Gastric cancer in Mexico is ranked third in both males and females. Most patients present clinically with advanced disease and treatment options are sparse. HER2 overexpression in gastric cancer is related to poor outcome. Immunohistochemical testing for HER2 is becoming the standard of care for guiding adjuvant treatment of gastric cancer with trastuzumab.
ObjectivesTo determine the frequency of HER2 overexpression in patients with gastric cancer in the Hospital de Oncología del Centro Médico Nacional, Siglo XXI and its association with other histopathological findings.
Material and methodsPatients with gastric cancer who underwent surgery between March 12, 2006 and August 31, 2011, were enrolled in this retrospective study. Diagnosis was confirmed by review of slides and immunohistochemistry with anti-HER2 antibody was performed. Scoring was done by Hoffman scoring system. Medical records were evaluated.
ResultsNinety-three patients were included in the study, with 43 (46.2%) male and 50 (53.7%) female patients. The median age was 64 years. HER2-positive tumours were identified in 6 patients (6.45%) and located most frequently in the proximal stomach. There was no difference in HER2 overexpression in relation to age, gender or histologic type.
ConclusionIn our study, about 7% of patients with gastric cancer were HER2-positive on immunohistochemistry.
El cáncer gástrico ocupa en México el tercer lugar en incidencia tanto en hombres como en mujeres. La mayoría de los pacientes se presentan clínicamente con una enfermedad avanzada y pocas opciones de tratamiento. La sobreexpresión del HER2 se asocia con mal pronóstico, por lo que su evaluación con inmunohistoquímica se propone como un método de rutina que puede ayudar a seleccionar a pacientes que podrían beneficiarse del tratamiento con trastuzumab.
ObjetivosDeterminar la frecuencia de la sobreexpresión del HER2 en pacientes con cáncer gástrico tratados en el Hospital de Oncología del Centro Médico Nacional Siglo XXI y su asociación con otros factores histopatológicos.
Material y métodosSe incluyó en el estudio a todos los pacientes con cáncer gástrico operados entre el 12 de marzo de 2006 y el 31 de agosto de 2011. Se revisaron las laminillas originales para confirmar el diagnóstico y la evaluación del HER2 se hizo mediante inmunohistoquímica. Se utilizó el método de Hoffman para valorar la expresión del HER2. Los datos clínicos se obtuvieron de los expedientes de cada paciente.
ResultadosSe incluyó en el estudio a 93 pacientes, 43 hombres (46.2%) y 50 (53.7%) mujeres. La mediana de edad fue de 64 años. El HER2 fue positivo en 6 pacientes (6.45%) y se observó con mayor frecuencia en tumores localizados en estómago proximal y en carcinomas de alto grado. No se observaron diferencias entre la sobreexpresión de HER2 y edad, género o tipo histológico.
ConclusiónAlrededor de un 7% de los pacientes con cáncer gástrico fueron HER2 positivos en nuestro estudio.
Gastric cancer is a public health problem, it is the fourth most frequent and the second cause of cancer deaths worldwide.1 Sánchez-Barriga reports that, between 2002 and 2010, 69,107 patients died from gastric cancer in Mexico. In 2000, there were 5003 deaths due to gastric cancer and there were 5459 deaths due to the disease in 2012.2
Helicobacter pylori infection is the most significant factor in sporadic distal gastric cancer development.3 Some patients with persistent Helicobacter pylori develop gastric atrophy, followed by intestinal metaplasia, which might eventually progress to dysplasia and adenocarcinoma.4,5
The Epstein–Barr virus is also associated with gastric cancer, and is found in 80% of the neoplastic cells and not in the normal epithelial cells of lymphoid stroma-rich gastric adenocarcinomas.6 Around 10% of gastric cancers can occur in the same family.7 Finally, hereditary cases constitute from 1% to 3% of all gastric cancers.8
Most early gastric cancer patients are asymptomatic, therefore the disease is most frequently diagnosed at advanced stages.9 There are few therapeutic options for advanced cancer, and consequently the 5-year survival rate is 20%.10
According to the ToGA study, an international, phase III, randomised trial, chemotherapy in combination with trastuzumab significantly increases the survival of patients with advanced gastric cancer with HER2 overexpression.11 Trastuzumab is a monoclonal antibody that binds selectively to the human epidermal growth factor receptor type 2 (HER2).12
The oncogene HER2neu (c-erb-B2) is located on chromosome 17 (17q11.2-q12) and encodes 185-kDA protein with 3 domains, extracellular rich in cysteine residues, one transmembrane and one intracellular domain with tyrosine kinase activity. HER2 acts as a receptor on the cell surface and belongs to the erbB family, comprised of 4 members (HER1 or EGFR, HER2 itself, HER3 and HER4). The overexpression of HER2 molecules facilitates the spontaneous formation of dimers on the surface of the tumour cell, triggering the activation of various intracellular signalling pathways, the consequences of which are increased cell proliferation, longer cell survival through apoptosis evasion, loss of cell cycle control, greater dedifferentiation and increased cell migration.13,14
Numerous studies have demonstrated that HER2 in breast cancer plays an important prognostic and predictive role, which, in addition, correlates with the progression of the disease.15 On the other hand, HER2 overexpression as a prognostic factor for gastric adenocarcinoma is controversial. This is because initial studies did not demonstrate a correlation between overexpression of this oncoprotein and the prognosis of the disease.16,17 However, other studies report a direct correlation between HER2 overexpression and poor prognosis of the disease.18
The frequency of HER2 overexpression according to the literature is variable: between 7% and 27%.19 At present it is recommended that HER2 status should be evaluated in all gastric carcinomas at time of diagnosis, to select the patients that might benefit at a given time from treatment with trastuzumab.20
The aim of this study was to evaluate the frequency of HER2 overexpression in gastric cancer patients and its association with other histopathological characteristics.
Material and methodsStudy designA retrospective, observational, descriptive, cross-sectional study.
Patients and clinical-pathological informationAll the surgical specimens from total gastrectomies with a diagnosis of carcinoma studied in the period between 12 March 2006 and 31 August 2011 were selected from the pathology archives of the Oncology hospital of the Centro Médico Nacional Siglo XXI, of the Mexican Social Security Institute (IMSS). None of the patients had received neoadjuvant chemotherapy or any other type of treatment prior to the operation. All the cases had their respective histopathological reports and the original slides of each case were reviewed to confirm the diagnosis of carcinoma (IAC and DVR). Clinical parameters were assessed such as the patients’ age and gender, and pathological parameters such as tumour topography, size, stage and histological grade. The carcinomas were classified according to the Lauren21 system on intestinal and diffuse-type adenocarcinomas.
Cases with malignant nonepithelial tumours (lymphoma, sarcoma, neuroendocrine tumours, etc.) partial gastrectomies, cases with incomplete material or with no paraffin blocks or material with major processing artefacts were excluded.
Construction of microarraysThe most representative tumour slide was selected from each case the (areas with a solid architectural pattern with >75% cancer cells, with no presence of necrosis) and then the block corresponding to each. Tissue cylinders of 2mm in diameter were taken from each paraffin block with a punch biopsy forceps, of the type used for skin lesions. The cylinder was inserted in a new block (recipient block), which contained 8 tumour samples divided into 4 columns and 4 rows.
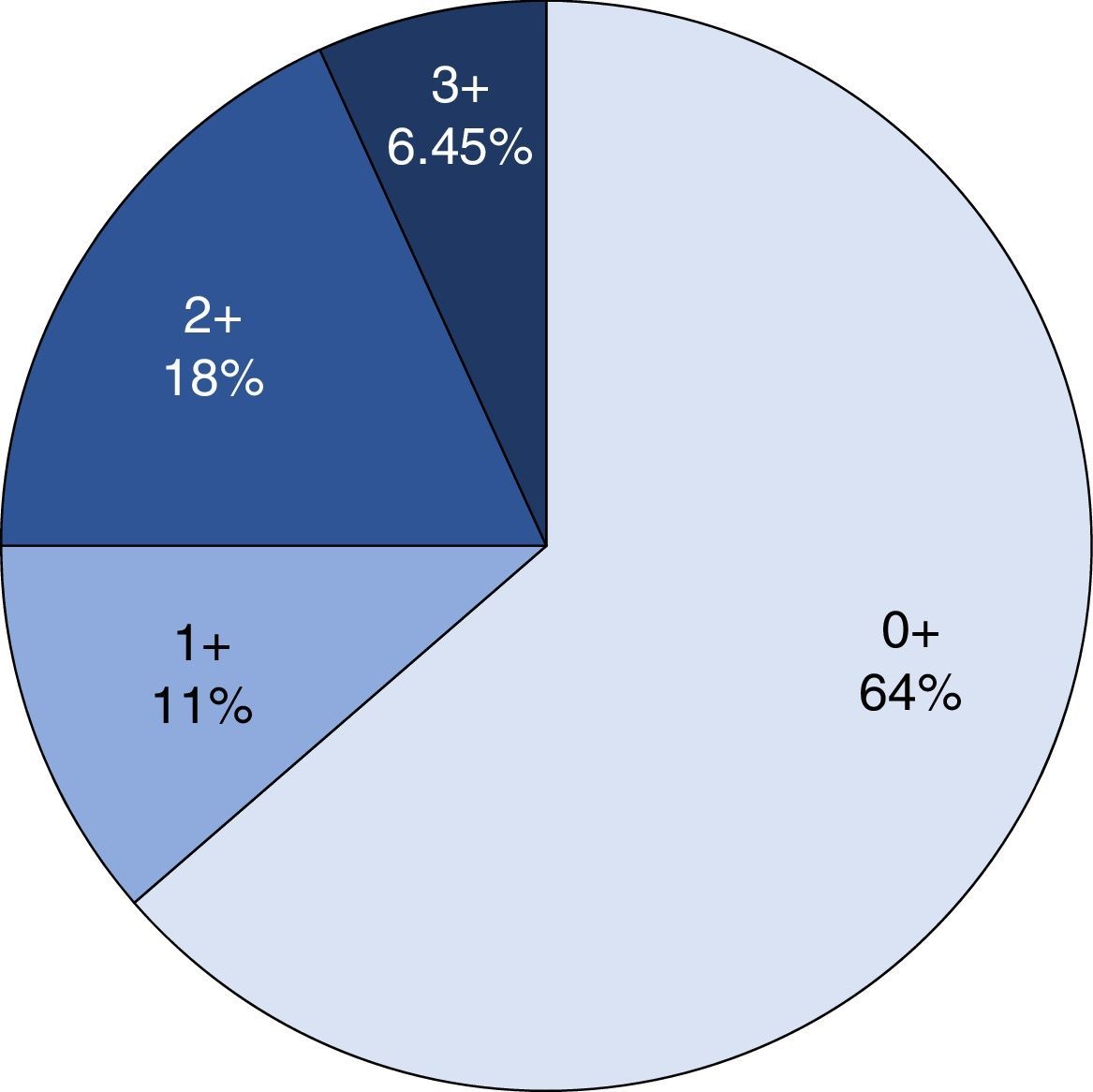
ImmunohistochemistryHER2 expression was assessed with immunohistochemistry and the anti-HER2 monoclonal antibody (Ao485, DAKO) was used. The results were analysed by 3 pathologists (IAC/SGH/ARP) simultaneously using a multi-head microscope. HER2 expression was assessed using the system of Hoffman et al.22 The cases were divided into 4 groups, depending on the extension and intensity of expression: 0, with no positivity or positivity in the cell membrane in <10% of the cells; 1+, weak or hardly perceptible stain in >10% of the cells; 2+, weak to moderate, complete or in the basolateral membrane in >10% of the cells; 3+ complete, intense, positive or in the basolateral membrane in >10% of the cells. Only the cases of the 3+ category were considered positive for HER2 overexpression, and cases 0 and 1+ were considered negative. The 2+ cases were catalogued as erroneous for HER2 expression.
The appropriate descriptive measure, means and proportions were used for the data analysis, and their 95% confidence intervals.
Because the study was conducted with material studied in the Pathology Department, we do not consider that ethical considerations are required. However, the patient data will be kept confidential
ResultsA total of 93 cases of gastric carcinoma were included in the study. The age of the patients at time of diagnosis ranged from 39 to 88 years (mean: 64), 43 (46.2%) were male and 50 (53.7%) female. Eighty-six percent of the tumours were located in the proximal stomach. The tumour size varied between 1.9 and 16.0cm (average: 6.44cm), and 74% of cases were in advanced stages (categories, pT2, pT3 and pT4).
A total of 36 (38.7%) adenocarcinomas were intestinal and 57 (61.2%) diffuse type. With regard to the histological grade of the intestinal-type adenocarcinomas, 6 cases (16.6%) were well differentiated, 20 (55.5%) moderately differentiated and 10 (27.7%) were poorly differentiated.
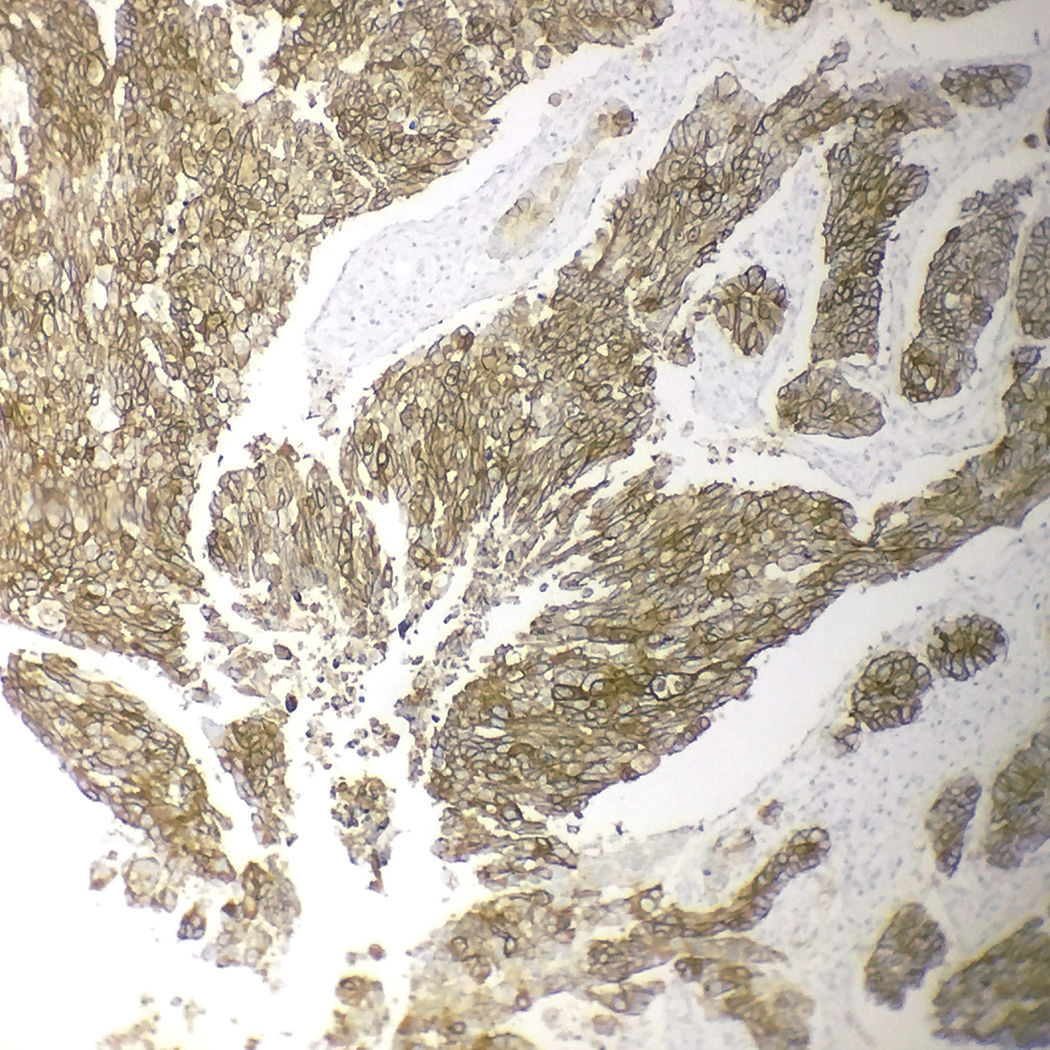
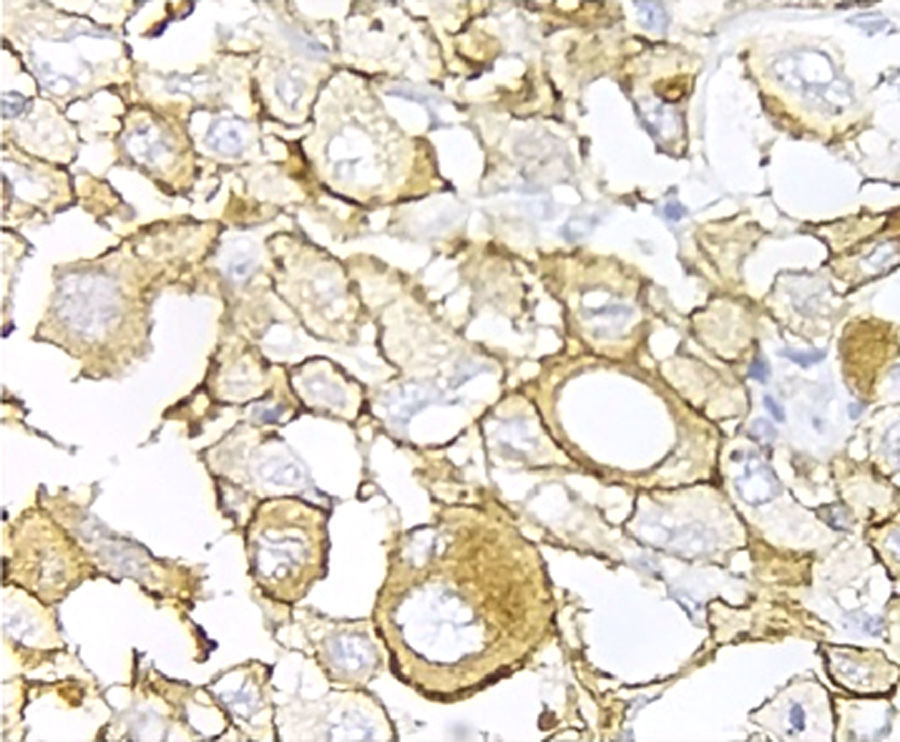
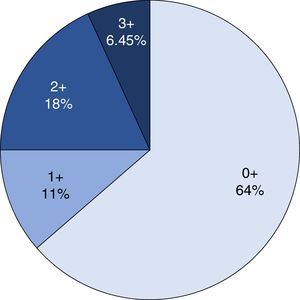
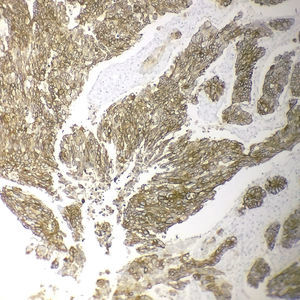
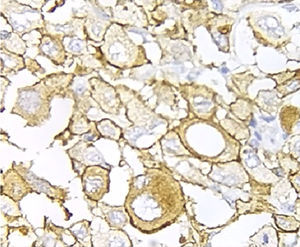
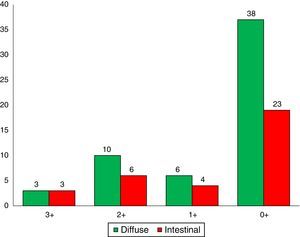
Immunohistochemical analysis revealed 6 cases positive to HER2 overexpression (3+; 6.45%); 16 cases were erroneous (2+); 10 were 1+ and 61 cases were negative (Fig. 1). Of the positive tumours, 3 cases were intestinal (Fig. 2) (one moderately differentiated and 2 poorly differentiated) and 3 diffuse type (Fig. 3). Of the HER2 positive cases, 5 were located in the proximal stomach and one in the distal. Moreover, the majority of the carcinomas that were negative to HER2 were diffuse type (Fig. 4).
The incidence of gastric cancer varies by geographical region. It occurs more frequently in East Asia, Eastern Europe and South America, with lower percentages in North America.23 In Mexico, gastric cancer is the third cause of death due to cancer. The high mortality associated with this disease and the poor quality of life it causes, makes gastric cancer a public health problem in Mexico that requires research studies to establish measures for its prevention, diagnosis and treatment.24
Overexpression of the HER2 oncoprotein in gastric cancer was first described in 1986.25 Since then numerous research studies have been conducted to analyse the role this oncoprotein plays in gastric cancer development, and to seek other biomarkers to establish its molecular profile.12
The ToGA study11 to date has assessed HER overexpression in many cases. This international project included 24 countries, with a combined total of 3807 patients with a diagnosis of gastric cancer; the frequency of HER2 expression was 22.1%.
Positive HER2 expression in gastric cancer is different in various parts of the world.26 In a study undertaken with Brazilian patients positive HER2 was found in 10.5% of cases,27 Beltrán Garate et al.28 found a HER2 positivity rate of 9% in Peruvian patients, while García-Ramírez et al.29 found positivity of 11.2% in a Columbian patient population. In this series, which included 93 patients with gastric cancer, HER2 positivity was 6.45%.
In our study, diffuse-type carcinoma occurred more frequently than intestinal-type adenocarcinoma (61.2 vs. 38.7%, respectively). Moreover, 86% of cases occurred in the proximal stomach. These figures contrast with those reported by Santos Laboissiere et al.30 in a Brazilian patient population, where the most frequent histological subtype was intestinal (61 cases, 49.2%). Moreover, 80.6% of the carcinomas occurred in the distal stomach.
Three of the HER2 positive tumours in this study were intestinal-type adenocarcinomas and 3 diffuse. With regard to histological type, several reports indicate that HER2 positivity occurs more frequently in intestinal-type adenocarcinomas compared to diffuse or mixed-type. It has been reported that HER2 overexpression can be one of the molecular alterations associated with gastric cancer, principally the intestinal-type.31
ConclusionsIn our patient population, HER2 positivity occurred in 6 cases (6.45%) and there were no differences between the histological type (intestinal and diffuse) and HER2 immunoreactivity. All the patients with tumours that were positive to the oncoprotein had poorly differentiated adenocarcinomas.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this stud.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors have no conflict of interests to declare.
Please cite this article as: Alvarado-Cabrero I, Gil-Hernández S, Ruelas-Perea A, Villaverde-Rodríguez D, Montes-Ochoa JR, Medrano-Guzmán R. Evaluación por inmunohistoquímica de la expresión del HER2 en cáncer gástrico. Estudio clínico-patológico de 93 casos. Cir Cir. 2017;85:504–509.