The incidence of anastomotic stricture varies due to the different definitions given to the condition. In most cases they are asymptomatic, and if there are symptoms, they are usually those of a partial intestinal obstruction.
Case reportThe case is presented of an 80 year old patient who underwent a lower anterior resection for rectal neoplasm. After ileostomy closure, he presented with subocclusive symptoms caused by stenosis of colorectal anastomosis. This stenosis was managed with endoscopic dilations, and one of these dilations produced an anastomotic perforation with pneumoperitoneum, retropneumoperitoneum, and pneumothorax. Once the patient was clinically and haemodynamically stable, the perforation was treated with conservative measures, resolving the complication satisfactorily.
ConclusionsThe literature describes several management options for colorectal anastomoses strictures, such as surgical resection, rubber dilators, endoscopic dilation, all of which might produce colonic perforation. Its management ranges from conservative measures to surgical intervention.
La incidencia de estenosis anastomótica tras cirugía colorrectal es variable por las diferentes definiciones que existen de ella. En la mayoría de las ocasiones son asintomáticas y en el caso de que presenten sintomatología se manifiestan como cuadro suboclusivo.
Caso clínicoPresentamos el caso de un paciente de 80 años intervenido de neoplasia de recto que, tras cierre de ileostomía, presentó cuadro suboclusivo ocasionado por estenosis de anastomosis colorrectal. Esta estenosis se trató con dilatación endoscópica, ocasionando una perforación anastomótica con neumoperitoneo, retroneumoperitoneo y neumotórax. Tras la estabilización del paciente, la perforación se manejó con medidas conservadoras que lograron resolver el cuadro de manera satisfactoria.
ConclusionesSe han descrito diversas opciones terapéuticas para el tratamiento de las estenosis anastomóticas, entre las que destacan: resección quirúrgica, empleo de dilatadores, colonoscopia dilatadora. Todas las opciones terapéuticas pueden conllevar una perforación colónica. La resolución en función de la estabilidad del paciente permitirá desde un manejo conservador, como el caso que presentamos, hasta una intervención quirúrgica.
The incidence of iatrogenic perforation during colonoscopy varies from 0.016%,1 described in diagnostic colonoscopy, to 5% in therapeutic colonoscopy. Management of this type of perforation varies and includes different strategies depending on the patient's clinical condition and the support means available in the environment, with the possibility of various strategies ranging from conservative treatment to a surgical approach.
We present a clinical case with iatrogenic perforations; conservative treatment in hospital was possible for this patient, despite the dramatic clinical/radiological picture, with intensive monitoring over the first hours.
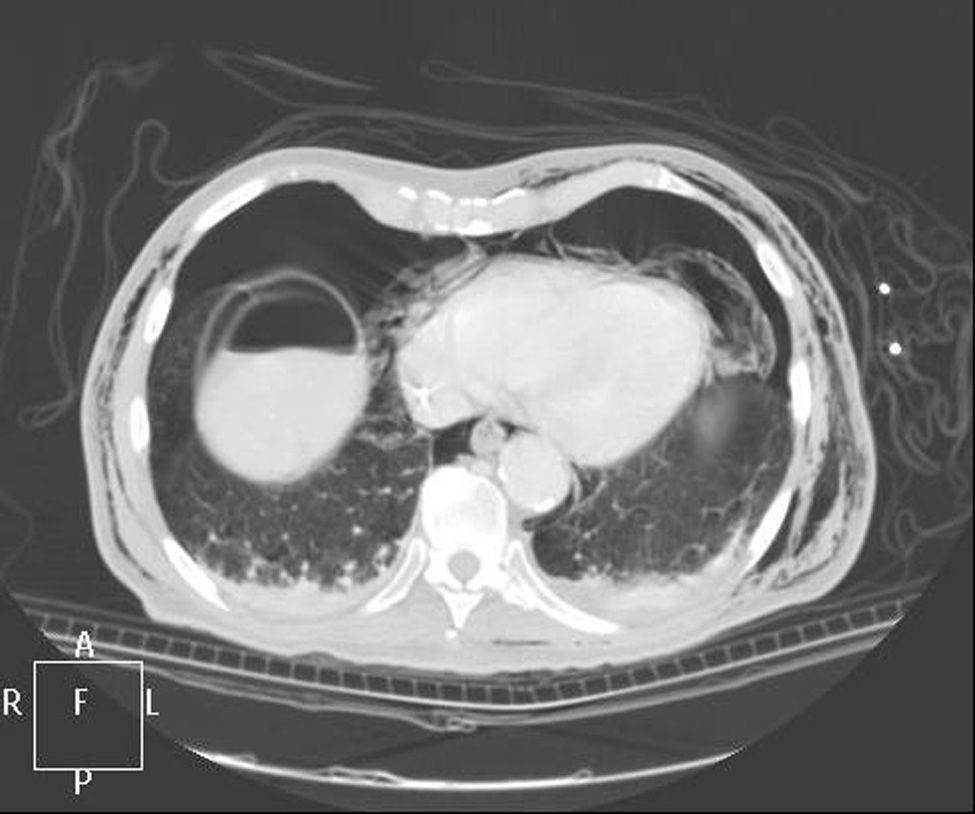
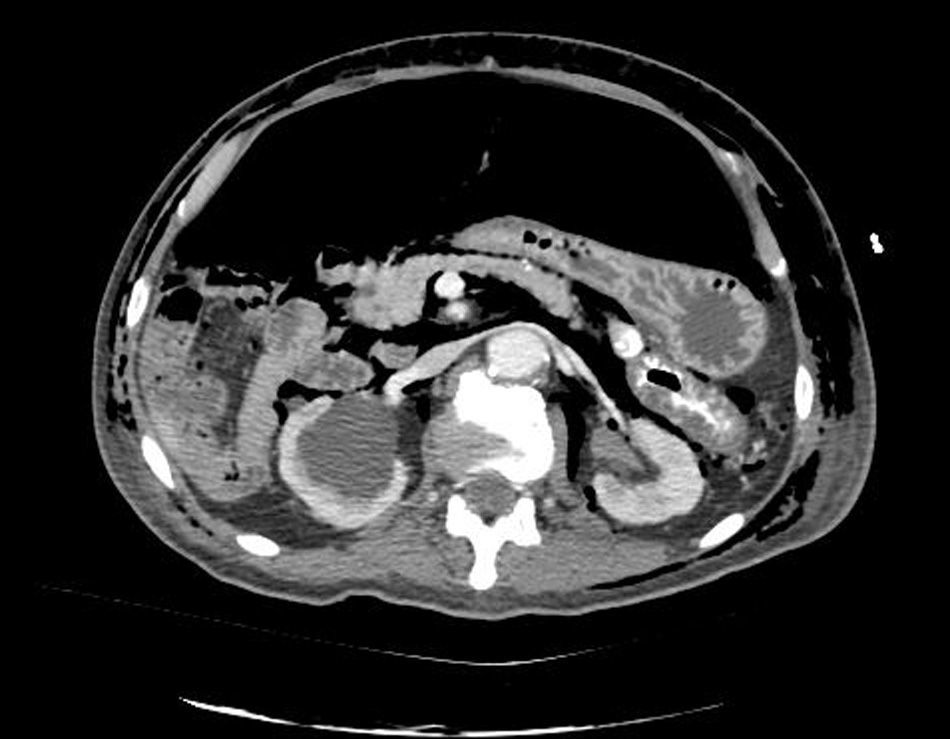
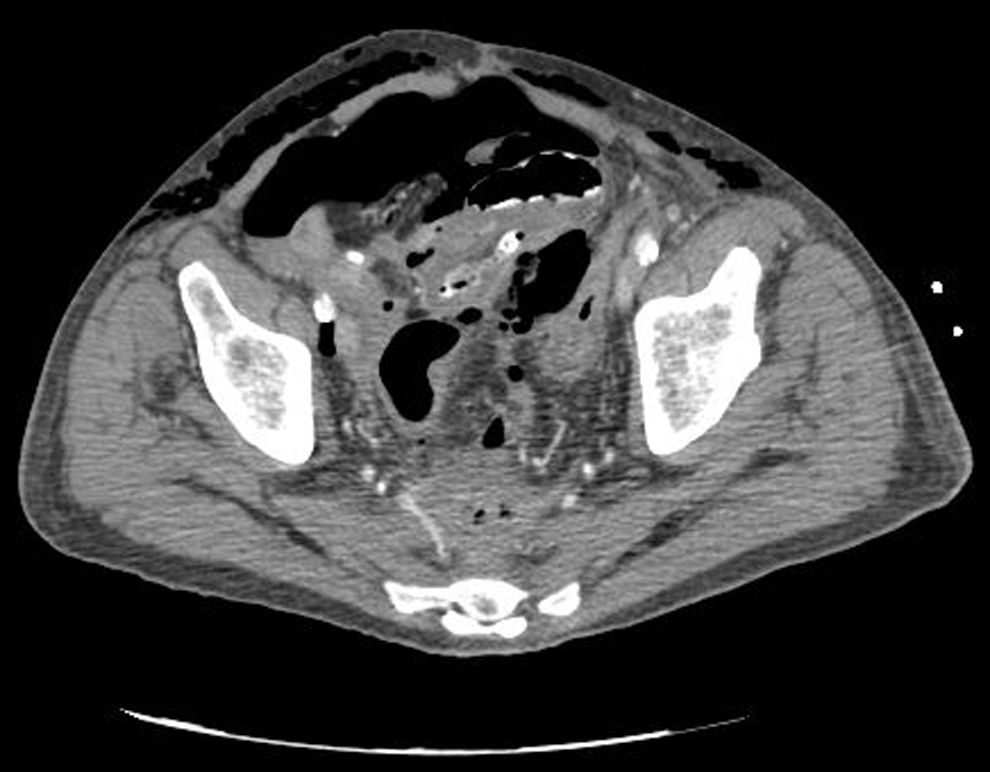
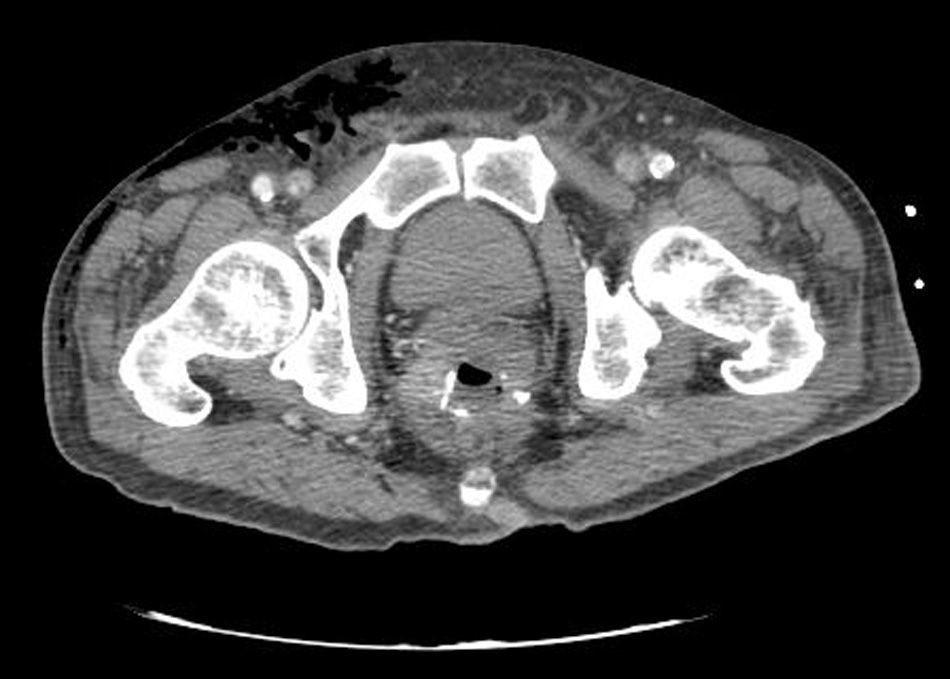
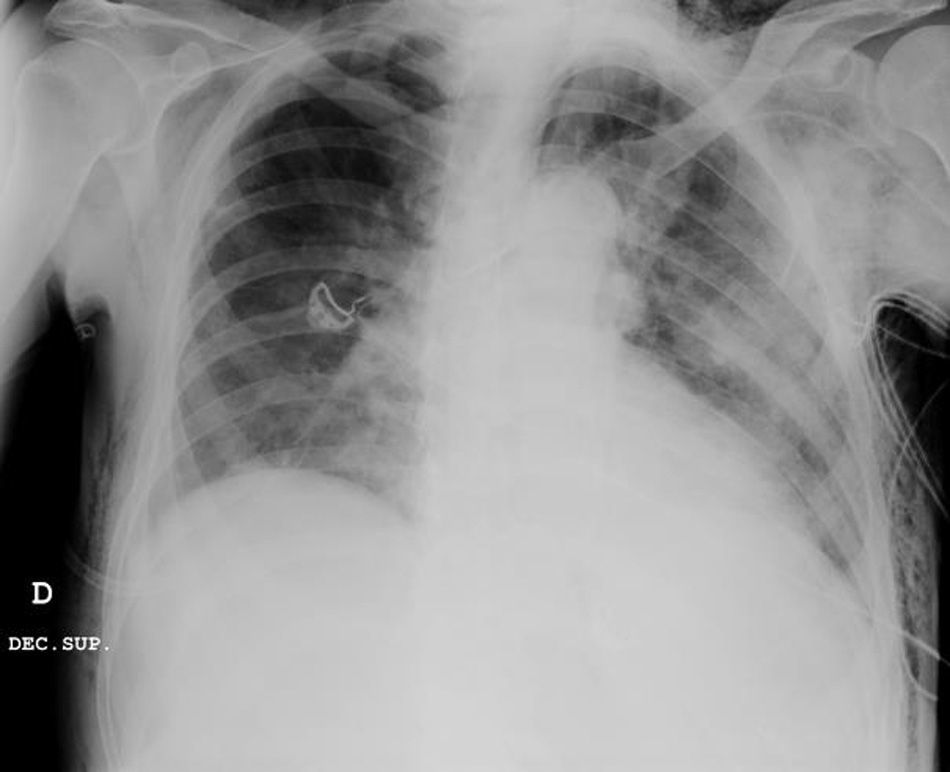
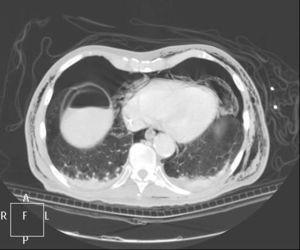
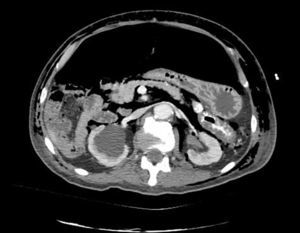
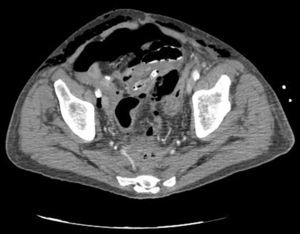
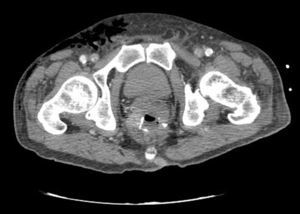
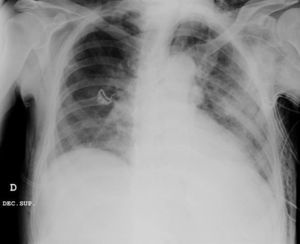
Clinical caseWe present the case of an 80-year-old woman with a history of systemic high blood pressure, operated in 2003 for an infrarenal abdominal aortic aneurysm. After assessment in the Digestive Department for occasional rectorrhagias, she was diagnosed endoscopically with high grade rectal adenomacarcinoma 6–7cm from the anal verge. The study was completed with axial computed tomography that revealed a rectal neoplasm with involvement of the peri-rectal lymph nodes and infrarenal abdominal aortic aneurysm with intraluminal thrombus. After their assessment, the Oncology Department considered that neoadjuvant treatment would not be worthwhile, and therefore the patient underwent a low anterior rectal resection with laparascopic total mesorectal excision and reconstruction of the tract with mechanical side- to- end anastomosis (circular stapler, 28mm) and loop ileostomy at the level of the right iliac fossa. The anatomical pathological result was high grade rectal adenocarcinoma pT3N2a (GL 5+/14) Dukes’ stage C. A subclinical leak was observed in the postoperative period which was treated and resolved conservatively. Three months later, closure of ileostomy was scheduled after checking the integrity and normality of the anastomosis by rectal examination and anoscopy. On the second post-operative day, after reconstruction of the digestive tract, the patient presented abdominal distension, nausea and vomiting. Abdominal X-ray revealed hydroaeric levels in the small and large intestine. A rectoscopy was performed because of the suspicion of anastomotic stenosis, the suspicion was confirmed and pneumatic dilation was performed by colonoscopy. This dilation was laborious, it was difficult to identify the anastomosis (probably because it was side-to-end) and the blind pouch of the side closure. The patient was sedated during the procedure and only at that time was major abdominal distension noted which was attributed to colonic distension due to the huge amount of air insufflated during the long procedure. At no point did the endoscopist perceive any perforation. Two hours after the procedure, the nursing staff reported that the patient was presenting clinical signs of high blood pressure, tachycardia and sweating. Physical examination revealed abdominal distension with palpable crepitus at the level of the chest and lower hemiabdomen, accompanied by haemodynamic instability and the patient was mildly obtunded. She was therefore transferred to the Intensive Care Unit, where she was stabilised using the necessary measures without administering vasoactive drugs. The diagnostic study included axial slice thoracoabdominopelvic computed tomography which revealed a large pneumo- and retropneumoperitoneum with pneumodediastinum (Figs. 1–4). And a single study with plain chest X-rays revealed a discreet bilateral pneumothorax (Fig. 5).
Due to the patient's age and because it had been possible to stabilise her with conservative treatment, it was decided to continue with nil by mouth, nasogastric tube, hydrotherapy, oxygen therapy and broad spectrum antibiotics. Once the patient had been stabilised, the pneumoperitoneum was eliminated by percutaneous puncture with Abbocath No. 14 at epigastric level. The patient was kept under strict supervision, by monitoring and serial laboratory and radiological tests.
The third day after her admission to the Intensive Care Unit, the patient made good clinical progress, confirmed by the relevant laboratory and radiological tests, and was transferred to the Surgical department, where her intestinal transit returned to normal and she was discharged 10 days after her admission. After 6-months’ follow-up as an outpatient, where she underwent serial rectoscopies, no endorectal pneumatic dilations were necessary; despite the fact that the location of the perforation had not been found.
DiscussionThe incidence of symptomatic anastomotic stenosis that manifests as partial or complete intestinal obstruction is estimated at between 4% and 10%.1
The first therapeutic option in patients with either benign or malignant anastomotic stenosis is endoscopic dilation of the anastomosis with or without the placement of expanding stents or the endoscopic resection of stenotic tissues if there is a high- grade benign stenosis2 and, in cases where these measures are not effective, surgical resection will be necessary. Although endoscopic dilation is the treatment of choice, this might trigger a series of unwanted adverse effects such as restenosis, the formation of abscesses or perforation, which is the most serious complication following this procedure.
The incidence of perforation is around 5% after therapeutic colonoscopy, according to the different series.3 After a diagnosis of perforation following endoscopic dilation, various therapeutic strategies can be chosen: conservative management, endoscopic endoanal repair of perforation3,4 or surgical intervention. In the case we present, colonoscopic dilation was extremely laborious, perhaps due to the type of anastomosis, (side-to-end), and the previous leakage and, although ultimately effective, it resulted in a secondary effect of a perforation which was managed conservatively. This type of treatment should be reserved for patients who are in a good general condition, have had bowel preparation, have no signs of peritonitis and are clinically stable. It could be considered for unstable patients, as in our case. However, the necessary support measures of the Intensive Care Unit are recommended for the strict supervision and monitoring of these patients under conservative management, otherwise revision surgery should be performed.
Conservative management comprises digestive resting, hydrotherapy and the administration of broad-spectrum antibiotics and other necessary measures depending on the patient's clinical manifestations. The success of conservative treatment ranges from 33% to 73% and should be apparent 24–48h after it has been started, otherwise a more aggressive approach is required.3,5,6 The conservative option was chosen for this patient because of the availability of an Intensive care Unit that enabled her to be monitored strictly over the first 48h, during which time the pneumoperitoneum was drained percutaneously and the patient was given the necessary support measures. A good clinical response was achieved, which enabled conservative management to be kept as the first therapeutic option, thus avoiding surgical revision with its consequent mortality and morbidity. If conservative measures had failed in our patient, or if the stenosis had reoccurred, given her age and the fact that this was a low anastomosis, our approach would have been to perform a resection with colostomy in the left iliac fossa.
Restenosis has an incidence of 15% of patients operated for rectal cancer according to Suchan,7 although this data is very variable in the literature due to different and irregular follow-up methods.
Furthermore, benign stenoses, with an incidence ranging from 3% to 30%8 are directly associated with the following risk factors: (A) mechanical anastomosis which is associated with greater collagen deposit and inflammation, which can encourage the onset of stenosis.8 (B) End-to-end anastomosis rather than side-to-end, the latter should be chosen when the anastomosis is below 8cm.9 (C) having presented signs of anastomotic leak or subclinical leak, with the consequent pelvic sepsis that promotes the onset of fibrosis of the perianastomotic tissues.10,11 (D) being male, due to the technical difficulties in certain cases posed by the male pelvis.12 (E) having a protective stoma,12 due to the lack of intestinal transit in this region.
The standard practice in our unit whenever we perform low rectal resections and providing the patient's anatomical and surgical characteristics allow, is to perform a mechanical side-to-end tension-free anastomosis with a 31mm device, avoiding smaller devices and systematically checking the seal of the anastomosis, reinforcing it if necessary with a number of sutures (3/0). Our patient, although she underwent a mechanical end-to-side anastomosis using a device of a size we considered appropriate (circular stapler, 28mm), presented a postoperative ileus after the first operation, with purulent output through the drain. We interpreted this as a subclinical leak which was well-tolerated and resolved under conservative treatment; over time we considered this to be the main cause of the anastomotic stenosis. If we add to this the use of medium-sized head, we believe that on certain occasions and with certain postoperative complications following colorectal surgery, outpatient imaging studies of the anastomosis13,14 and routine rectoscopy is not only compulsory15 but also allows dilation prior to ileostomy closure, which reduces morbidity during the postoperative period after the second surgical stage.
To conclude, we believe that it is important to check all low colorectal anastomosis prior to ileostomy closure. This is a simple procedure which can be performed as an outpatient by digital examination, anoscopy and rigid rectoscopy. However, as in the case that we present, this check did not rule out stenosis. Furthermore, with an anastomotic perforation after endoscopic manipulation, it is worth considering the possibility of conservative treatment in a hospital environment with the appropriate resources, if the patient's clinical situation allows.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Echazarreta-Gallego E, Córdoba-Díaz de Laspra E, Elía-Guedea M. Retroneumoperitoneo secundario a dilatación endoscópica de anastomosis colorrectal: ¿permite un manejo conservador? Cir Cir. 2016;84:420–424.