Retinopathy of prematurity (ROP) is an eye disease caused by an alteration in retinal vasculogenesis that may lead to partial or complete vision loss with a harmful impact in terms of neurodevelopment. The purpose of the present study was to determine the neurodevelopment in patients with type i retinopathy of prematurity treated with intravitreal bevacizumab.
Material and methodsCase series. The inclusion criteria were: patients with type I ROP treated with a dose of 0.625mg/0.025ml of intravitreal bevacizumab. Demographic data and comorbidities were documented. Neurodevelopment was evaluated with the screening test of the Bayley Scale of Infant Development (BSID) in all patients between 11 and 28 weeks of age.
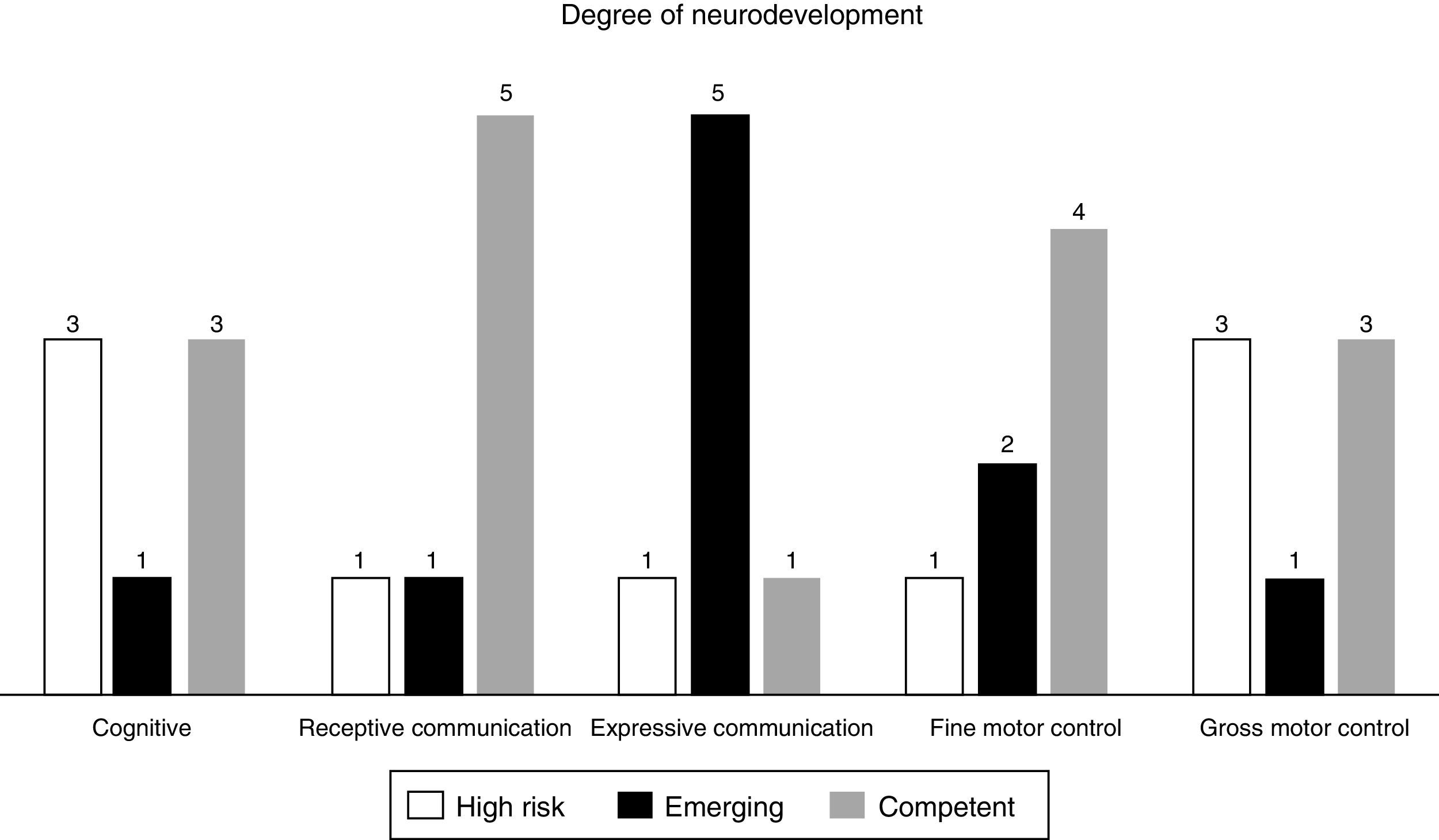
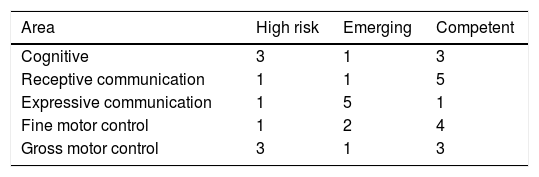
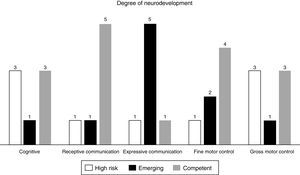
ResultsSeven patients were included in the study. Four patients showed normal neurodevelopment according to the overall scores of the BSID scale. The distribution of high risk for neurodevelopmental delay in the different areas evaluated were as follows: 3 patients presented it in the cognitive area, one in the receptive communication area, one in the expressive area, one in the fine motor skills and 3 patients in the gross motor skills area.
ConclusionsIn these case series, the majority of patients treated with intravitreal bevacizumab for ROP showed normal neurodevelopment scores.
La retinopatía del prematuro (ROP) es una enfermedad ocular provocada por una alteración en la vasculogénesis de la retina que puede ocasionar pérdida parcial o total de la visión, con impacto deletéreo en cuanto a neurodesarrollo se refiere. El propósito de este estudio fue el de cuantificar con la escala de Bayley el neurodesarrollo en los pacientes diagnosticados con ROP de tipo 1 tratados con bevacizumab intravítreo.
Material y métodosEstudio de serie de casos. Los criterios de inclusión fueron: niños con ROP de tipo 1 tratados con una dosis de 0.625 mg/0.025 ml de bevacizumab intravítreo. Se documentaron los datos demográficos y enfermedades asociadas. Se evaluó el desarrollo psicomotriz de los pacientes con la aplicación de la prueba de detección de la Escala de Bayley del Desarrollo Infantil (BSID), la cual se aplicó entre las 11 y 28 semanas de edad en los pacientes estudiados.
ResultadosSe incluyeron a 7 pacientes en el estudio. De ellos, 4 presentaron desarrollo normal o competente en el puntaje total. La distribución de alto riesgo de retraso en el neurodesarrollo de acuerdo con las diferentes áreas o esferas evaluadas fue la siguiente: 3 pacientes lo presentaron en el área cognitiva, uno en comunicación receptiva, uno en expresiva, otro en motricidad fina y 3 en motricidad gruesa.
ConclusionesEn esta serie de casos, se encontró un desarrollo psicomotriz normal en la mayoría de los pacientes tratados con bevacizumab intravítreo por ROP.
Retinopathy of prematurity (ROP) is one of the principal causes of childhood blindness.1 The entity, that presents in preterm infant, is often accompanied by other comorbidities that can have an adverse effect on their neurodevelopment.2 Treatments that can improve the prognosis of visual function can also have a favourable effect on this condition.
In recent years, cryotherapy and laser therapy have been used with limited success. Nevertheless, there are current treatment options that have been shown to be safe and efficacious, such as intravitreal antiangiogenic therapy. One of the drugs in this treatment modality is bevacizumab, a recombinant humanised monoclonal antibody, whose principal action mechanism is to block vascular endothelial growth factor (VEGF). The latter, in some of its isoforms, is involved in the formation and growth of new vessels. And these, in turn, comprise some of the key histopathological lesions that predispose to haemorrhage in the vitreous cavity, the formation of fibroglial tissue and tractional retinal detachment in patients with ROP.
In ROP, intravitreal antiangiogenic therapy has shown encouraging results as treatment in the threshold stage as monotherapy or as adjuvant treatment of laser photocogulation.3–6
Information is scarce and occasionally contradictory about the systemic effects of this drug, especially on the central nervous system and neurodevelopment.7 Therefore, there is a need to design studies with a larger number of patients, cases and controls, and prospective cohort studies that help to clarify the harmful and beneficial effects of this drug. The main objective of our study was to establish the psychomotor development in children with ROP treated with bevacizumab.
Material and methodsAn ambispective case study was undertaken in preterm infants who were born in the intensive care unit of the IMSS Hospital General Regional Número 1, between April 2013 and May 2015. The inclusion criteria were as follows: premature neonates of less than 36 weeks’ gestation weighing less than 2000g, patients with type 1 ROP (defined as ROP of any stage, in zone 1 with disease plus; stage 3 with or without disease plus in zone 1; stage 2 or 3 in zone 2 with disease plus) treated with a dose of 0.625mg/0.025ml, aged under 3 months, under ophthalmological and neurological monitoring and in follow-up for at least 2 months, with informed consent from their parents. The study was approved by the respective hospital ethics committee of the hospital.
Assessment of neurodevelopmentThe Bayley Scales of Infant Development, third edition (BSID III) screening test was used. This test is an indicator of neurodevelopment that is widely used for research and considered the gold standard for the early detection of psychomotor delay in paediatric patients. The test is designed to evaluate the level of development in three 3 subtests: cognitive, language and motor.
The different scores obtained were quantified and used to classify each patient: for the ages from 9 months and 16 days to 12 months and 15 days, neurodevelopment was considered “at risk” if the score was 57–136; for the ages from 18 months and 16 days to 24 months and 15 days, the score considered “at risk” was from 0 to 65, “emerging” if the total score was 42–52 and “competent” if the score was 57–136; for the ages from 18 months and 16 days to 24 months and 15 days, the score considered “at risk” was from 0 to 65, “emerging” from 70 to 84 and “competent” from 89 to 136. Finally, for the ages from 24 months and 16 days to 30 months and 15 days, the score considered “at risk” was from 0 to 76, as “emerging” from 81 to 99 and as “competent” from 104 to 136. All the patients were assessed by the same paediatric neurologist.
Data were collected from the clinical records on the prenatal, perinatal and postnatal conditions of each patient.
Statistical analysisDescriptive statistics were used for the continuous variables. Means were calculated and their respective standard deviation. The variables of nominal qualitative characteristics were represented with percentages. The data was processed using the Statistical Package for Social Sciences (SPSS v. 20.0). The results are presented in different tables.
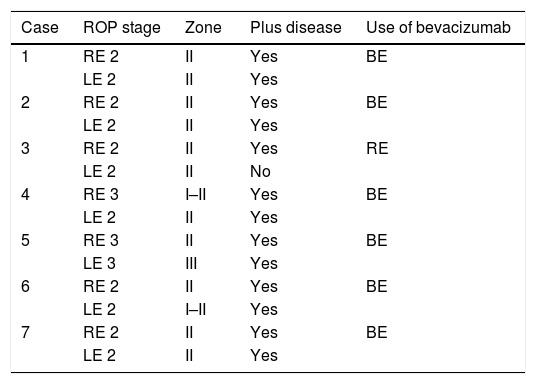
ResultsSeven patients were included in the study. All the patients presented bilateral ROP type 1, for which intravitreal bevacizumab was applied in both eyes, except for one case. In total, 13 eyes were treated (Table 1). The ROP was monitored in all the patients with no significant complications due to the application of intravitreal bevacizumab.
Patients ROP characteristics.
| Case | ROP stage | Zone | Plus disease | Use of bevacizumab |
|---|---|---|---|---|
| 1 | RE 2 | II | Yes | BE |
| LE 2 | II | Yes | ||
| 2 | RE 2 | II | Yes | BE |
| LE 2 | II | Yes | ||
| 3 | RE 2 | II | Yes | RE |
| LE 2 | II | No | ||
| 4 | RE 3 | I–II | Yes | BE |
| LE 2 | II | Yes | ||
| 5 | RE 3 | II | Yes | BE |
| LE 3 | III | Yes | ||
| 6 | RE 2 | II | Yes | BE |
| LE 2 | I–II | Yes | ||
| 7 | RE 2 | II | Yes | BE |
| LE 2 | II | Yes |
BE: both eyes; RE: right eye; LE: left eye; ROP: retinopathy of prematurity.
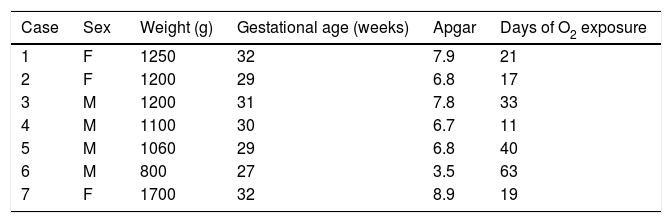
Four male and 3 female patients were included. The average gestation age at birth was 30±1.82 (range from 27 to 32) weeks, and the corrected age at time of neurological testing was de 22.57±5.62 months, with an average birth weight 1187.1±271g; the mean Apgar at one minute and at 5min was 6.7.
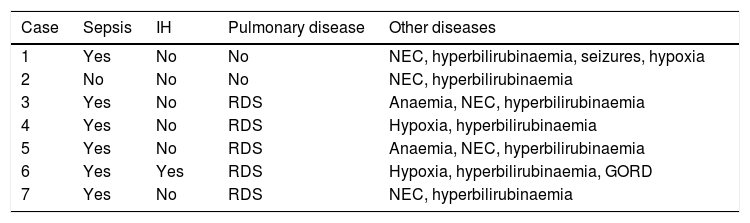
Of the risk factors associated with the retinopathy, oxygen exposure was found in all the patients with an average of 29.14±17.9 days (range 11–63); the frequency of lung disease was 74.1%, which included respiratory difficulty syndrome, pulmonary bronchodysplasia and meconium aspiration syndrome. There was neonatal sepsis in 85.7%; hyperbilirubinaemia was present in all the children; hypoxia in 42.9%; necrosing enterocolitis in 71.4%, one patient had intraventricular haemorrhage, and only one patient required a transfusion (Tables 2 and 3).
Intercurrent diseases of the patients.
| Case | Sepsis | IH | Pulmonary disease | Other diseases |
|---|---|---|---|---|
| 1 | Yes | No | No | NEC, hyperbilirubinaemia, seizures, hypoxia |
| 2 | No | No | No | NEC, hyperbilirubinaemia |
| 3 | Yes | No | RDS | Anaemia, NEC, hyperbilirubinaemia |
| 4 | Yes | No | RDS | Hypoxia, hyperbilirubinaemia |
| 5 | Yes | No | RDS | Anaemia, NEC, hyperbilirubinaemia |
| 6 | Yes | Yes | RDS | Hypoxia, hyperbilirubinaemia, GORD |
| 7 | Yes | No | RDS | NEC, hyperbilirubinaemia |
NEC: necrosing enterocolitis; GORD: gastro-oesophageal reflux disease; IH: intraventricular haemorrhage; RDS: respiratory difficulty syndrome.
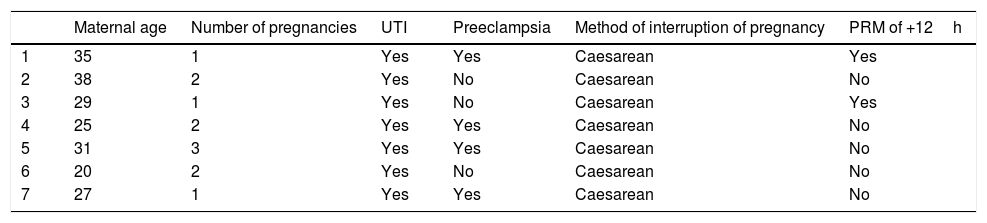
The average maternal age was 29.2±6.1 years, the disease found in the mothers of the children with retinopathy were: premature rupture of membranes (42.9%), preeclampsia (42.9%), HELLP syndrome (14.3%) (Table 4).
Maternal characteristics.
| Maternal age | Number of pregnancies | UTI | Preeclampsia | Method of interruption of pregnancy | PRM of +12h | |
|---|---|---|---|---|---|---|
| 1 | 35 | 1 | Yes | Yes | Caesarean | Yes |
| 2 | 38 | 2 | Yes | No | Caesarean | No |
| 3 | 29 | 1 | Yes | No | Caesarean | Yes |
| 4 | 25 | 2 | Yes | Yes | Caesarean | No |
| 5 | 31 | 3 | Yes | Yes | Caesarean | No |
| 6 | 20 | 2 | Yes | No | Caesarean | No |
| 7 | 27 | 1 | Yes | Yes | Caesarean | No |
UTI: urinary tract infection; PRM: premature rupture of membranes.
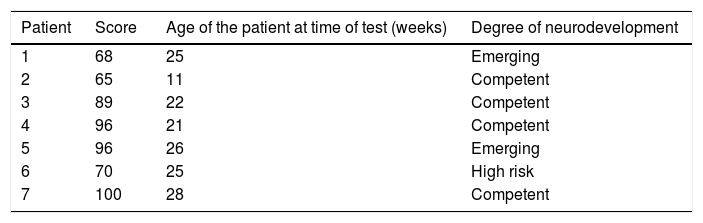
Four patients were found to have normal development, 2 with emerging risk and one with a risk of delayed neurodevelopment. The distribution of the patients at high risk of delayed development in the different areas evaluated was as follows: 3 patients in the cognitive area, one in receptive communication, one in expressive communication, the other in fine motor control and 3 in gross motor control (Fig. 1, Tables 5 and 6).
Intravitreal antiangiogenic therapy forms part of the revolution in the treatment of both ocular and systemic diseases that has been taking place for several years now and that has changed, in many cases drastically, the course and prognosis of many diseases, including ROP.3
There have been numerous publications demonstrating the safety and efficacy of bevacizumab in the treatment of this entity, in terms of functional visual and anatomical variables in both the short and the long term.3
However, the impact on the neurodevelopment of patients with this disorder is an issue that has probably not been tackled sufficiently in the different studies. This is perhaps in part because it is so difficult to evaluate this development in patients who, in addition to their eye disease, have multiple intercurrent systemic diseases that might also have some impact. Therefore it is difficult to establish whether a particular comorbidity and its treatment have a specific impact on the neurodevelopment of preterm infants.
Araz-Ersan et al.8 performed a study where they found 3 patients with abnormal development, from a group of 13, who were receiving intravitreal bevacizumab as adjunct treatment to laser. In another group laser alone was used: they reported significant cognitive delay in 30.8%. By contrast, in our study 47.4% of the children were found to have normal development and there were 3 children with low cognitive development, which corresponds to 42.85%. In terms of language, the children in said study presented language delay of 23.1% and there was significant motor delay of 38.5%. In our study, 28.5% of the cases had language delay. Finally, there was motor delay in 42.8%: this, together with the cognitive, was one of the most affected areas.
Furthermore, Orozco-Gómez et al.9 performed a study where they used laser and ranibizumab with a follow up of 3 years, where they evaluated both the efficacy of this combined therapy in preterm patients with threshold, prethreshold and “plus” disease, as well as infantile development disorders, in 34 patients. The results showed normal development in 23.5%, psychomotor delay but normal mental development in 29.4%, and delayed mental but normal psychomotor development in 23.5%.
Our studies’ limitations include the fact that it is a case series – a study design with a low level of evidence – and we did not include a control group or perform a calculation of the sample.
With the growing use of bevacizumab in treating ROP, questions have been raised about its use in preterm infants in terms of its safety for the central nervous and the pulmonary systems; the latter, partly because pulmonary angiogenesis takes place after birth at term and might be compromised.3
The maturation processes of the central nervous system take place at different times and in different areas of the brain, therefore the critical period of this maturation might be specific to each area of the brain.4
However, the dosage of ROP treatment is still under debate. Martínez-Castellanos et al.10 reported the adverse effects they found using 1.25mg/0.05ml of bevacizumab, including a worsening of retinal detachment and raised intraocular pressure, among others. These reduced when 0.03ml were used. The children in our study showed no systemic or ocular complications after giving the drug at the dosage used. However, in the same study by Martínez-Castellanos, the patients with neurodevelopmental problems presented interventricular haemorrhage, which does coincide with our study, since the only patient with altered neurodevelopment also had a grade 2 intraventricular haemorrhage.
One study included 150 children with plus ROP at stage 3 who received bilateral intravitreal injections of 0.625mg bevacizumab. In terms of safety, the authors concluded that 2800 patients were required to assess mortality and an even greater sample for local or systemic toxicity and that the study was too small to tackle the question as to whether intravitreal bevacizumab has an acceptable safety profile.6
In our study, only 7 patients were assessed; this sample is even smaller and insufficient to conclude whether treatment with the drug has a favourable impact or otherwise on neurodevelopment, and it is not possible to appropriately assess the drug's safety profile. The only patient found to be at high risk of delayed neurodevelopment had various added circumstances in the prenatal period, such as a lower gestational age than the rest, and being the only infant with intraventricular haemorrhage. These are factors that might well alone have significantly contributed to child's low Bayley score.
ConclusionsSurvival rates are increasingly higher in neonates of low gestational age and weight, which causes an increase, among other factors, in the incidence of patients with ROP. Intravitreal bevacizumab has proved effective in controlling this disorder short and long term. It is likely that appropriate control of the disease using this drug will have a favourable impact on the neurodevelopment of patients in the long term, irrespective of the associated diseases in these preterm infants. A question mark still hangs over the drug's safety profile. However, it is also very likely that it is acceptable according to the evidence generated to date.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingNo financing was received in undertaking this study.
Conflict of interestThe authors have no conflict of interests to declare.
The authors would like to thank the staff at the Biomedical Research Centre of the Mexican Social Security Institute, Dr in Sciences Anel Gómez García and analyst mathematician Carlos Gómez Alonso.
Please cite this article as: Martínez-García SM, Hernández-Da Mota SE, Rubio-Rangel A, Rojas-Flores I, Vieyra-López ME, Martínez-Castellanos MA, et al. Neurodesarrollo en pacientes con retinopatía del prematuro tratados con bevacizumab intravítreo. Serie de casos. Cir Cir. 2017;85:478–484.