The risk of post-operative pneumonia is a latent complication. A study was conducted to determine its risk factors in abdominal surgery.
Material and methodsA cross-sectional study was performed that included analysing the variables of age and gender, chronic obstructive pulmonary disease and smoking, serum albumin, type of surgery and anaesthesia, emergency or elective surgery, incision site, duration of surgery, length of hospital stay, length of stay in the intensive care unit, and time on mechanical ventilation. The adjusted odds ratio for risk factors was obtained using multivariate logistic regression.
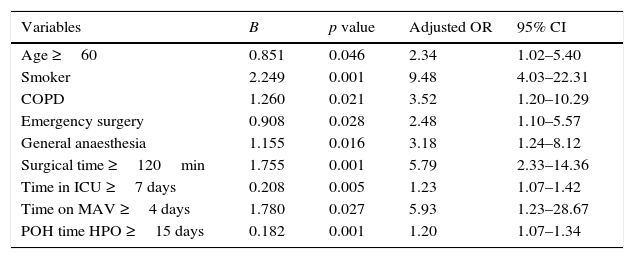
ResultsThe study included 91 (9.6%) patients with pneumonia and 851 (90.4%) without pneumonia. Age 60 years or over (OR=2.34), smoking (OR=9.48), chronic obstructive pulmonary disease (OR=3.52), emergency surgery (OR=2.48), general anaesthesia (OR=3.18), surgical time 120min or over (OR=5.79), time in intensive care unit 7 days or over (OR=1.23), time on mechanical ventilation greater than or equal to 4 days (OR=5.93) and length of post-operative hospital stay of 15 days or over (OR=1.20), were observed as independent predictors for the development of postoperative pneumonia.
ConclusionsIdentifying risk factors for post-operative pneumonia may prevent their occurrence. The length in the intensive care unit of greater than or equal to 7 days (OR=1.23; 95% CI 1.07–1.42) and a length postoperative hospital stay of 15 days or more (OR=1.20; 95% CI 1.07–1.34) were the predictive factors most strongly associated with lung infection in this study.
El riesgo de neumonía postoperatoria es una complicación latente. Realizamos una investigación para definir sus factores de riesgo en cirugía abdominal.
Material y métodosMediante un estudio transversal analizamos la edad y género, enfermedad pulmonar obstructiva crónica y tabaquismo, albúmina sérica, tipo de cirugía y de anestesia, operación de urgencia o electiva, sitio de la incisión, duración de la operación; tiempo de hospitalización, en la Unidad de Cuidados Intensivos y, en Ventilación Mecánica Asistida. Por regresión logística multivariado obtuvimos la odds ratio ajustada para los factores de riesgo.
ResultadosEstudiamos a 91 (9.6%) pacientes con neumonía y, 851 (90.4%) sin neumonía. Identificamos la edad≥60 años (OR=2.34), el tabaquismo (OR=9.48), la enfermedad pulmonar obstructiva crónica (OR=3.52), la intervención quirúrgica de urgencia (OR=2.48), la anestesia general (OR=3.18), el tiempo quirúrgico ≥120min (OR=5.79), el tiempo en la Unidad de Cuidados Intensivos ≥7 días (OR=1.23), el tiempo en ventilación mecánica asistida ≥4 días (OR=5.93) y el tiempo de hospitalización postoperatoria ≥15 días (OR=1.20) como factores predictivos independientes para el desarrollo de neumonía postoperatoria.
ConclusionesLa identificación de factores de riesgo para la neumonía postoperatoria puede prevenir su aparición. El tiempo en la Unidad de Cuidados Intensivos ≥7 días (OR=1.23; IC del 95%, 1.07–1.42) y de hospitalización postoperatoria ≥15 días (OR=1.20; IC del 95%, 1.07–1.34) fueron los factores predictivos más fuertemente asociados con la infección pulmonar en nuestro estudio.
Despite the advances in surgical and anaesthetic techniques, the risk of developing postoperative pneumonia remains a latent complication, probably owing to an ageing population, with higher morbidity, and who undergo more complex surgery. In this regard, one study reported that 36% of the episodes of nosocomial pneumonia in their series of patients were diagnosed in the operating theatre.1 This disease is defined as an infection of the pulmonary parenchyma which is neither present nor in the incubation period, at the time the patient is admitted to hospital, and develops 48h after admission.2 After urinary tract infections, pneumonia is the second most common of all nosocomial infections, with a frequency of 13–18%.2,3 However, it is responsible for 20–70% of deaths associated with hospital-acquired infection, especially in patients admitted to intensive care units who receive assisted mechanical ventilation.2,4 In this context, between 9% and 40% of patients who undergo abdominal surgery present, at least one pulmonary complication,5,6 with an overall mortality, specifically for pneumonia, of between 19% and 45%, and up to 65% when there is a history of intra-abdominal infection.7
Because postoperative pneumonia incurs high morbimortality, high costs and prolonged hospital stay,2 we undertook an investigation study to define the impact of possible risk factors for pneumonia in patients undergoing abdominal surgery. The information obtained will enable health programmes to be set up for the prevention, and control of this infection in surgical departments.
Material and methodsBetween 3 January 2011 and 30 December 2013, we performed an analytical cross-sectional study to determine risk factors associated with postoperative nosocomial pneumonia. All elective and emergency postoperative patients with intra-abdominal oncological and general surgical conditions; men and women >18 years of age, and with a hospital stay >48h post surgery. Patients who underwent laparoscopic surgery, and those in whom trauma was the reason for their abdominal surgery, patients with a preoperative diagnosis of pneumonia or any other identifiable infection, patients who were under or had had assisted mechanical ventilation prior to their surgical intervention, those who had undergone abdominal reoperation during the same hospital stay, and patients with a postoperative abdominal infection were excluded from the study. For the analysis of the risk factors associated with the disease, we identified all the consecutive patients with postoperative pneumonia in the study period as response cases, whereas patients without pneumonia were the control group cases. The potential risk factors were selected based on the clinical experience of the authors and previous studies.5,7
The Dr. Valentín Gómez Farías hospital is a regional reference centre with third level healthcare and teaching services in the Guadalajara Metropolitan Area, Mexico. It has 204 hospital beds and 15 intensive care beds with mechanical ventilation equipment, and invasive haemodynamic monitoring equipment for adult surgical, medical, and trauma conditions. There were 34,717 hospitalised patients during the study period, of whom 3724 had undergone general surgical procedures, and 1066 oncological surgery.
This study was approved by the Teaching and Research department and the main hospital's Ethics Committee and was performed in compliance with the clinical record guidelines set out in Norma Oficial Mexicana NOM-004-SSA3-2012 and the Ley General de Salud en Materia de Investigación para la Salud de los Estados Unidos Mexicanos. The patients were not asked for their informed consent because the study did not alter the strategies for their diagnosis or treatment.
Standard electronic forms in Microsoft Excel 2007 (Microsoft Inc., Redmond, WA, EE. UU.) were used to obtain information from the medical records, laboratory data and radiological reports; these were collected by a general surgical intern.
The investigation variables included the presence or absence of postoperative pneumonia as a dependent variable, while the independent variables were: the patients’ age and gender, history of obstructive pulmonary disease and smoking, preoperative albumen level, type of surgery (oncological or general), if the procedure was emergency or elective, the type of anaesthesia (general or local), abdominal incision site, the duration of the operation, and postoperative hospitalisation time, and the time spend in the intensive care unit, and on assisted mechanical ventilation.
Postoperative pneumonia was considered nosocomial when it was diagnosed >48h after surgical intervention, and before the patient was discharged from hospital after the same intervention. The disease was diagnosed when a new, persistent or progressive pulmonary infiltrate, with no other explanation, appeared in 2 or more serial X-rays.8–10 In addition to at least 2 of the following criteria: body temperature >38°C or <35°C; leukocytosis >12,000mm−3 or leukopenia <4000mm−3; purulent bronchial sputum, and minimal presence of a micro-organism at a concentration of at least 104colony-forming units/ml in bronchoalveolar lavage.8–10 The pneumonia was considered to be associated with the ventilator when it occurred >48h after endotraqueal intubation, and on commencing assisted mechanical ventilation, but within 72h of starting said ventilation.2,11 For this study, age ≥60, surgical time ≥120min, stay in intensive care unit ≥7 days and on mechanical assisted ventilation ≥4 days, as well as a postoperative hospital stay ≥15 days, were considered high risk factors for postoperative pneumonia; values lower than those cited were the benchmark. Likewise, in order to adjust the effect of confusing or intervening variables, and to codify the categories for logistic regression analysis, we considered high risk factors in men compared to women, smokers compared to non smokers, the presence of chronic obstructive pulmonary disease compared to its absence, oncological surgery compared to general surgical procedures, emergency intervention compared to elective, and high abdominal incision taking low incision as the benchmark, and general anaesthesia compared to local. A patient was considered a smoker if they reported smoking a minimum of one cigarette a day for >1 year, were smoking at the time of the study or had stopped smoking 8 weeks before the study started.12 Chronic obstructive pulmonary disease was defined on previously published criteria.13 Surgical interventions were defined as oncological when a preoperative histopathological diagnosis was available in order to classify them as such; none of the patients in this category had undergone radiotherapy, chemotherapy or immunosuppressant medication prior to the surgical intervention. The incision was defined as high or low when made, respectively, above or below an imaginary line on the anterior abdominal wall which passed through the umbilical scar transversally; simultaneous supra- and infra-umbilical incision was categorised as high for this study. The length of postoperative stay was defined as the total number of days in hospital, from the date of surgery to the date of discharge or death during the same hospital stay as that of the operation. Serum albumin <2.2mg/dl was the high-risk cut-off point for association with the presence of pneumonia based on a previous report.14
For the statistical analysis, the data were described as numbers and means±standard deviation. The categorical variables were analysed using the Pearson's chi-squared test, and the Student's t-test for comparing means and normally distributed data. The statistically significant value was placed a priori at p=≤0.05, with 95% confidence intervals (95% CI). Independent variables were identified by univariate logistic regression as potential risk factors for postoperative pneumonia. Therefore, only variables with p=≤0.05, or clinically significant, were entered into a multivariate logistic regression analysis (methods Enter and Backward: RV) in order to obtain the final model. For these factors, the results are presented as odds ratio (OR) and 95% CI. Social Science software was used for the statistical analyses (SPSS® 19.0; SPSS Inc., Chicago, U.S.A.) for Windows™ with EPIDAT version 3.1 (Pan American Health Organisation).
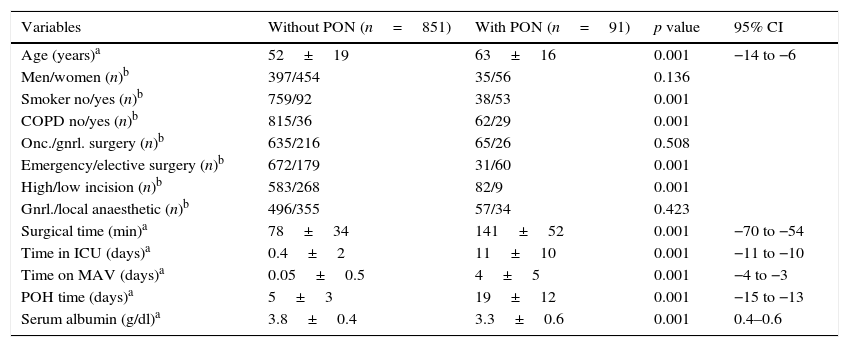
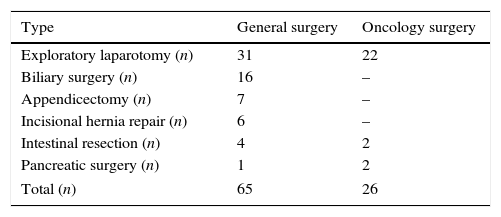
ResultsDuring the 3-year study period (2011–2013), the number of patients who underwent general surgical operations who met the inclusion and exclusion criteria was 700, 65 (9.2%) with pneumonia and 635 (90.8%) without pneumonia, whereas the number of patients who underwent oncological surgery was 242, 26 (10.7%) with pneumonia and 216 (89.3%) without pneumonia. Thus, the total number of patients analysed was 942, 91 (9.6%) with pulmonary infection and 851 (90.4%) without. Table 1 shows that the average age of the patients in the group without post-operative pneumonia was 52±19 and in the group with pneumonia 63±16, and that, within the variables investigated, only the men: women ratio (p=0.136), oncological surgery: general (p=0.508) and general: local anaesthesia (p=0.423) ratios had no statistically significant difference. There were 6 (9.2%) deaths in the general surgery group and 2 (7.7%) in the oncology surgery group. In the 8 cases (total mortality of 8.8%), the deaths were directly contributed to pulmonary infection. The types of surgical intervention undertaken in the general and oncology surgery groups are shown in Table 2. All the operations were undertaken using the conventional technique (not laparascopic) and exploratory laparotomy was the most common procedure in both categories (48% in the general surgery category, and 85% in the oncology surgery category).
Demographic data and perioperative variables.
| Variables | Without PON (n=851) | With PON (n=91) | p value | 95% CI |
|---|---|---|---|---|
| Age (years)a | 52±19 | 63±16 | 0.001 | −14 to −6 |
| Men/women (n)b | 397/454 | 35/56 | 0.136 | |
| Smoker no/yes (n)b | 759/92 | 38/53 | 0.001 | |
| COPD no/yes (n)b | 815/36 | 62/29 | 0.001 | |
| Onc./gnrl. surgery (n)b | 635/216 | 65/26 | 0.508 | |
| Emergency/elective surgery (n)b | 672/179 | 31/60 | 0.001 | |
| High/low incision (n)b | 583/268 | 82/9 | 0.001 | |
| Gnrl./local anaesthetic (n)b | 496/355 | 57/34 | 0.423 | |
| Surgical time (min)a | 78±34 | 141±52 | 0.001 | −70 to −54 |
| Time in ICU (days)a | 0.4±2 | 11±10 | 0.001 | −11 to −10 |
| Time on MAV (days)a | 0.05±0.5 | 4±5 | 0.001 | −4 to −3 |
| POH time (days)a | 5±3 | 19±12 | 0.001 | −15 to −13 |
| Serum albumin (g/dl)a | 3.8±0.4 | 3.3±0.6 | 0.001 | 0.4–0.6 |
The values are mean±SD and number of patients.
COPD: chronic obstructive pulmonary disease; gnrl./loc.: general/local; POH: post-operative hospitalisation; CI: confidence interval; PON: post-operative pneumonia; onco./grnl.: oncology/general; ICU: intensive care unit; AMV: assisted mechanical ventilation.
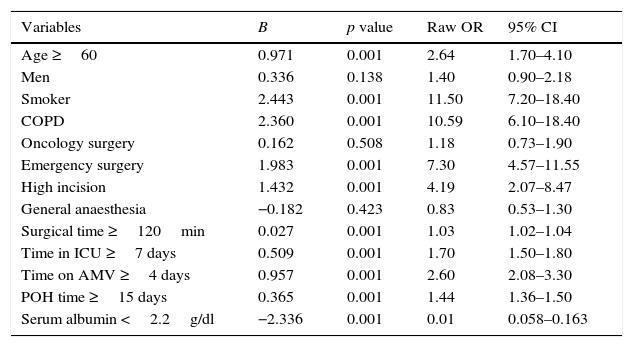
By means of univariate analysis each of the independent variables, with the numerical variables categorised, was compared individually with the dependent variable in order to identify the statistically significant value and the 95% CI of the risk (raw OR) (Table 3). The results show that the following variables had no significant difference between the groups of patients with or without pneumonia: men compared to women (p=0.138), oncology surgery compared to general surgery (p=0.508), and general compared to local anaesthesia (p=0.423). The risk factors which presented a statistically significant association in the univariate analysis, as well as clinical significance, were selected for inclusion in the multivariate analysis. The final logistic regression model (Table 4) identified age ≥60 (OR=2.34; 95% CI 1.02–5.40), smoking (OR=9.48; 95% CI 4.03–22.31), chronic obstructive pulmonary disease (OR=3.52; 95% CI 1.20–10.29), emergency surgical intervention (OR=2.48; 95% CI 1.10–5.57), general anaesthesia (OR=3.18; 95% CI 1.24–8.12), surgical time ≥120min (OR=5.79; 95% CI 2.33–14.36), time spent in the intensive care unit ≥7 days (OR=1.23; 95% CI 1.07–1.42), time spent on assisted mechanical ventilation ≥4 days (OR=5.93; 95% CI 1.23–28.67) and postoperative hospitalisation time ≥15 days (OR=1.20; 95% CI 1.07–1.34) as independent predictive risk factors associated with the development of postoperative pneumonia. We did not find this association between the patients’ gender, type of surgery (oncology/general surgery), incision site (high/low) or with serum albumin level <2.2g/dl and level ≥2.2g/dl. The latter parameter, although it showed a reduction in the risk of postoperative pulmonary infection in patients with serum albumin <2.2g/dl (B=−2.336), did not appear in our final multivariate model.
Condensed univariate analysis of the risk factors for pneumonia after abdominal surgery.
| Variables | B | p value | Raw OR | 95% CI |
|---|---|---|---|---|
| Age ≥60 | 0.971 | 0.001 | 2.64 | 1.70–4.10 |
| Men | 0.336 | 0.138 | 1.40 | 0.90–2.18 |
| Smoker | 2.443 | 0.001 | 11.50 | 7.20–18.40 |
| COPD | 2.360 | 0.001 | 10.59 | 6.10–18.40 |
| Oncology surgery | 0.162 | 0.508 | 1.18 | 0.73–1.90 |
| Emergency surgery | 1.983 | 0.001 | 7.30 | 4.57–11.55 |
| High incision | 1.432 | 0.001 | 4.19 | 2.07–8.47 |
| General anaesthesia | −0.182 | 0.423 | 0.83 | 0.53–1.30 |
| Surgical time ≥120min | 0.027 | 0.001 | 1.03 | 1.02–1.04 |
| Time in ICU ≥7 days | 0.509 | 0.001 | 1.70 | 1.50–1.80 |
| Time on AMV ≥4 days | 0.957 | 0.001 | 2.60 | 2.08–3.30 |
| POH time ≥15 days | 0.365 | 0.001 | 1.44 | 1.36–1.50 |
| Serum albumin <2.2g/dl | −2.336 | 0.001 | 0.01 | 0.058–0.163 |
Statistical significance p=≤0.05.
COPD: chronic obstructive pulmonary disease; POH: post-operative hospitalisation; CI: confidence interval; OR: odds ratio; ICU: intensive care unit; MAV: mechanically assisted ventilation.
Condensed multivariate analysis of the adjusted risk factors for pneumonia after abdominal surgery.
| Variables | B | p value | Adjusted OR | 95% CI |
|---|---|---|---|---|
| Age ≥60 | 0.851 | 0.046 | 2.34 | 1.02–5.40 |
| Smoker | 2.249 | 0.001 | 9.48 | 4.03–22.31 |
| COPD | 1.260 | 0.021 | 3.52 | 1.20–10.29 |
| Emergency surgery | 0.908 | 0.028 | 2.48 | 1.10–5.57 |
| General anaesthesia | 1.155 | 0.016 | 3.18 | 1.24–8.12 |
| Surgical time ≥120min | 1.755 | 0.001 | 5.79 | 2.33–14.36 |
| Time in ICU ≥7 days | 0.208 | 0.005 | 1.23 | 1.07–1.42 |
| Time on MAV ≥4 days | 1.780 | 0.027 | 5.93 | 1.23–28.67 |
| POH time HPO ≥15 days | 0.182 | 0.001 | 1.20 | 1.07–1.34 |
Statistical significance p≤0.05.
COPD: chronic obstructive pulmonary disease; POH: post-operative hospitalisation; CI: confidence interval; OR: odds ratio; ICU: intensive care unit; MAV: mechanically assisted ventilation.
The principal cause of nosocomial pneumonia is colonisation of the oropharynx and the gastrointestinal tract by pathogenic micro-organisms, chiefly gram positive and negative bacteria, followed by their aspiration, and the disease manifests when the host's defences are lowered.4 Endotracheal intubation for assisted mechanical ventilation is a recognised risk factor for developing this infection, as the vocal cords remain open enabling the aspiration of bacteria into the lungs.15 In addition to this condition, many other factors associated with the development of pneumonia have been described in the postsurgical population.16 However, as these can differ significantly from one hospital to another and therefore influence the measures implemented for its prevention, we performed a study to assess the predictive impact on postoperative pneumonia of the risk factors we consider the most relevant in our surgical department.
The role of age is not clearly defined in several studies.17,18 Specifically in patients with abdominal surgery, Smetana et al.19 reported that age >70 is an independent risk factor for the disease, whereas Tusman et al.20 place the cut-off age at >65. In our investigation we found that the risk presents at an earlier age, i.e., from age 60 (OR=2.34; 95% CI 1.02–5.40). Furthermore, although it is recognised that abdominal surgery is a risk factor for the development of postoperative pneumonia, when the intervention is an emergency the risk increases.2,13,21 Our findings support these reports, in that we found that emergency surgery was associated with a 2.48-fold higher probability (95% CI 1.10–5.57) than elective surgery of presenting pulmonary infection. In the same context of possible preoperative associations, it has been documented that general anaesthesia, compared to local anaesthesia, is an independent risk factor for postoperative pneumonia,5 we report a similar finding in our investigation (OR=3.18; 95% CI 1.24–8.12). Furthermore, despite having identified that durations of abdominal surgery >3–4h is an independent predictor of postoperative pneumonia,5,16,22,23 in our study we found that a surgical time ≥120min is already an important risk factor for the disease (OR=5.79; 95% CI 2.33–14.36), both in univariate and multivariate logistic analysis. Moreover, abdominal surgery patients are frequently admitted to intensive care units for various reasons, and also require assisted mechanical ventilation. The prevalence of pneumonia in these units, amongst all the hospital acquired infections, is between 27% and 31%,24 whereas the risk of presenting the disease is multiplied more than 20 fold in patients who receive mechanical ventilation.3 In fact, endotracheal intubation is considered the most important risk factor for pulmonary infection, especially during the patients’ first 8–10 days on mechanical ventilation.12,25 In our investigation, the risk of pneumonia in abdominal surgery patients admitted to the intensive care unit for ≥7 days was 1.23-fold greater (95% CI 1.07–1.42) compared to those who were admitted for <7days, whereas this risk was 5.93-fold greater (95% CI 1.23–28.67) with assisted mechanical ventilation for ≥4 days compared with <4 days. Moreover, during the postoperative hospitalisation period, the patients could have been colonised by antibiotic-resistant bacterial strains which can cause nosocomial infections.26,27 We found that the risk of pneumonia was 1.20-fold greater (95% CI 1.07–1.34) with ≥15 days of hospitalisation after the intra-abdominal procedure. Finally, similar to previous reports that chronic obstructive pulmonary disease is a risk factor for hospital-acquired pneumonia,28 this risk was significantly greater (OR=3.52; 95% CI 1.20–10.29) in our patients with chronic pneumonia. In contrast with the frequency of death by postoperative nosocomial pneumonia of 21–54% which is reported in literature,21,29 in our study we found a total mortality of only 8.8%, probably because most of our cases occurred in the early phase of the postoperative period (<5 days), which was associated with a better prognosis in comparison with pneumonias which presented later (≥5 days).2
It is important to highlight that serum albumin, commonly evaluated in hospitalised patients who have undergone abdominal surgery, is a reliable indicator of the clinical outcome of patients with infectious diseases.30 In fact, some investigators have found that a low preoperative serum level of this protein plays an important role in predicting the development of pneumonia.31,32 Our findings did not support these reports, or that supraumbilical incisions more than double the risk of pulmonary infection compared to infraumbilical incisions.13,33 It is probable that this was due to the P value of ≤0.05 that we chose as the statistically significant value (rather than the p value ≤0.1 which is usually chosen in other studies) for an independent variable to be considered in our multivariate logistic regression model, and also a relatively low number of cases in the group with pneumonia with the above-mentioned risk factors (only 7 cases with serum albumin <2.2g/dl, and 9 cases with a low incision). Other weaknesses and limitations of our study were that it was retrospective, that some cases in the study probably fell into the category of healthcare-associated pneumonia, the difficulty in diagnosing pneumonia in surgical patients,16 as well as the different and variable criteria for detecting this disease in hospitals.34 Furthermore, the heterogeneity of the procedures, and operating techniques that we included limited our possibility of predicting the risk of pneumonia in a particular patient, and for a specific type of surgical intervention. Finally, we did not consider other possible risk factors, described in medical literature,16 due to the lack of information on them in the clinical records that we reviewed.
ConclusionsIdentifying risk factors for postoperative pneumonia can help to plan, implement and evaluate the public health services in order to prevent them. In our study we found that age >60 (OR=2.34), smoking (OR=9.48), chronic obstructive pulmonary disease (OR=3.52), emergency surgery (OR=2.48), general anaesthesia (OR=3.18), surgical time ≥120min (OR=5.79), and assisted mechanical ventilation for ≥4 days (OR=5.93) were significantly associated with an increased risk of the disease. However time spent in the intensive care unit ≥7 days (OR=1.23; 95% CI 1.07–1.42, and postoperative hospitalisation time ≥15 days (OR=1.20; 95% CI 1.07–1.34) were the predictive factors which were associated most strongly with pulmonary infection. We consider that the final multivariate logistic regression model that we present can be used clinically to predict a greater risk of postoperative nosocomial pneumonia in patients who have undergone abdominal surgery.
Conflict of interestsThe authors have no conflict of interest to declare.
Please cite this article as: Evaristo-Méndez G, Rocha-Calderón CH. Factores de riesgo para neumonía nosocomial en pacientes con cirugía abdominal. Cirugía y Cirujanos. 2016;84:21–27.