Liver biopsy is the main diagnostic tool for the study of the liver, and as such, its inherent complications have been minimised as much as possible over the years, through the modification of several factors regarding its procedure, including post-biopsy recovery time.
The aim of this study was to evaluate the safety in the reduction of post-liver biopsy recovery time.
Material and methodsA non-blinded, randomised clinical trial was conducted in the “Hermanos Ameijeiras” Hospital from November 2011 to October 2012, on 128 patients in order to assess safety when reducing post-biopsy recovery times. The patients were randomised into 2 groups. Group A was allowed a 6-h recovery time, while Group B was allowed a 2-h recovery time after liver biopsy. Complications were fully recorded. The Chi squared test of homogeneity and Student t test was used as appropriate, in the statistical analysis, a significance level of 0.05 was set.
ResultsThe main biopsy indication was elevated plasma transaminases. Pain in the puncture site was the most recurrent complication (67.2%), and the most serious complication was subcapsular liver haematoma in two cases (1.6%). There were no differences regarding the liver biopsy technique that could have caused complications in any group.
ConclusionsThere were no significant differences between 2h and 6h post-liver biopsy recovery time in terms of complications, so it is considered that after 2h the patient is incorporated more quickly into their activities, and the institution spends less material and human resources.
La biopsia hepática es una herramienta diagnóstica para el estudio del hígado, por lo que en su evolución se han tratado de minimizar las complicaciones mediante la modificación de varios factores en relación con su realización, incluido el tiempo de reposo posbiopsia.
El objetivo de esta investigación fue evaluar la seguridad de la reducción del tiempo de reposo posbiopsia hepática.
Material y métodosSe realizó un ensayo clínico, aleatorizado, sin cegamiento, en el Hospital Clínico Quirúrgico «Hermanos Ameijeiras», en el período comprendido entre noviembre de 2011 y octubre de 2012. Se eligieron 128 pacientes que fueron asignados aleatoriamente a 2 grupos: grupo A con reposo de 6h y grupo B con 2h de reposo posbiopsia hepática. Se registraron las complicaciones. En el análisis estadístico se utilizó la prueba de chi cuadrado de homogeneidad y la t de Student según correspondiera; se fijó un nivel de valour estadístico significativo de 0.05.
ResultadosEl dolor en el sitio de punción resultó la complicación más frecuente (67.2%) y la más grave fue el haematoma hepático subcapsular con 2 casos (1.6%), sin diferencias con la técnica empleada en la biopsia para la aparición de complicaciones en ambos grupos.
ConclusionesNo existieron diferencias significativas entre el tiempo de reposo posbiopsia hepática de 2h y el de 6 en cuanto a complicaciones, por lo que se considera que con el de 2h el paciente se incorpora más rápidamente a sus actividades y la institución dedica menos recursos materiales y humanos.
Anatomopathological study of the liver has formed the basis of hepatology, and liver biopsy its principal tool, because it enables in vivo exploration and can be repeated sequentially.1 Percutaneous liver biopsy is a procedure which is used worldwide, both because of its safety and because it is highly useful in evaluating and managing patients with liver disease. Despite the fact that liver biopsy was used for the first time in Germany by Ehrlich in 1883, it was only after 1958 when Menghini,2 with his so-called “one-second biopsy” technique, managed to ensure the widespread use of this procedure. Liver biopsy started to be used increasingly from 1970 onwards due to the development and evolution of cytopathological techniques, due to the technical advances in imaging studies enabling effective and minimally invasive access to perform the procedure, and due to the advances in puncture needle technology; all of which have made this procedure really safe.3
At present, it is considered that liver biopsy is indicated in order to: (a) determine the cause of alterations in liver function tests, of no precise cause; (b) evaluate alcoholic liver disease and non-alcoholic liver disease; (c) investigate fever of unknown origin; (d) establish a diagnosis of multisystemic granulomatous and infiltrating disease; (e) confirm the aetiology of intrahepatic cholestasic disease; (f) support a diagnosis and to stage primary and secondary cancers in the liver, which also enables us to perform the relevant immunohistochemical study; (g) assess the extent of drug-induced liver damage; (h) achieve a diagnosis for hidden causes of hepatomegaly, jaundice and hereditary metabolic diseases of the liver; (i) establish a diagnosis in relation to the degree of activity and staging of chronic hepatitis and response to treatment; (j) follow-up and evaluate complications in transplanted patients; and (k) determine the cause of acute liver failure.4–11
As with any medical procedure which carries a risk, there are contraindications. These contraindications can be divided into absolute (failure to cooperate on the part of the patient, severe coagulopathy, infection of the liver bed and marked extrahepatic biliary obstruction) and relative (ascites, morbid obesity, vascular lesions, amyloidosis, hydatid disease).12–14 The available techniques are ultrasound-guided blind percutaneous biopsy, transjugular biopsy and the laparoscopic approach.7–15
Minor complications can occur in liver biopsy (30%), such as localised and temporary discomfort at the site of the biopsy, pain that requires analgesia and low blood pressure due to a vasovagal response. Major complications can also arise (0.3%), including intraperitoneal haemorrhage, intrahepatic or subcapsular haematoma and biliary peritonitis, which can even be fatal (0.03%).5–16 Different studies have shown that 61% of complications occur in the first 2h after the biopsy and 96% within the first 24h.16–21
Care after a liver biopsy has varied considerably over the years, especially with regard to rest time. Hospitalisation for the 24h following the procedure was indicated more than 20 years ago, during which time the patient was required to lie in the right lateral position or prone for 6h and then lie on either side for the remainder of the time.22 This rest time was later reduced to 6h in our centre – from the start of our department in 1983 – and as either an outpatient or inpatient, with no serious complications of mortality reported at all. In 1996 a study was performed in the National Gastroenterology Institute called “early mobilisation” after 4h rest, but with strict clinical monitoring for 24h.23
In recent years, studies have been published which argue that one hour's rest post biopsy under strict clinical monitoring is just as safe as rest for longer periods of time.24,25 These studies demonstrate that complications present in the first hours post biopsy, which was our motivation for undertaking this study where we propose shortening the post-biopsy rest time to only 2h, lying in the right lateral position, for the percutaneous technique after ultrasound marking or prone after laparoscopic-guided biopsy, under strict medical supervision which would result in the patient being able to resume their routines early, and reduce hospital costs.
Material and methodsA non-blinded, randomised clinical trial was carried out in the Gastroenterology Department of the “Hermanos Ameijeiras” Hospital between 1 November 2011 and 31 October 2012 including: all the patients who required a liver biopsy, as either inpatients or outpatients, whatever the cause, of either gender and over the age of 18. Patients who presented blood dyscrasia or any other disease affecting coagulation and predisposing them to haemorrhage, with haemoglobin levels below 8g/dl, thrombocytopenia of less than 70,000 platelets or a prothrombin deficiency with levels below 50%; or with vascular or cystic tumours of the liver were excluded from the study. Therefore, the sample comprised 128 patients, who were randomised into 2 groups: group A and group B; the doctor was informed during the surgical procedure itself. The random allocation was performed by a specialist in biostatistics, concealed in opaque envelopes with the patients’ number on the outside and the study group inside. These were kept in the Gastroenterology Department in the care of the Charge Nurse. After their liver biopsy, the patients in group A were told to rest in the right lateral position if they had had a percutaneous biopsy, and to lie prone if they had had a laparoscopic biopsy, for 2h, and then to lie on either side for 4 more hours. The patients in group B were told to rest for 2h only, in the same way. Both groups were strictly monitored and their blood pressure and heart rate taken every 15min for the first hour, then every 30min for the second hour and every hour for the 4 remaining hours for the patients in group A. The outpatients were discharged after the rest time, regardless of their study group, as long as they had not presented any major complication, and we contacted them by telephone in the following 24h to find out how they were progressing. All complications were fully recorded.
Statistical analysisPercentages were used for the qualitative variables and the mean with standard deviation for the quantitative variables. The Chi squared test and Fisher's exact test were used when there were 25% or more expected frequencies less than 5, to compare proportions in the qualitative variables. The means were compared using the Student t-test; a level of 0.05 was set for the comparison to be considered statistically significant.
The decision to include patients who had undergone an ultrasound-guided percutaneous biopsy with patients who had had a laparoscopic biopsy in one group was because they had all had liver biopsies with a Menghini needle and the monitoring procedure is the same. The difference between blind percutaneous biopsy and laparoscopic biopsy was only to identify the most appropriate site for the biopsy, taking into account the size of the liver and the absence of vascular malformations and cysts. Ultrasound was used in the percutaneous biopsies and it was identified visually in the laparoscopic biopsies, therefore there was no difference in the procedure for the puncture, or therefore in possible complications and monitoring times. We did not attempt to compare either form of guidance either.
The type of liver biopsy to be undertaken, percutaneous or laparoscopic, was randomly distributed, according to whether or not there was a need for the attending physician to assess the liver macroscopically. In order to perform the traditional “blind” percutaneous liver biopsy, first ultrasonic marking was performed to avoid puncturing any vascular malformations, the gallbladder or other viscera. Then, with the patient in the supine position with their right arm under their head, hepatic dullness was checked by percussing the intercostal space marked on the mid axillary line; after asepsis and antisepsis of the area, the puncture site was infiltrated with lidocaine 2%, first the epidermis and then a deeper level, then a millimetre, surface incision was made using the tip of a scalpel to allow entry of the lancet, which was used to open a passage through the intercostal space, until entering the abdominal cavity. Finally, after checking the permeability of the 14-Gauge Menghini needle (2.1mm in diameter), by expelling a small amount of physiological saline solution, the needle was introduced until it came into contact with the surface of the liver, where a further instillation was made, in order to expel any tissue that might have penetrated into the needle as it was being introduced; the patient was instructed to hold their breath and by applying intensive suction to the syringe, the Menghini needle was introduced and removed immediately to obtain the sample and deposit it in a vial containing 10% formalin, for anatomopathological assessment.
Liver biopsy under laparoscopic vision was started by performing a laparoscopy under local anaesthesia without sedation, in order to then introduce the Menghini needle through a surgical laparoscope to obtain a sample of liver tissue for histological study.
The following criteria governed the liver biopsies performed on outpatients: 1. They had to be able to return to the hospital within at least 30min. 2. They had to be accompanied by a family member or friend to have the procedure performed and during their first night at h ome. 3. They must not present any associated disease which would increase the risk of complications. 4. They would be hospitalised if more than one dose of analgesia was required within the first hours.
All the patients in both groups were informed of the specific objective of the procedure and possible complications, and they gave their consent and approval according to the principles of the II Helsinki declaration.
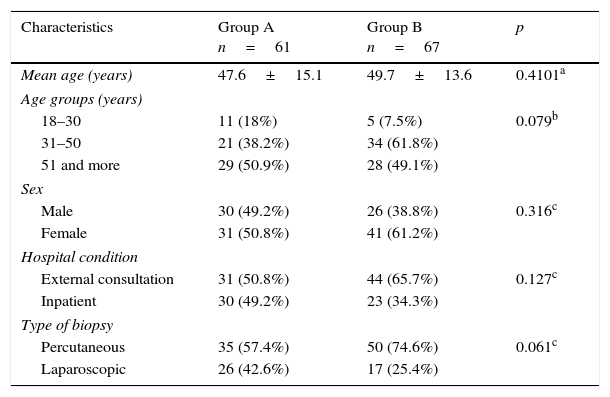
ResultsTable 1 presents the characteristics of the patients distributed in both study groups, it was observed that there were no significant differences (p>0.05) between them in terms of age, sex, hospital condition or type of biopsy performed.
Patient characteristics according to study group.
| Characteristics | Group A n=61 | Group B n=67 | p |
|---|---|---|---|
| Mean age (years) | 47.6±15.1 | 49.7±13.6 | 0.4101a |
| Age groups (years) | |||
| 18–30 | 11 (18%) | 5 (7.5%) | 0.079b |
| 31–50 | 21 (38.2%) | 34 (61.8%) | |
| 51 and more | 29 (50.9%) | 28 (49.1%) | |
| Sex | |||
| Male | 30 (49.2%) | 26 (38.8%) | 0.316c |
| Female | 31 (50.8%) | 41 (61.2%) | |
| Hospital condition | |||
| External consultation | 31 (50.8%) | 44 (65.7%) | 0.127c |
| Inpatient | 30 (49.2%) | 23 (34.3%) | |
| Type of biopsy | |||
| Percutaneous | 35 (57.4%) | 50 (74.6%) | 0.061c |
| Laparoscopic | 26 (42.6%) | 17 (25.4%) | |
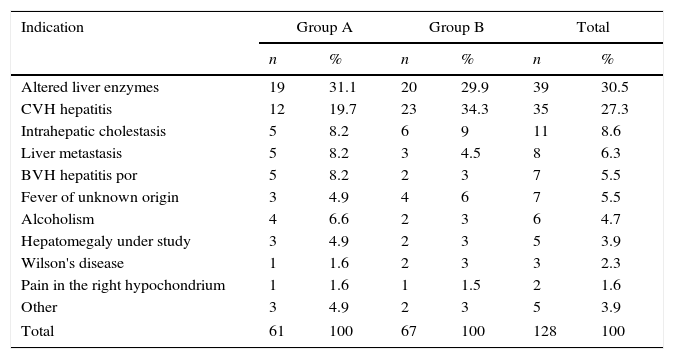
With regard to the indication for a liver biopsy in both study groups, most were performed on patients with altered hepatic enzymes, followed by hepatitis C; other causes were much less frequent (Table 2).
Indication for biopsy according to study group.
| Indication | Group A | Group B | Total | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Altered liver enzymes | 19 | 31.1 | 20 | 29.9 | 39 | 30.5 |
| CVH hepatitis | 12 | 19.7 | 23 | 34.3 | 35 | 27.3 |
| Intrahepatic cholestasis | 5 | 8.2 | 6 | 9 | 11 | 8.6 |
| Liver metastasis | 5 | 8.2 | 3 | 4.5 | 8 | 6.3 |
| BVH hepatitis por | 5 | 8.2 | 2 | 3 | 7 | 5.5 |
| Fever of unknown origin | 3 | 4.9 | 4 | 6 | 7 | 5.5 |
| Alcoholism | 4 | 6.6 | 2 | 3 | 6 | 4.7 |
| Hepatomegaly under study | 3 | 4.9 | 2 | 3 | 5 | 3.9 |
| Wilson's disease | 1 | 1.6 | 2 | 3 | 3 | 2.3 |
| Pain in the right hypochondrium | 1 | 1.6 | 1 | 1.5 | 2 | 1.6 |
| Other | 3 | 4.9 | 2 | 3 | 5 | 3.9 |
| Total | 61 | 100 | 67 | 100 | 128 | 100 |
BVH: B virus hepatitis; CVH: C virus hepatitis.
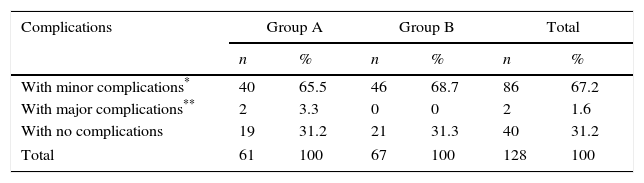
As can be observed in Table 3, minor complications arose in most of the patients in both study groups almost equally, at 65.5% for group A and 68.7% for group B, giving 67.2% for the total number of patients studied. Major complications only occurred in group A with 2 cases corresponding to 3.3% of the patients in this group and to 1.6% of the total number of patients in both groups. The 2 major complications that presented were 2 cases with subcapsular haematoma which progressed satisfactorily with conservative treatment and follow-up with imaging studies for 72h. There were no significant differences (p>0.05) on comparing the percentage of major and minor complications between the 2 study groups.
Distribution of patients according to complications and study group.
| Complications | Group A | Group B | Total | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| With minor complications* | 40 | 65.5 | 46 | 68.7 | 86 | 67.2 |
| With major complications** | 2 | 3.3 | 0 | 0 | 2 | 1.6 |
| With no complications | 19 | 31.2 | 21 | 31.3 | 40 | 31.2 |
| Total | 61 | 100 | 67 | 100 | 128 | 100 |
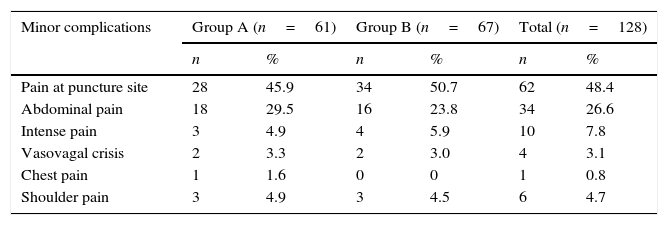
With regard to minor complications, it was seen that both in general (48.4%) and per study group (45.9% vs. 50.7%), most patients had pain at the puncture site; next in order of frequency was abdominal pain at 29.5% and 23.8% respectively for groups A and B. Another minor but less frequent complication, was severe shoulder pain which required more than one dose of analgesia, and then there were more isolated cases such as vasovagal crisis and chest pain, there was only once case of the latter (Table 4).
Patients according to the presence of minor complications and study groups.
| Minor complications | Group A (n=61) | Group B (n=67) | Total (n=128) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Pain at puncture site | 28 | 45.9 | 34 | 50.7 | 62 | 48.4 |
| Abdominal pain | 18 | 29.5 | 16 | 23.8 | 34 | 26.6 |
| Intense pain | 3 | 4.9 | 4 | 5.9 | 10 | 7.8 |
| Vasovagal crisis | 2 | 3.3 | 2 | 3.0 | 4 | 3.1 |
| Chest pain | 1 | 1.6 | 0 | 0 | 1 | 0.8 |
| Shoulder pain | 3 | 4.9 | 3 | 4.5 | 6 | 4.7 |
On comparing the presence of complications according to the biopsy technique used, it was observed that the biopsies via the laparoscopic route had slightly more minor and major complications, but this difference was not significant in either situation (p>0.05).
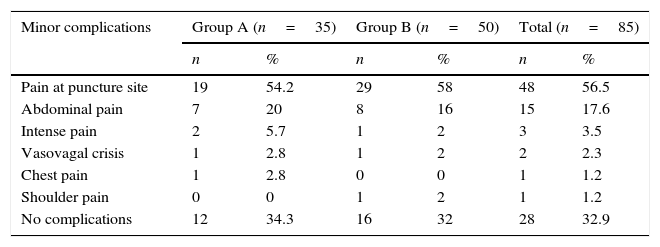
From the detail of the minor complications presented by the patients who underwent percutaneous biopsies in both study groups, it can be observed that pain at the puncture site is still the most frequent, both generally (56.5%) and per study group (54.2% vs. 58%) (Table 5).
Patients with percutaneous biopsy according to minor complications and study group.
| Minor complications | Group A (n=35) | Group B (n=50) | Total (n=85) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Pain at puncture site | 19 | 54.2 | 29 | 58 | 48 | 56.5 |
| Abdominal pain | 7 | 20 | 8 | 16 | 15 | 17.6 |
| Intense pain | 2 | 5.7 | 1 | 2 | 3 | 3.5 |
| Vasovagal crisis | 1 | 2.8 | 1 | 2 | 2 | 2.3 |
| Chest pain | 1 | 2.8 | 0 | 0 | 1 | 1.2 |
| Shoulder pain | 0 | 0 | 1 | 2 | 1 | 1.2 |
| No complications | 12 | 34.3 | 16 | 32 | 28 | 32.9 |
The most frequent minor complication in the patients who underwent laparoscopic biopsy, unlike those who underwent percutaneous biopsy, was abdominal pain (44.2%), this was similar in both groups (42.3% vs. 47.1%). The large number of patients with shoulder pain is striking (11.6%), which might be explained by the effect of the pneumoperitoneum required to undertake a laparoscopic procedure.
Of the total number of patients included in the study, 34 (26.6%) required 2 punctures to obtain liver tissue, and of these 85.3% had minor complications; only 5 patients (3.9%) required 3 punctures, presenting minor complications in all cases. The patients who required a single puncture presented the fewest complications (58.4%). It is striking that the only 2 major complications occurred in the patients who underwent 2 punctures.
DiscussionLiver biopsy has made it possible to gain better knowledge of the anatomopathological basis of liver disease, its evolution and response to treatment. This is why this procedure remains in constant use despite the risk of complications and the appearance of sophisticated diagnostic imaging methods, in an attempt to substitute the procedure. However, over time, various aspects have changed in terms of its use per se and principally concerning post-biopsy care. This study is based on the latter and with the latest data available from the global scientific environment we consider that rest after a liver biopsy should not exceed 2h in selected patients.
De la Barra et al.26 performed a retrospective study in Chile in 2010, where 23 patients underwent percutaneous liver biopsies as outpatients. The average age of the patients studied was 53.2, with a range from 27 to 68. Our research study had a greater number of patients with a broader age range and average age almost 5 years younger, similar to the data on age published by Beddy et al.27 in 2007, where 500 patients were studied with an average age of 43 and a range from 18 to 76.
There is little published research comparing the results and complications of percutaneous biopsy with those of laparoscopic biopsy. However, there is evidence of a study performed by Denzer et al.15 in 2007 which compares laparoscopic and percutaneous biopsies in diagnosing cirrhosis; to do this, 857 patients were randomised into 2 groups, one group underwent percutaneous biopsy (415) and the other laparoscopic biopsy (442) with very equal figures as can be observed, unlike those we present in this study, where the percutaneous technique was used in a much higher number of patients. This is because, unlike Denzer et al.15 we did not allocate the biopsy technique randomly, but rather according to whether or not there was a need to assess the liver macroscopically as outlined in the section “Material and methods”.
There are few publications which compare 2 study groups according to post-liver biopsy complications with different post-biopsy rest periods. However, there is one very important study performed by Firpi et al.28 published in 2005, where a 9-year study of 3214 patients was undertaken, in which the post-biopsy rest period was shortened over the years from 6h in 1995 to 1h in 2004, recording the complications that occurred in the different post-biopsy rest periods. The percutaneous biopsy technique was used and the minor complications which occurred in general in this study included: pain at the puncture site (13%), abdominal pain (18%) and vasovagal crisis (0.5%); these percentages are smaller than those presented by the patients who underwent percutaneous biopsies in our study, with the exception of that of abdominal pain which is a similar figure. It is worth highlighting the much greater number of patients who presented pain at the puncture site in this study compared to Firpi et al.28 study, although it coincides with another study performed by Eisemberg et al.29 published in 2003, which reported that 84% of patients who underwent percutaneous biopsy experienced this pain.
The percentage of major complications reported by Firpi et al.28 was 0.9%, with 2 deaths (0.06%), this figure is slightly lower than this series in terms of major complications and there was not a single death. On comparing the complications occurring in the different post-biopsy rest periods, Firpi et al.28 found no significant differences, and neither did we in this study. The fact that these studies report a lower percentage of complications than the data found in this work might be explained by the following: first, the small size of the sample, given that most reports are based on large caseloads; second, the procedure being carried out by interns in training, because the experience of the surgeon is a factor in the appearance of complications,18,30 although in 2004 Chevallier et al.31 published a study refuting this concept; third, the diameter of the Menghini's needle used in our study is larger than that of the needles used in the abovementioned studies, according to Sterling et al.32 and Plecha33 et al., this factor might increase the amount of complications; and four, most of the biopsies in these studies were guided by ultrasound in real time and not after ultrasound marking as in our study, although, according to a study published in 2007 by Manolakopoulos et al.34 this factor should not influence complications.
In order to compare the presence of complications according to the biopsy technique used, we again cite the study published by Denzer et al.15 which observed that the percentage of minor complications reported in the percutaneous biopsy group was 5.8% of the patients, pain (1.7%) and vasovagal crisis (1.4%) being the main complications, percentages less than 8.8% presented in the laparoscopy group, where pain also came first at 3.2% followed by agitation and restlessness, and other at 2.7%; however these differences were not statistically significant. The percentages of minor complications in our study were greater both with the percutaneous and the laparoscopic techniques than those reported by Denzer et al.15 but, like that study, with no significant differences between either study group. On evaluating the major complications, Denzer et al.15 reported 1% in the percutaneous biopsy group (intra-abdominal haemorrhage 0.7%, and haemobilia 0.3%), this percentage is much higher than that found in the laparoscopy group at only 0.2% in one case of haemobilia, with no death reported, on this occasion a significant difference presented between both study groups (p=0.025). Although these percentages are still lower than those reported in our study, where there was no significant difference between the patients who underwent percutaneous biopsy and those who underwent laparoscopic biopsy in relation to major complications, they reflect the opposite to that found in Denzer et al.15 study, which justified integrating both groups to reassess the monitoring procedure.
With regard to the number of punctures required, Firpi et al.28 reported that 20% of their patients required 2 punctures and 0.2% required 3; these results are slightly lower than those found in our study, however, like Firpi et al.28 it was demonstrated that the complications were more frequent when more than one puncture was required.
As established in the previous paragraphs, this research study has certain limitations, but the authors consider that the main limitations are the few current publications on this subject in the scientific world and the lack of standardisation in terms of reporting complications post liver biopsy, especially with regard to pain, as there is no international consensus on this factor. Therefore, correlation between the different series published world-wide is difficult, if not impossible.
ConclusionsRandomly allocating the study groups ensured their homogeneity in terms of demographic variables and hospital condition, and this ensured correct comparison. There were no differences between the 2 study groups with regard to the presence of minor and major complications, and therefore, post-biopsy care of 2h is feasible as a better option. We recommend that this safe reduction in post-liver biopsy care should be generalised to all secondary and tertiary level institutions.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Nodarse-Pérez PO, Pérez-Menéndez R, Heredia-Andrade ED, Noa-Pedroso G, Araluce-Cordoví R, Fernández-Sotolongo J. Seguridad de la reducción del tiempo de reposo posbiopsia hepática percutánea y por vía laparoscópica. Cirugía y Cirujanos. 2016;84:196–202.