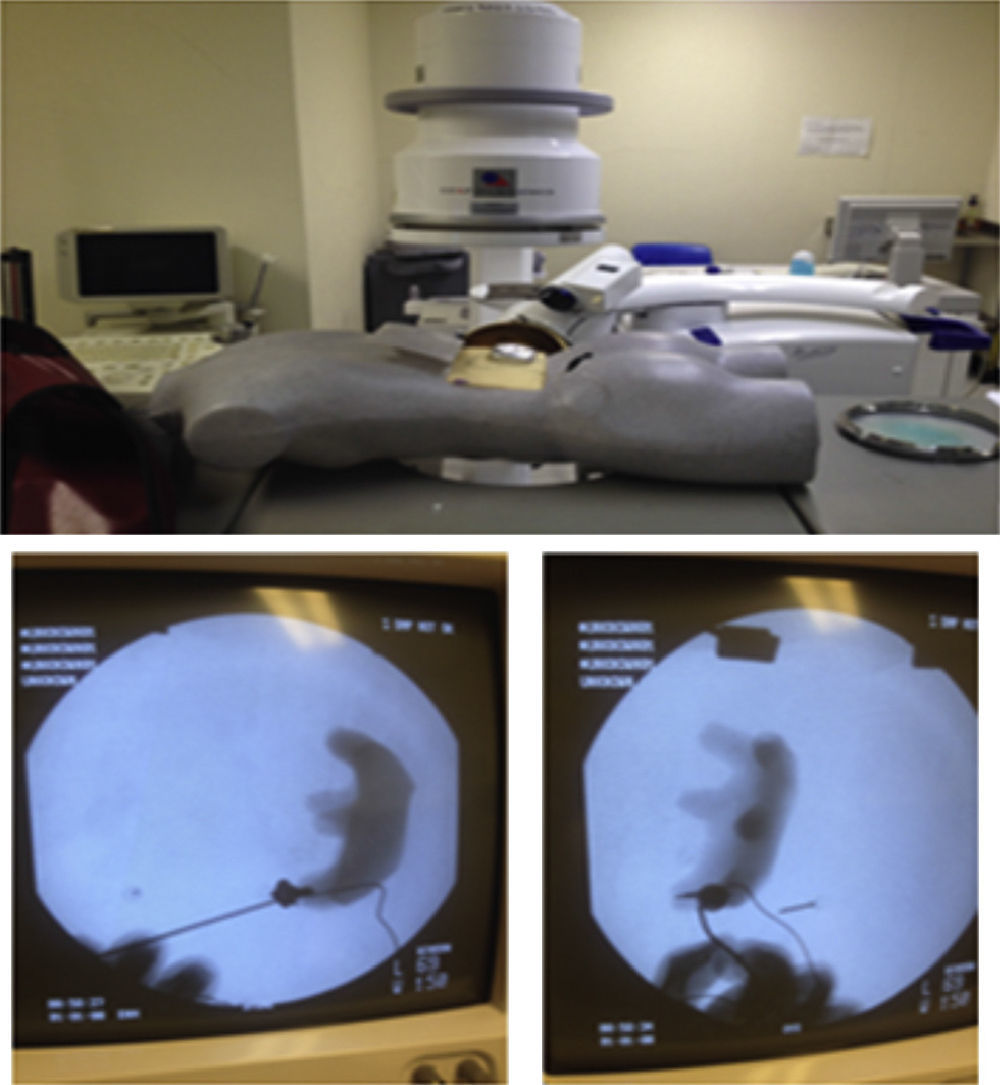
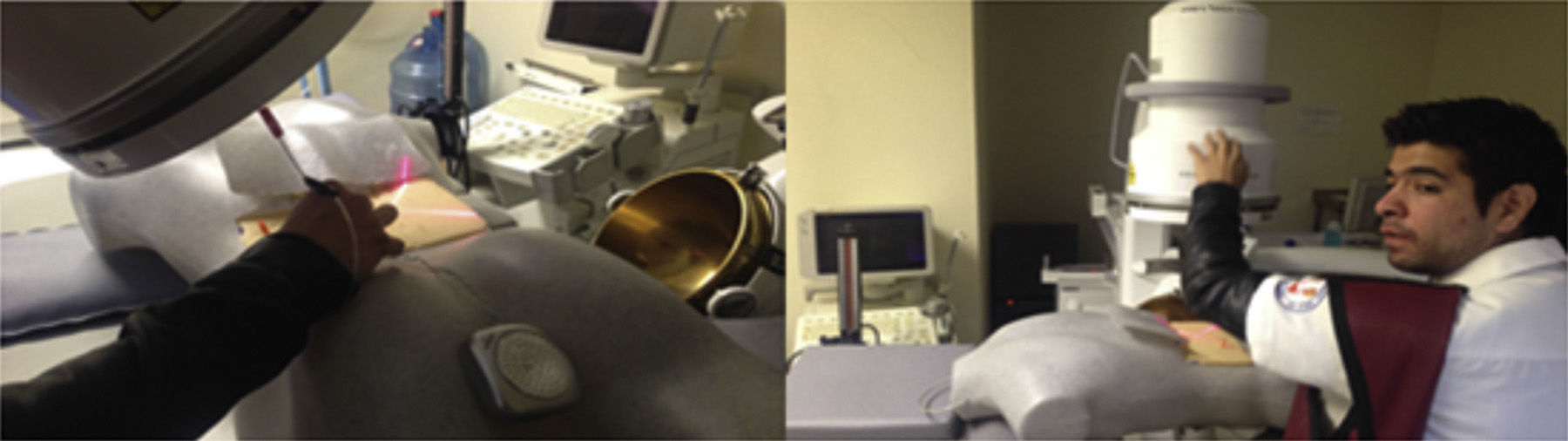
The interns carried out 20 practice sessions on one of the 2 inanimate models as training in percutaneous fluoroscopy-guided renal access with triangulation technique. The first, called iPERC (patent pending), enables the simulation of fluoroscopic emission on emitting light through a 15W lamp (direct current) when a switch is activated and it has a rotating arm in the neutral position (0°) and turn position at 30°, with an upper platform on which to place a mobile telephone (with camera and video recording function) and to use its video camera to visualise the manoeuvres on its screen in real time. It uses a pyelocalyceal system of radio-opaque resin connected to an electrical circuit which closes and activates an indicator light when it reaches the target to be punctured, contained in an opaque acrylic cube with a polyethylene puncturing surface. The second model is called ManiPERC, it is a three-dimensional training model with a polystyrene dummy which enables the same circuit and resin pyelocalyceal system used in the iPERC to be placed, with the angle characteristics of the human pyelocalyceal system, inside a radiolucent polyurethane foam cube inside the dummy, using fluoroscopic images at 0° and 30° obtained with the C-arm of the extracorporeal lithotripsy table, Edap Technomed Vision model of the Urology Department of the Hospital de Especialidades Centro Médico Nacional Siglo XXI, del Instituto Mexicano del Seguro Social. The fluoroscopic images and the adjustments of the arm and the puncture needle in real time are recorded by video camera. The model is shown in Fig. 1.
The resin moulds of the pyelocalyceal system were produced in series and were the same for all the procedures in order to prevent bias generated by puncturing systems of varying complexity.
The punctures were made after the theoretical bases of the procedure had been explained. The interns worked in pairs to perform the percutaneous access procedures on both inanimate models. Student 1 performed the puncture and student 2 contributed by moving the C-arm and the “fluoroscopy” emission when requested by the student doing the puncture, starting with marking the access point to the lower collecting system on the puncture surface with a vision angle at 0°, and then marking the access point with a vision angle at 30° using the triangulation technique. The needle was subsequently directed adjusting the route with changes of vision angle of the fluoroscopy arm until accessing the lower and posterior collecting system of the pyelocalyceal system, with visual monitoring on the fluoroscopy screen for the ManiPERC and on the mobile phone screen for the iPERC. Images are obtained on the iPERC by activating a button, and on the ManiPERC, with a pedal.
Recordings were made of the initial and final practice sessions after repetitions and access time skills, vision time (fluoroscopy), C-arm movements and needle adjustments at 0° and 30° were analysed. For the ManiPERC model a subjective validation questionnaire was completed by the doctor undergoing training to give the model a score as unfavourable, favourable, good or excellent.
All the doctors practising on the ManiPERC inanimate model were supplied with jackets and collar pads for protection against radiation and were asked to give informed consent in order to participate in the study.
Statistical analysisShapiro's normality test was performed on numerical variables. The statistical differences between each group of research subjects were identified using the Student's-t-test for normally distributed dependent samples, and Wilcoxon's test was used for variables of free distribution. The F test (ANCOVA) was used to compare the access and fluoroscopy (image or vision) time between the 2 groups to homogenise the different initial times between the groups. The remaining nominal variables are described in percentages. The software package SPSS® v. 21 (SPSS, Inc., Chicago, IL, USA) was used for the statistical analysis.
ResultsSixteen interns took part in the study. They were assigned purely in terms of their availability, 8 undertook practice sessions on the iPERC model (Fig. 2) and 8 on the ManiPERC (Fig. 3). The ages of the research subjects were comparable: 29.50 (29–31.5) for the iPERC model and 29.00 (29.00–30.00) for the ManiPERC (p=0.574). Each group's demographic characteristics were as follows: for the iPERC group (n=8), 3 interns (37.5%) were in their fifth year of the speciality, 2 (25%) in the fourth, one (12.5%) in the third year and 2 (25%) in the second; in the ManiPERC training group (n=8), 3 interns (37%) were in their fifth year, 4 (50%) in the fourth, 1 (12.5%) in their third year and there were no second year interns.
An improvement in access time was observed for the iPERC model from 133.88±41.40 to 76±12.62s (p=0.006). The simulated fluoroscopy time decreased from 78.71±37.25 to 39.88±11.34s (p=0.007). The C-arm movements reduced from 8.5 (RIC 3.00–10.50) to 4.0 (IQR 3.00–5.00) (p=0.027); improvement in the number of adjustments of the needle at 0° was 8.5 (IQR 1.00–4.00) to 1.0 (IQR 0.00–2.00) (p=.031) and there was no improvement in adjustments at 30° (p=0.344).
For the ManiPERC there was an improvement in access time, from 176.5±85.81 to 68.75±18.40s (p=0.007); a reduction in fluoroscopy time from 65.63±59.50 to 21.75±11.87s (p=0.037); an improvement in C-arm adjustments and adjustments of the needle at 0° and 30° (p=0.021, p=0.012 and p=0.27, respectively). The results for the skills assessed in each model are shown in Table 1.
Improvement in skills assessed with both of the training models.
| iPERC (n=8) | ManiPERC (n=8) | |||||
|---|---|---|---|---|---|---|
| Skill | Initial | Final | Pa | Initial | Final | Pa |
| Access time, mean (SD) | 132.8 (41.4) | 76 (12.6) | 0.006 | 176.7 (85.8) | 68.7 (18.4) | 0.007 |
| Fluoroscopy time, mean (SD) | 78.7 (37.2) | 39.8 (11.3) | 0.007 | 65.6 (59.5) | 21.7 (11.8) | 0.037 |
| C-arm adjustments, median (IQR) | 8.5 (3–10.5) | 4 (3–5) | 0.027 | 7 (4–12.7) | 4 (1–5) | 0.21 |
| Needle adjustments in 0°, median (IQR) | 3.5 (1–4) | 1 (0–2) | 0.031 | 6.5 (2–11) | 1 (1–2) | 0.012 |
| Needle adjustments in 30°, median (IQR) | 4.5 (2–7) | 3.5 (2–4.7) | NS | 12 (3–16) | 4 (2–6.5) | 0.027 |
SD, standard deviation; NS, not significant; IQR, interquartile range.
The access and fluoroscopy time is reported in seconds and the adjustments of the C-arm and the needle are reported in absolute numbers.
Both models were demonstrated as equivalent in improvement percentage for access time to the kidney, 38.09±20.40 vs. 55.77±18.17% for iPERC and ManiPERC, respectively (p=0.089); the improvement in fluoroscopy time was equivalent, 43.91±16.90 vs. 59.73±14.29% for iPERC and ManiPERC, respectively (p=0.063).
Because the initial access times and fluoroscopy (vision) time were different at the start on the iPERC and the ManiPERC, we used ANCOVA to make comparisons, using it to standardise the initial time in both groups. For the access time, the result of the F-test for the model product and the initial access time assume homogeneity of regression, model F* initial access time 1.12=0.46 (p>0.05), and therefore, since there was no interaction, the net effect of both models on the access time at the end of the practice sessions was the same, model F 1.13=1.598 (p=0.228).
For the fluoroscopy time (vision time) the result of the F-test for the model product and the initial fluoroscopy time assume homogeneity of regression, model F* initial fluoroscopy time 1.12=0.314 (p>0.05), and therefore, since there was no interaction, the net effect of both models on the fluoroscopy time at the end of the practice sessions was different. Model F 1.13=25.53 (p=0.000), better for ManiPERC.
In terms of the subjective validation by the research subjects for the ManiPERC model, 50% (n=4) considered that the model's performance was good and 50% (n=4) considered it excellent, all of them (100%) considered exposure to radiation the model's major disadvantage and the movement of the puncture surface. For the iPERC model all the subjects assessed (n=8) considered that the main advantage was the lack of radiation, but that the lack of support material between the puncture surface and the resin model of the pyelocalyceal system was a disadvantage.
DiscussionPercutaneous renal surgery is a minimally invasive therapeutic option for treating most renal calculi.2 This type of surgery poses serious risks to the patient until the learning curve in this procedure has been completed. Because the learning curve has to be completed directly there and then by the surgeon when operating on patients, it is not generally used by urologists in Mexico, and therefore they do not offer the procedure to their patients despite its being regarded as the gold standard.27
Both our models offer a significant improvement in access time and fluoroscopy time skills, and when they are compared with each other, it can be observed that both models are equivalent. Although the ManiPERC is more realistic according to the subjective validation by the research subjects, the iPERC model has the advantage of no exposure to radiation. Although the fluoroscopic (imaging) exposure time was shorter for the ManiPERC model by 18s, the improvement percentage with respect to initial time was equivalent in both models, this might be associated with the growing awareness of the accumulated radiation dosage in the ManiPERC model group (real radiation).
With regard to the secondary outcomes of the study, which were the comparison of the C-arm movements and the necessary adjustments of the needle at 0° and 30°, both initial and final, there was no significant difference between either model, except for the number of needle adjustments at 30° at the start, which were greater with the ManiPERC model; this might be associated with the ease in orientating the needle in the iPERC model, as it occasionally allows direct visualisation through the translucent puncture surface. However, this might be an advantage for urologists who are new to this surgery.
Progress made over the last century has dictated that the concept of “experimental animal” should be replaced by the much wider concept of “experimental model”. As is logical, the quality of the information to be gained from a model is directly linked with its complexity. When the model is more realistic, it is therefore more complex, it offers the researcher less freedom in setting the factors that they wish to study. Thus, as we are faced with the study of a specific problem, in this case percutaneous fluoroscopy-guided renal access, it is probable that different experimental models will need to be created – as each have their own limitations – in order to eventually confirm the technique in human beings.37–40
There are numerous models, such as those published by De Sá Earp and Imkamp, amongst others, which offer simulation in pigs’ kidneys with good results in most of their skills in up to 83% of their cases. However, they all pose a risk from exposure to biological material, and in the case of De Sá Earp's model, exposure to radiation.27,32,33 In our case, both models offer the possibility of providing training with the advantage that the research subjects are not exposed to biological materials, they imitate human calyceal anatomy in a more precise way, and are easily reproducible; they are low cost, and are easily accessible; all of which are advantages for teaching.
Because all the models of the pyelocalyceal system were identical in order to prevent bias from punctures in systems of varying complexity, we believe that the improvement in skills might also be due in part to spatial learning.
The ManiPERC model is realistic; however it has the drawback of exposure to radiation, which restricts its continuous use. The iPERC model does not have this drawback; however it loses a little in similarity with the real clinical scenario. The acquisition of skills after training on each model needs to be validated in in vivo surgery.
ConclusionsBoth models offer an equivalent significant improvement of the skills studied, with the advantages of no exposure to biological material, and although the ManiPERC model imitates fluoroscopic manoeuvres more exactly, and therefore is more realistic, it has the great drawback of exposure to real radiation, which is the advantage of the iPERC model.
Both models and their results need to be validated by in vivo practice.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Maldonado-Alcaraz E, García FG-M, Serrano-Brambila EA. Evaluación de 2 modelos inanimados para mejorar el tiempo de acceso renal percutáneo guiado por fluoroscopia. Cir Cir. 2015;83:402–408.