Cancer of the head and neck comprises a group of neoplasms that share a similar anatomical origin. Most originate from the epithelium of the aerodigestive tract and 90% correspond to squamous cell carcinoma. In the last 15 years, an increase in the incidence of squamous cell carcinoma induced by human papillomavirus (HPV) has been seen, mainly types 16 and 18, which are the most frequent found in cancers of the oral cavity and oropharynx, and types 6 and 11 in laryngeal cancer. There are reports in the literature that show HPV as the leading cause of oropharyngeal squamous cell carcinoma.
ObjectiveDetermine the prevalence of infection with high-risk HPV in patients diagnosed with squamous cell carcinoma of the oral cavity, oropharynx and larynx.
Material and methodsAn observational, cross-sectional, descriptive, unblinded study was performed. Prevalence of HPV infection was determined by polymerase chain reaction (PCR) in DNA samples from tumour tissue of patients with squamous cell carcinoma of the oral cavity, oropharynx and larynx. Typing was subsequently performed in HPV positive samples in order to detect types 18, 16, 11 and 6, using custom primers.
ResultsA total of 45 patients were included. The association between laryngeal squamous cell carcinoma and HPV was established in two patients, which represented an overall prevalence of 4.4% in our population, and 10% for laringeal tumours.
ConclusionsThere is a low prevalence of HPV infection in squamous cell carcinoma of the oral cavity, oropharynx and larynx, in our population. Prospective studies on younger patients could provide more information.
El carcinoma de cabeza y cuello comprende un grupo de neoplasias que comparten un origen anatómico similar. La mayoría se originan de la mucosa del tracto aerodigestivo y más del 90% corresponden al carcinoma espinocelular. En los últimos 15 años se observó un incremento en la incidencia de carcinoma espinocelular inducido por el virus del papiloma humano (VPH) en jóvenes, principalmente los serotipos 16 y 18, los cuales son los más estudiados en cáncer de cavidad oral y orofaringe, y los serotipos 6 y 11 en cáncer de laringe. Existen reportes en la literatura sobre el VPH como principal causa de carcinoma espinocelular, principalmente de orofaringe.
ObjetivoDeterminar la prevalencia de infección por VPH de alto riesgo en pacientes con diagnóstico de carcinoma espinocelular de cavidad oral, orofaringe y laringe.
Material y métodosEstudio observacional, transversal, descriptivo, no ciego. Se determinó la prevalencia de infección por VPH por medio de la reacción en cadena de la polimerasa en muestras de ADN de tejido tumoral en pacientes con carcinoma espinocelular de cavidad oral, orofaringe y laringe. Se realizó tipificación de serotipos de alto riesgo.
ResultadosSe incluyó en el estudio un total de 45 pacientes. La asociación entre el carcinoma de células escamosas de laringe con VPH se ailo en 2 pacientes, lo cual represento una prevalencia global del 4.4% en nuestra población y del 10% para los tumores de laringe.
ConclusionesExiste una baja prevalencia de carcinoma espinocelular de la cavidad oral, orofaringe y laringe, asociado a infección por VPH en nuestra población. Estudios prospectivos en población más joven con cáncer de cabeza y cuello podrían aportar mayor información sobre la influencia del VPH en dicha patología.
Cancer of the head and neck comprises a group of neoplasms that share a similar anatomical origin. Most originate from the mucous membrane encasing the epithelium of the aerodigestive tract and over 90% correspond to squamous cell carcinoma. They rank sixth in worldwide neoplasms of all locations and the most commonly affected site is the oral cavity.1
It has been calculated that approximately 400,000 new cases per year are diagnosed worldwide, with higher prevalence in males. The most common sites of origin are the oral cavity and the oropharynx. In the oral cavity the most affected area is the tongue, followed by the gums and the floor of the mouth. In the oropharynx it is the tonsillar región.2
In Mexico, 5% of all malignancies correspond to the epidermoid carcinoma of the head and neck, which in general is diagnosed in advanced stages, particularly in the tongue.3
Several mechanisms have been reported in the genesis of tumours which originate in the upper aerodigestive tracts, with the most outstanding being the carcinogenic effects of alcohol and tobacco as the main risk factors and which play a major role in their aetiopathogenesis due to their potential to induce mutations in tumour suppressor gene p53.4
However, the role of the human papillomavirus has now been recognised as an independent factor in the development of these neoplasms. During the last 15 years an increase in the incidence of squamous cell carcinoma induced by the human papillomavirus (HPV) has been observed, with its frequency increasing in young patients and in non smokers or drinkers, especially associated with high risk serotypes such as viral 16 sub-type. This has been supported by reports in the literature which show HPV as the primary cause of oropharyngeal squamous cell carcinoma.5,6
There are over 180 known viral sub-types, of which over 40 sub-types of HPV are sexually transmitted and infect the anogenital tract and are thus transmitted to the oral cavity.7
HPV have been classified as high, intermediate and low risk, depending on their association with malignant neoplasms. Serotypes 16 and 18 are considered high risk and they are linked to cancer of the oral cavity and oropharynx, whilst serotypes 6 and 11 are considered low risk and have been linked to cancer of the larynx.8
The mechanism by which the virus induces head and neck squamous cell carcinoma is not altogether clear and is subject to controversy. The potential oncogenic for inserting specific DNA fragments (E6 and E7) in the host genome leads to suppression of tumour suppressor factors p53 or Rb and the ability to induce dysplastic lesions and malignant neoplasms.9
The majority of immune competent individuals are able to eliminate infection without any clinical signs and only 10% develop lesions. The integration of the DNA of the virus in the host genome is a predictor of the progression of an infection to the formation of a neoplasm.10
Despite the increase in the risk of squamous cell carcinoma of the head and neck, better prognosis exists for patients in whom the viral DNA is detected, and who therefore have greater control and a better rate of survival. One study conducted in the United States in 2010 showed that HPV positive squamous cell carcinoma presented a 58% reduction in the risk of death, whilst age, smoking rates and invasion of the lymph nodes continue being the determining factors in survival.11
The aim of this article is to determine the prevalence of high risk HPV (16, 18) and low risk HPV (6, 11) in patients diagnosed with squamous cell carcinoma of the oral cavity, oropharynx and larynx.
Material and methodsAn observational, cross-sectional, descriptive, unblinded study was performed which included all patients who had been histopathologically diagnosed with squamous cell carcinoma of the oral cavity, oropharynx and larynx who attended the Servicio de Otorrinolaringología y Cirugía de Cabeza y Cuello in the Hospital Universitario José Eleuterio González between 1st January 2011 and 31st December 2013. The study included a total of 45 patients.
Patients of both sexes who had been histopathologically diagnosed with primary squamous cell carcinoma of the head and neck were included in the study.
Those patients with carcinoma of the oral cavity, oropharynx and larynx with a different histological type to squamous cell carcinoma were excluded, as were patients under 18 years of age and patients who had previously received radiotherapy or chemotherapy.
A complete medical history of each patient was conducted to search for risk factors related to the development of squamous cell carcinoma, such as alcoholism, a tobacco habit, a periodontal disease, the presence of laryngeal papillomatosis or a family history of squamous cell carcinoma of the head and neck. A complete ENT examination was carried out, including examination with a flexible endoscopy using Karl Storz RP1® fibre optic material and the Endodigi® digitalisation system. A search for HPV genetic material was made using the polymerase chain reaction technique at the final point of the reaction.
DNA extraction of fresh tumour tissues was performed on 9 patients, and tissue stored in paraffin blocks was obtained for the other patients. Paraffin blocks of 10μm thickness were obtained, and collected in 1.5ml tubes. The paraffin was removed and they were subsequently dehydrated. DNA extraction was performed using the proteinase K/phenol method. The DNA concentration was determined through spectrophotometry in the NanoDrop 2000 (Termo Fisher Scientific, Inc., Wilmington, DE) and a quantity was extracted by 1% agarose gel electrophoresis in order to visually evaluate its quality. For HPV detection the PCR end point technique was used, with the general initiators MY09/MY11 and GP5/GP6 for detection of the L1y CpI/CpII region for detection of gene E1. An Eppendorft Mastercycler Gradient thermal cycler (Eppendorf AG, Hamburg, Germany) was used for the PCR.
The presence of the viral subtypes 6, 11, 16 and 18 were traced through the positive samples for the human papillomavirus using specific HPV initiators.
DNA sample and analysis from chain reaction of the polymeraseThe DNA was extracted from 8 fresh tumour samples of biopsies and 36 tumour biopsies embedded in paraffin. Both samples were extracted using the K/phenol protocol. However, the paraffin blocks were cut into 10μm thick slices and deparaffinised with xylitol prior to the digestion of proteinase K. The DNA concentration was determined by spectrophotometry using NanoDrop 2000 (Thermo Fisher Scientific, Inc., Wilmington, DE), and a small quantity was extracted by 1% agarose gel electrophoresis in order to evaluate its quality. For HPV detection the PCR end point technique was used, with the general initiators MY09/MY11 (MY09: 5′-CGTCCMARRGGAWACTGATC-3′, MY11: 5′-GCMCAGGGWCATAAYAATGG-3′) and GP5/GP6 (GP5 5′-TTTGTTACTGTGGTAGATACTAC-3′; GP6 5′-GTAGTATCTACCACAGTAACAAA-3′) to detect the viral gene L1 and CpI/CPII (CpI: TTATCAWATGCCCAYTGTACCAT-3′; CpII: 5′-G ATGTTAATWSAGCCWCCAAAATT-3′) and the initiators for the detection of gene E1. PCR was performed using the Eppendorft Gradient thermal cycler (Eppendorf AG, Hamburg, Germany) and GoTaq-GreenMaster Mix (PROMEGA, USA.), in accordance with protocols recommended by the manufacturing company.
Once the positive samples for the HPV had been identified by any of the general initiators (MY, GP and CP),the presence of the viral subtypes 6, 11, 16 and 18 were determined by PCR using initiators specifically designed to identify a fragment within the viral genome of the long region of specific control for said subtypes (HPV-6 5′-TATTCGTACCGGTGTTAAGCGCC-3′/5′-TTGGCAGGGCAATGTGTACCTG-3′, HPV-11 5′-GCTGTGTCTAAGCCCTCTACAG-3′/5′-GTACCTTGGCACACAACATAT GG-3′, HPV-16 5′-ACCTCATCTACCTCTACAACTGC-3′/5′-GGCAAGCAGTGCAGGTC AGG-3′ y HPV-18 5′-AACCTGCCAAGCGTGTGCGTG -3′/5′-TAAGCCAAAGGCAACCGAAATCG-3′).
Statistical analysisFor analysis of the sample characteristics, descriptive statistics using means and ranges for continuous variables and percentages for categorical variables were used, in addition to the means and standard deviations depending on the variables described. The statistical programme IBM SPSS Statics version 20.0 was used for the analysis.
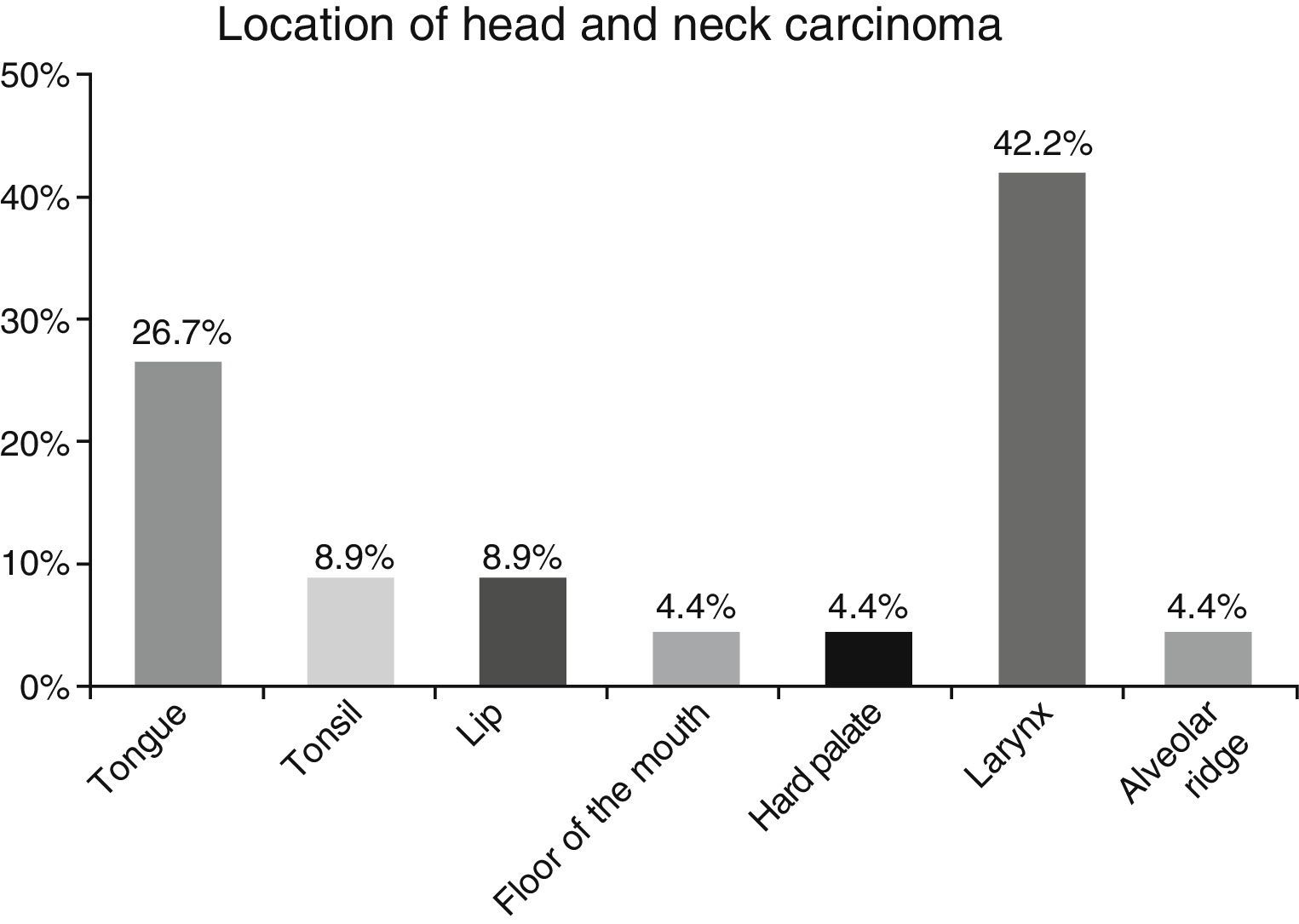
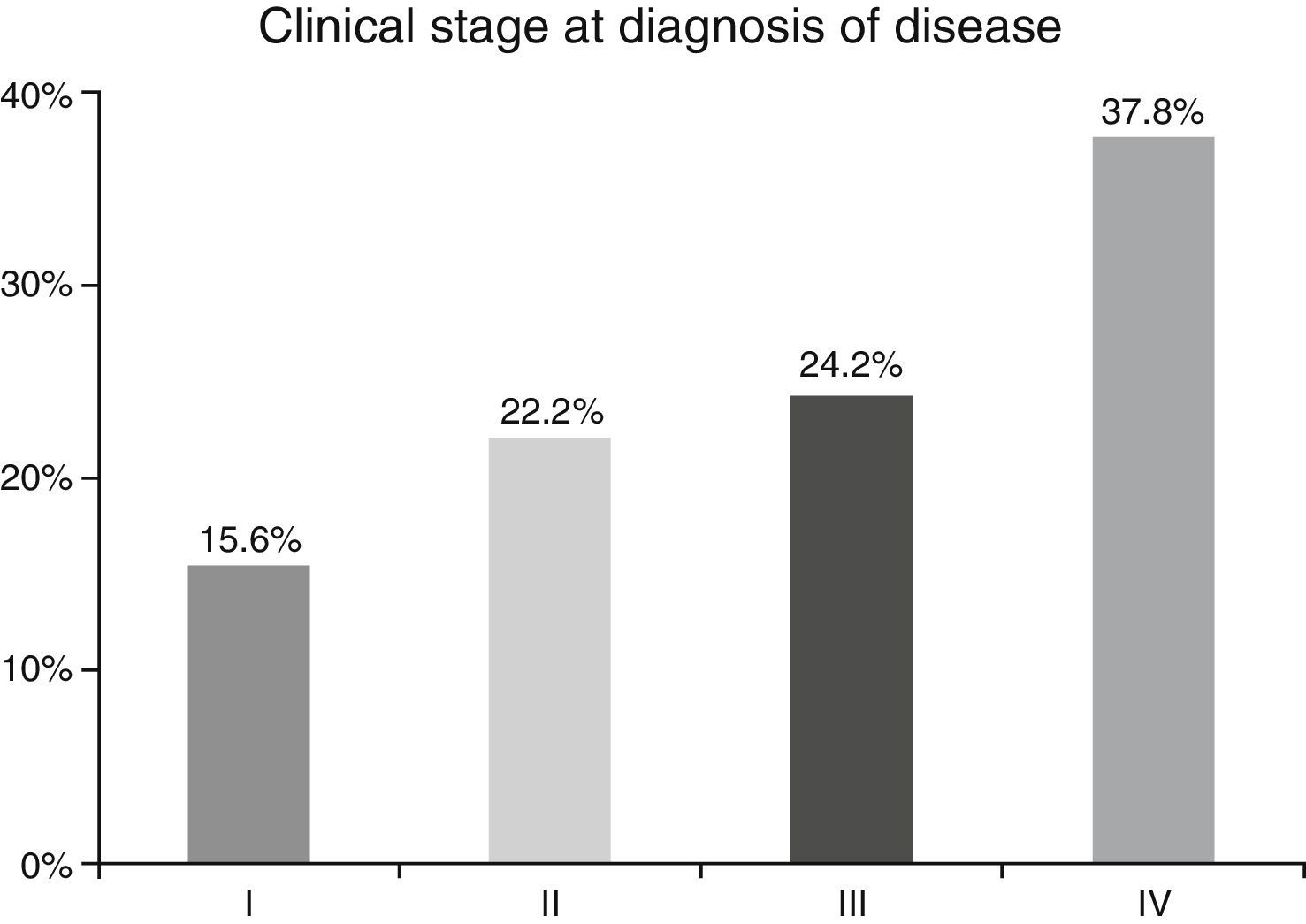
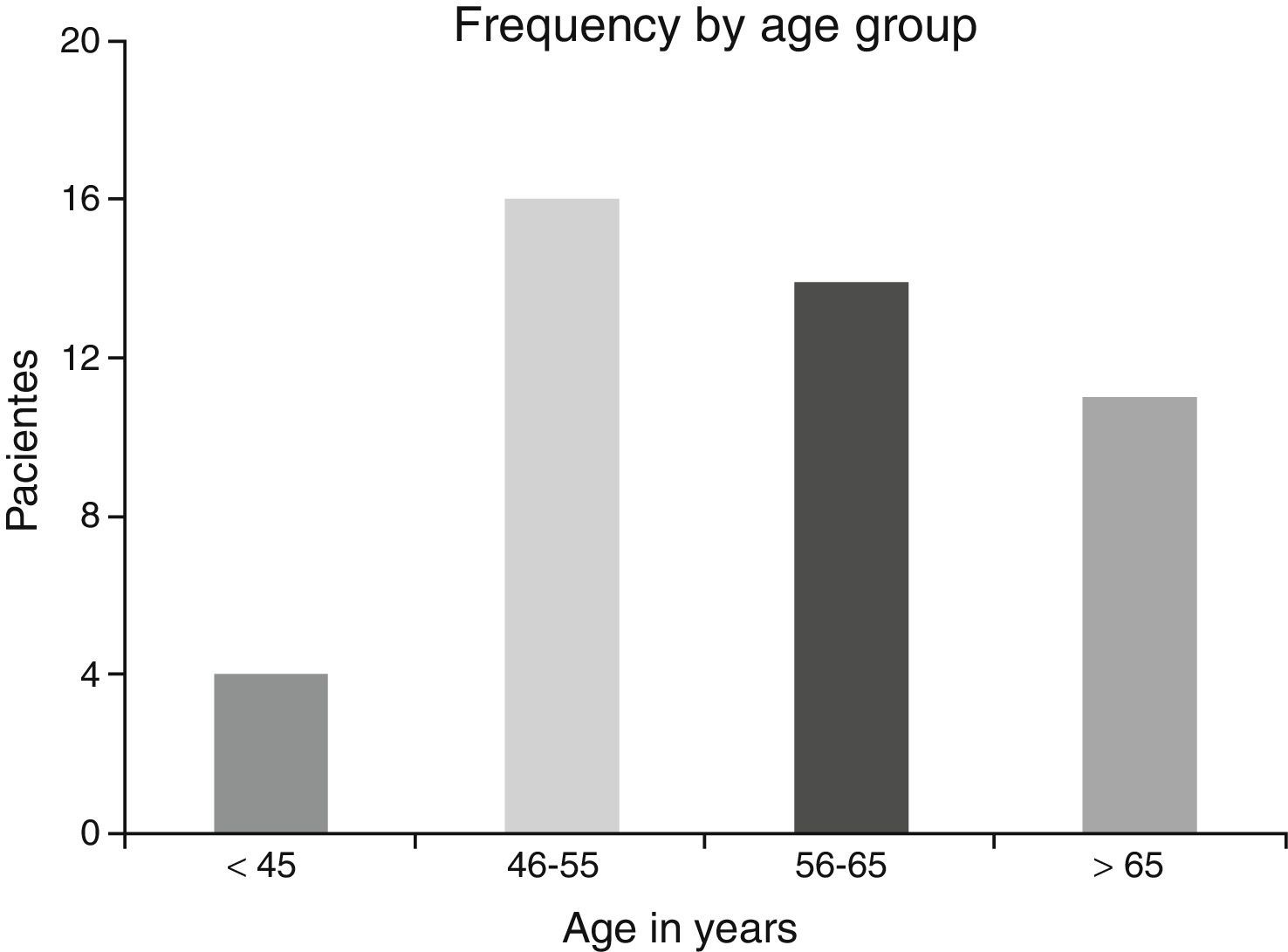
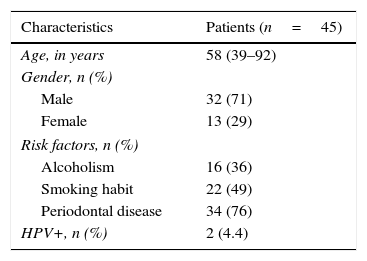
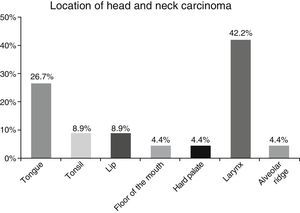
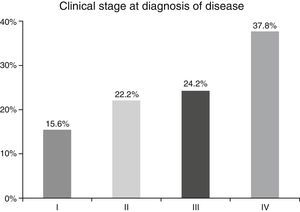
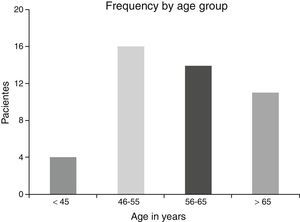
ResultsA total of 45 patients (32 males and 13 females) were included, with a median age of 58. 49% of patients presented with a history of smoking, 36%, with a history of alcoholism and periodontal disease was found in 76% of the patients. The other demographic characteristics are shown in Table 1. The sites found were: larynx in 19 patients, representing 42.2%; tongue in 12 patients; tonsil and lip in 4 patients respectively; floor of the mouth, hard palate and alveolar ridge in 2 patients (Fig. 1). Distribution by clinical disease stage on diagnosis was: 17 clinical stage IV patients, 11 stage III patients, 10 stage II and 7 stage 1 patients (Fig. 2). Infection by HPV was detected in 2 patients (4.4%), both diagnosed with cancer of the larynx. The viral subtype detected in both patients was type 11; Fig. 3 shows distribution by age group on diagnosis of the disease.
The prevalence of head and neck cancer according to location, as described by Parkin et al.1 in Globocan 2000, highlights the oral cavity as the most commonly affected site (50%), matching our results (57.8%). Cancer of the larynx was detected in 42.2% of our sample, unlike the 32% reported by this same study.
In the majority of our patients (62.2%) an advanced clinical stage was detected (stages III and IV); this is reflected in the conditions of our sample, characterised by a low socio-economic level and difficult access to specialised hospital services, resulting in the disease advancing to later stages.
Smoking is the key factor for the development of squamous cell carcinoma of the head and neck. 90% of oral cavity cancers in men and 60% in women are attributed to the consumption of tobacco.12 The habit of placing tobacco in the oral mucous membrane (under the tongue or in the cheek) increases the risk from 4 to 6 times of oral cavity cancer in lips, tongue and cheek mucosa. This is an infrequent habit in Mexico but popular in several regions of the United States and Europe.13 we did not find any patients with this habit in our sample.
Alcohol consumption is also associated with the increase in the risk of suffering from squamous cell head and neck carcinoma, and is the second most important aggressor after tobacco. The risk of cancer in drinkers is 6 times higher than in non drinkers and the risk of death from oropharyngeal cancer is 4 times greater in drinkers. There is therefore a strong relationship with squamous carcinoma originating in the oral cavity, oropharynx, hypo-pharynx and supraglottis.14
Alcohol and tobacco intoxication is synergetic in the genesis of squamous cell carcinoma, raising the relative risk sixteenfold when they are combined.15
In our sample we observed a prevalence of 35% drinkers and 51% smokers. There was also a 71% prevalence of cancer in males, which matches that described in the literature. We know that there is an increase in the incidence of head and neck cancer in females owing to the increase in smoking, which increased from a 6:1 male/female ratio in the 1960s to a 4:1 ratio at the present time.16,17 In our study the approximate male to female ratio was 3:1.
At the beginning of the 1980s, a little after recognition of HPV as an aetiological factor of cervical and uterine cancer, the participation of this virus in cancer of the head and neck was acknowledged.18
However, despite the efforts to lower the rate of tobacco usage, the development of cancer in the oropharynx and palatine tonsils has increased threefold, both in United States and Europe.19,20
Meta-analysis conducted in 2008 by Termine et al.21 established a 34.5% prevalence of the HPV in cancer of the head and neck originating from the oral cavity, pharynx or larynx.
The most frequent viral subtypes in head and neck cancer are 16 and 18, with their ability to immortalise the infected cell due to oncogenes E6 and E7 and suppression factors RB and p53.22,23 There is proof in the literature of a better prognosis for survival (33%), greater in patients with squamous cell carcinoma with HPV(+) status versus HPV(−) status.24
Infection caused by HPV was detected in 2 patients (4.4%), both diagnosed with cancer of the larynx. The viral sub-type detected in both patients was type 11. For the rest of the tumours no association with HPV was established, which differs from that reported in the literature. The patients with HPV had a history of laryngeal papillomatosis with severe dysplasia which progressed to squamous cell carcinoma. The cancer of these patients was detected at an early stage (clinical stages I and II).
The possible reasons for which HPV was not isolated in the majority of our sample may be due to several factors: first the virus is epigenetic at the start of the infection and consequently it is possible to detect it in cellular DNA a long time before dysplasic tumours are induced. Once it has integrated to the bone genome where the changes in cellular morphology have taken place its detection is more complicated, as reported by Koskinen et al.25 in a study conducted in 2003. Another point to consider is the median age of our patients, which was 58, since HPV has been generally isolated in a younger population.5,6
ConclusionThe frequency by location of the different squamous cell head and neck carcinomas was similar to that reported in the literature, with the oral cavity predominating as the most affected site. There was a high incidence of typically mentioned risk factors (smoking, alcoholism periodontal disease), with periodontal disease being the most commonly found. HPV was only isolated in 4.4% of our patients. However, the median age of the sample studies was high and HPV was isolated in the youngest sample diagnosed with head and neck carcinoma. For a more precise clarification of the role of this virus in this disease it is therefore important to carry out prospective studies in minors.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestsThe authors have no conflict of interests to declare.
We would like to thank the Centro de Investigación y Desarrollo en Ciencias de la Salud of the Universidad Autónoma de Nuevo León and the Servicio de Anatomía Patológica of the Faculty Medicine and the Hospital Universitario, Dr. José Eleuterio González for his support in conducting this research and to all the patients who agreed to participate in this study.
Please cite this article as: Villagómez-Ortíz VJ, Paz-Delgadillo DE, Marino-Martínez I, Ceseñas-Falcón LÁ, Sandoval-de la Fuente A, Reyes-Escobedo A. Prevalencia de infección por virus del papiloma humano en carcinoma espinocelular de cavidad oral, orofaringe y laringe. Cir Cir. 2016;84:363–368.