Primary repair of Fallot tetralogy has been performed successfully for the last 45 years. It has low surgical mortality (<5%), with excellent long-term results. However, there are delayed adverse effects: progressive right ventricular dilation and dysfunction, arrhythmia, and sudden death. In our centre, Fallot tetralogy is the most common form of cyanotic congenital heart disease (including transannular patch) and accounts for 7.5% of all cardiovascular surgical procedures. The mid-term follow-up results are reported.
Material and methodsCase series. The study included patients who had complete repair of Fallot tetralogy with transannular patch from January 2000 to December 2009. An analysis was performed on the clinical variables, morbidity and mortality.
ResultsThere were 52 patients in the study, with mean age 4±2 years. Perioperative mortality in 6 patients, with 5 associated with residual right ventricular obstruction and, 1 associated with further surgery. The survival rate was 88% (46) patients, with a follow-up 75±26 months. Late morbidity occurred in 14, due to right ventricular dysfunction in 11, recurrent distal obstruction in 2, and residual ventricular septal defect in 1. Associated risk factors were severe pulmonary insufficiency (p=0.001); QRS>160ms, (p=0.001); cardiothoracic>0.60 index (p=0.048), and tricuspid regurgitation (p=0.001).
ConclusionsThere was reasonable long-term survival and excellent quality of life after total correction of Fallot tetralogy; however, progressive right ventricular dysfunction requires continuous monitoring, as well as the choice of optimal timing of pulmonary valve replacement.
La reparación quirúrgica de la tetralogía de Fallot se ha realizado exitosamente en los últimos 45 años, con mortalidad inferior al 5% y con resultados satisfactorios a largo plazo; sin embargo, existen efectos adversos tardíos como: insuficiencia progresiva ventricular derecha, arritmias y muerte súbita. En el Hospital Infantil de México es la cardiopatía cianógena más frecuente y su corrección quirúrgica (incluido el parche transanular) corresponde al 7.5% de toda la cirugía cardiaca. El propósito de este informe es reportar el seguimiento a 6 años en niños tratados en esta institución.
Material y métodosSerie de casos. Se incluyeron pacientes intervenidos de corrección total de tetralogía de Fallot con parche transanular entre enero de 2000 a diciembre de 2009. Se analizan variables clínicas, morbilidad y mortalidad.
ResultadosSe incluyeron 52 pacientes. Edad 4±2 años. Mortalidad perioperatoria 6, asociada a obstrucción residual ventricular derecha 5 y, reoperación por isquemia miocárdica en 1. Sobrevida 46 (88%) pacientes, seguimiento 75±26 meses. La morbilidad tardía se presentó en 14, debido a insuficiencia ventricular derecha en 11, obstrucción recurrente distal 2 y comunicación interventricular residual uno. Factores de riesgo asociados de insuficiencia ventricular derecha: insuficiencia pulmonar grave (p=0.001); complejo QRS>160ms (p=0.001); índice cardiotorácico>0.60 (p=0.048) e insuficiencia tricuspídea (p=0.001).
ConclusionesEncontramos una sobrevida razonable a largo plazo y calidad de vida excelente, posterior a la corrección total de tetralogía de Fallot; sin embargo, la insuficiencia progresiva ventricular derecha obliga a un continuo seguimiento para elegir el momento óptimo de reemplazo valvular pulmonar.
Fallot Tetralogy is the most common form of cyanotic congenital heart disease, and accounts for 7–10% of all congenital cardiopathies.1–7 If it is not corrected surgically, Fallot tetralogy is progressive with high mortality (>35% die in the first year of life, and 50% at 3 years of age),3,4,7 total correction is the treatment of choice, and this has low perioperative mortality (2–5%), even in neonates,7–12 and high long term survival rates (95.7% at 10 years, 93.5% at 20, and 85% at 36).7,13,14 It is considered the cyanogenic cardiopathy with the longest survival (mean age of 30).7
Total correction has diverse complications, which include: progressive ventricular dilatation due to residual obstruction of the outflow tract; severe pulmonary valve insufficiency, other residual lesions, arrhythmia, and sudden death. It is observed that reoperation is needed in 6–10% of cases up to 10–20 years from the initial correction.13
The need to insert a transannular patch during reconstruction surgery of the right ventricular outflow results in pulmonary insufficiency which, associated with other residual defects (interventricular communication, obstruction in the pulmonary branches), creates volume overload, ventricular dysfunction requiring subsequent reinterventions, and even pulmonary valve replacement. It has been demonstrated that the surgical technique determines postoperative recovery, and long-term results.14–17
Over the past decade, 338 cases (26%) have been treated in the Hospital Infantil de México Federico Gómez, of whom 4.4% of the total number of congenital cardiopathies had Fallot tetralogy.
The purpose of this study was to assess the patients who underwent total correction of Fallot tetralogy with a transannular patch between January 2000 and December 2009.
Material and methodsA case series was used which included fifty-two patients with Fallot tetralogy, treated surgically by total correction with transannular patch in the Cardiovascular Surgery Department of the Hospital Infantil de México Federico Gómez, between 1 January 2000 and 31 December 2009. Twenty-three boys and 29 girls were registered. The mean age during the correction was 4±2 years of age. Four patients presented genopathies (Down's syndrome: 3; Williams syndrome: one).
The diagnosis of Fallot tetralogy was made by echocardiogram in all cases. Twenty-two cases were reported to have associated cardiac anomalies: right-sided aortic arch presented in 10 patients; single coronary ostium in 4 cases; double superior vena cava in 4 cases; agenesis of valves, and left branch of the pulmonary artery in 2 patients; complete atrioventricular canal defect in one patient, and finally, situs inversus in one patient.
The data were obtained from the last medical consultation, which included: full physical examination, standard electrocardiogram (12 lead) and/or 24h ambulatory cardiac monitoring (Holter), chest X-ray, echocardiogram (two-dimensional, M mode, three-dimensional, conventional and tissue Doppler).
The study variables were: perioperative morbidity and mortality occurring within the first 30 postoperative days, determined by: (1) causes associated with the surgical procedure: residual obstruction of the proximal and distal right ventricular outflow tract (gradient >50mmHg and right ventricle/left ventricle pressure ratio >0.75), residual interventricular communication with haemodynamic repercussions, cardiac conduction and/or rhythm disorders, diaphragm paralysis; (2) causes not associated with the surgical procedure: acute cardiac failure, pleural effusion, acute renal failure, pneumonia, neurological alterations, pancreatitis; and (3) reoperations.
Late morbidity and mortalityMorbidity and mortality after the first 30 postoperative days was considered, and included: (1) right ventricular dysfunction due to pulmonary insufficiency, with/without stenosis of the pulmonary branches; (2) residual/recurrent obstruction of the proximal or distal right ventricular outflow tract, with/without right ventricle dysfunction; (3) residual interventricular communication with haemodynamic repercussions; (4) reoperations; and (5) arrhythmias and sudden death.
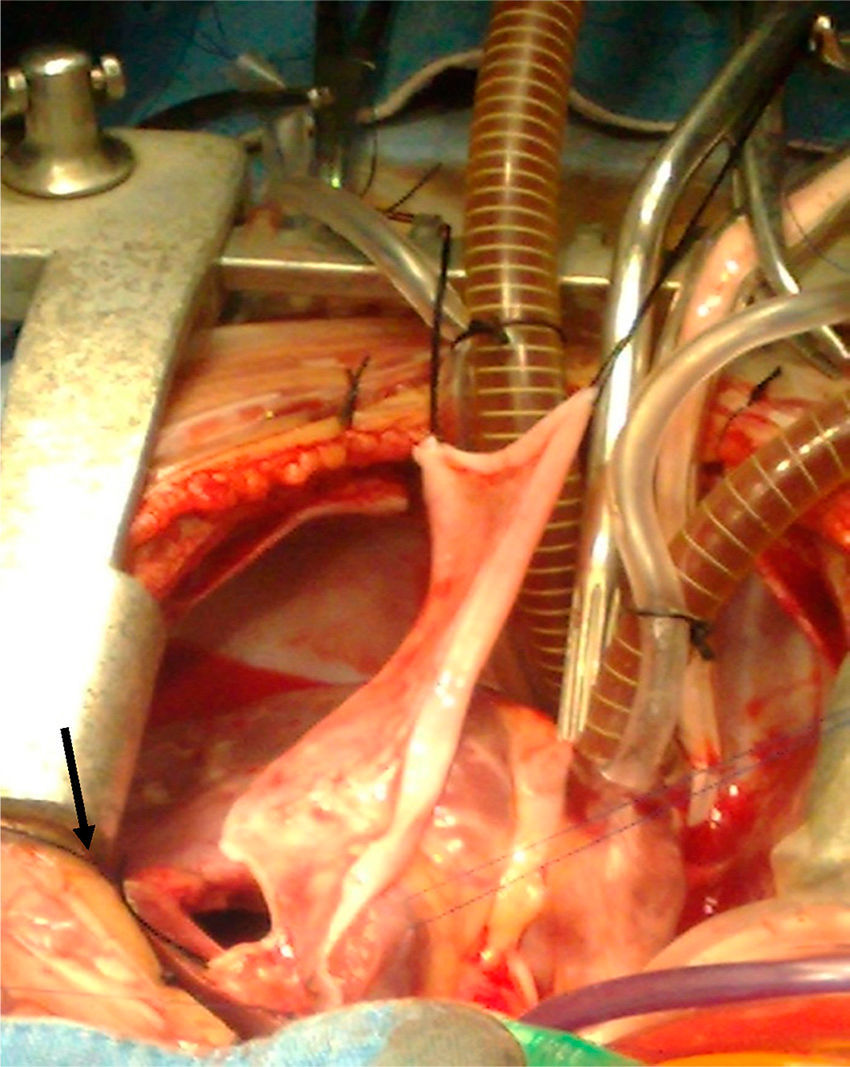
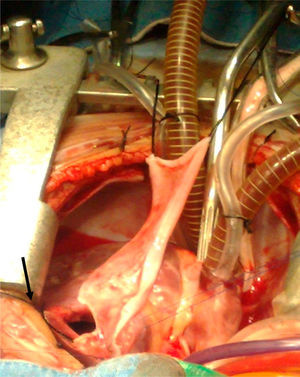
Surgical techniqueThe heart was exposed by midline sternotomy, a patch of pericardium was resected (treated with 0.6% glutaraldehyde for 10min and washed with saline solution), to reconstruct the right ventricular outflow tract, as can be seen in Fig. 1. Previous systemic-to-pulmonary shunts were ligated. Extracorporeal circulation and moderate hyperthermia were used (naso-pharyngeal temperature of 28–32°C), circulatory arrest with profound hypothermia was used in 2 cases to enable visualisation during closure of the interventricular communication, and correction of the complete atrioventricular canal. Myocardial protection was achieved with intermittent administration (every 30min) of cardioplegic solution (Benson Roe) and local hypothermia.
The interventricular communication was closed through the right atrium in 37 patients and through a right ventriculostomy in 15; a Dacron patch was used in all cases. Muscle was resected from the free infundibular wall and/or fibrous tissue, annular hypoplasia was confirmed, and hypoplasia of the pulmonary branches with Hegar's graduated dilators. The right ventricular outflow tract was exposed by ventriculostomy which was as limited as possible (5mm approximately), just in order for the patch to effectively widen the pulmonary ring. In the case of stenosis, the transannular incision was increased up to the pulmonary branches, extending the patch beyond the stenosis, which in the case of the left branch was after the ductus arteriosis.
Simultaneously, on widening the ring, the pulmonary valve function was preserved as far as possible, in 3 cases a monocuspid valve was made with autologous pericardium. We use the strategy of the limited transannular patch; i.e., the transannular patch was made to restrict the diameter of the pulmonary ring at a z value of ≥2 using a Hegar's dilator as a guide.
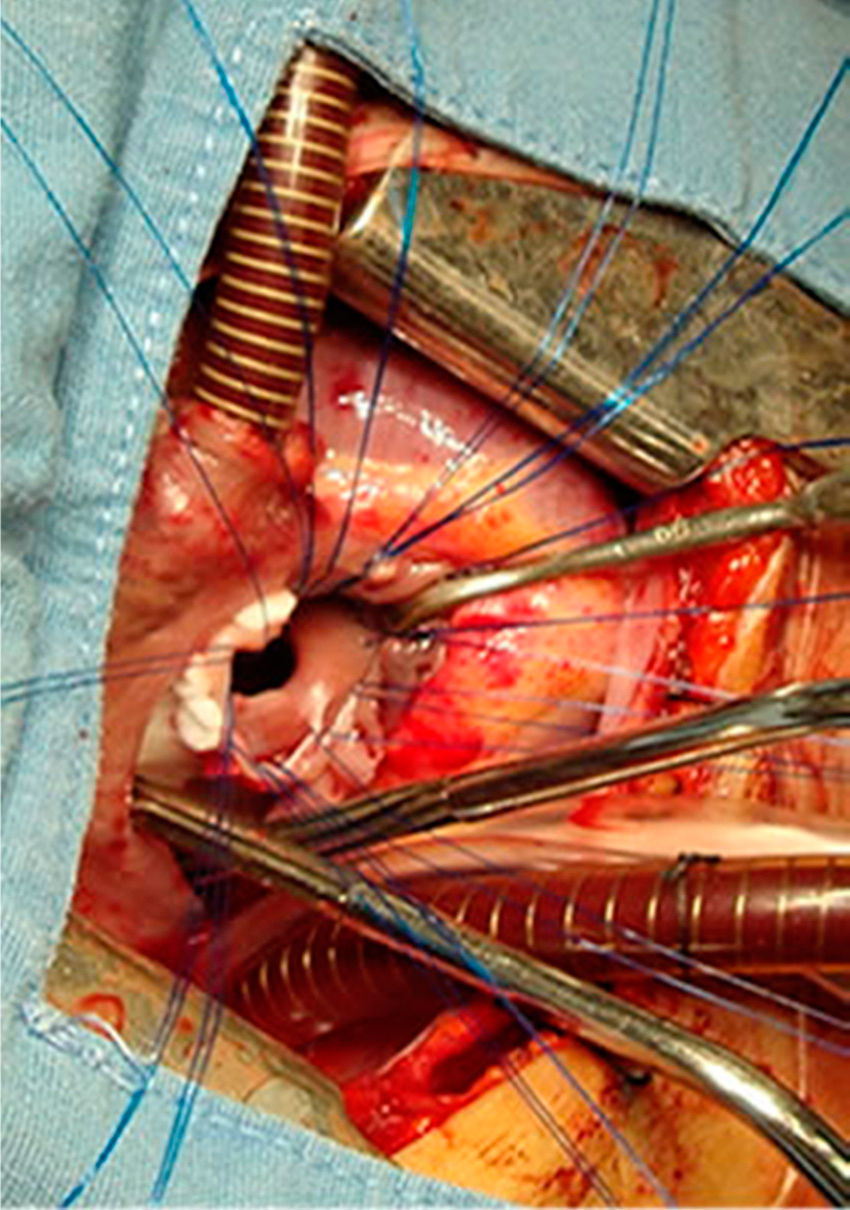
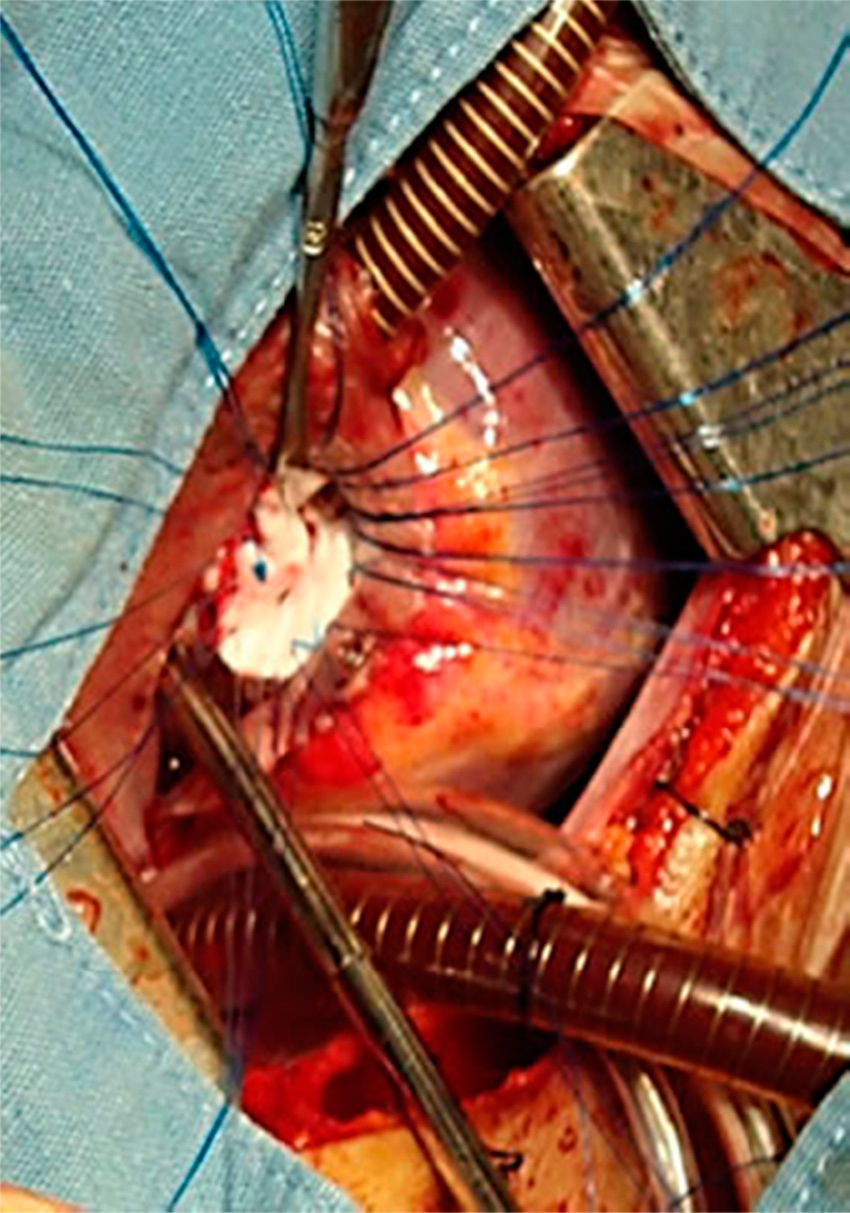
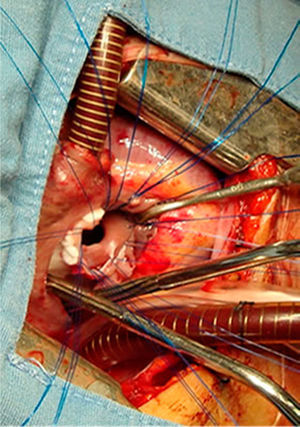
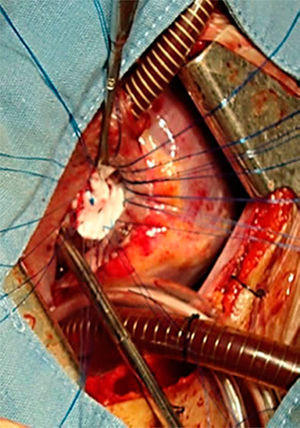
All the atrial septal defects were closed, as can be seen in Figs. 2 and 3. During the transoperative period right ventricular/left ventricular pressure ratio measurement>two-thirds systemic pressure (measured directly), and right ventricular outflow tract gradient >50mmHg (estimated by transoperative echocardiogram) occurred in 7 patients who required enlargement of their ventriculostomy, beyond the length of the infundibular septum (5 cases), and extension of the patch to the level of the left branch (2 cases). Extracorporeal circulation was stopped, cannulae removed, and the sternotomy closed. One patient was managed with an open chest.
Statistical analysisDescriptive statistics; raw numbers and proportions for qualitative variables; means and their standard deviation for quantitative variables. Inferential statistics by univariate analysis, and determination of odds ratios, and 95% confidence intervals.
Windows SPSS (version 17; IBM, Armonk, NY, USA) was used. All p values<0.05 were considered statistically significant.
Ethical considerationsEthical aspects: the basic principles according to the 2002 Helsinki Declaration and the Ley General de Salud (General Health Act) on research were followed, in order to ensure the maximum safety of the study population. The protocol was approved beforehand by the Local Health Research Committee of the main hospital.
ResultsIn our series there were 52 cases with total transannular patch or arterioventricular repair.
Fifteen patients presented with perioperative complications associated with surgical correction, which included: residual obstruction of the right ventricular outflow tract in 8 cases, myocardial ischaemia in 2, rhythm and/or conduction disorders in 4, diaphragm paralysis in one case.
Nine of the cases required early reoperation. Six cases due to residual obstruction documented during the transoperative period by echocardiogram, and direct measurement of right ventricular/left ventricular pressure; 4 required enlargement of the right ventricular outflow tract, and 2 of the left pulmonary branch: 2 cases had atrioventricular block, reoperated in order to insert a permanent pacemaker, and one case to perform a diaphragmatic plication.
Late morbidity occurred in 14 cases. Severe right ventricular dysfunction due to severe pulmonary insufficiency occurred in 9 patients, and due to severe pulmonary insufficiency with recurrent distal obstruction in 2 cases; recurrent distal obstruction with mild-moderate ventricular dysfunction occurred in another 2 cases. Three of these patients were reoperated: closure of residual interventricular communication in one case, and pulmonary valve replacement with bioprosthesis in 2.
There were six cases of periooperative mortality in the immediate postoperative period, 5 due to residual obstruction of the right ventricular outflow tract, and one due to myocardial ischaemia. Early reoperation preceded 50% of the fatal cases (OR=5.5, CI 95%: 1.3–22.5).
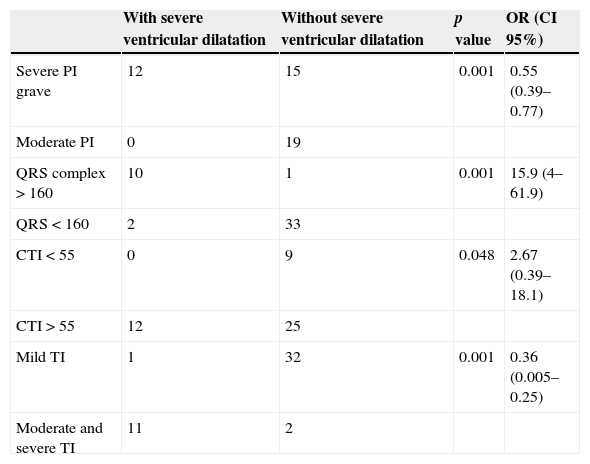
The results were analysed by univariate analysis of the risk factors for severe ventricular dilatation of the 46 surviving patients. Statistically significant differences were found with pulmonary insufficiency (p=0.001); QRS complex>160ms (p=0.001); cardiothoracic index>0.60 (p=0.048) and tricuspid insufficiency (p=0.001). Forty-six patients survived initial repair of Fallot tetralogy with transannular patch at 10 years (Table 1).
Univariate analysis of risk factors for the development of severe ventricular dilatation.
| With severe ventricular dilatation | Without severe ventricular dilatation | p value | OR (CI 95%) | |
|---|---|---|---|---|
| Severe PI grave | 12 | 15 | 0.001 | 0.55 (0.39–0.77) |
| Moderate PI | 0 | 19 | ||
| QRS complex>160 | 10 | 1 | 0.001 | 15.9 (4–61.9) |
| QRS<160 | 2 | 33 | ||
| CTI<55 | 0 | 9 | 0.048 | 2.67 (0.39–18.1) |
| CTI>55 | 12 | 25 | ||
| Mild TI | 1 | 32 | 0.001 | 0.36 (0.005–0.25) |
| Moderate and severe TI | 11 | 2 |
95% CI, 95% confidence interval; CTI, cardiothoracic index; PI, pulmonary insufficiency; TI, tricuspid insufficiency; OR, odds ratio.
Total repair of Fallot tetralogy is the method of choice for most cases. There are few studies on repair in neonates, and they are associated with a long stay in the intensive care unit, with increased use of circulatory arrest and transannular patch, and a high reoperation rate of 25% to 30% at 5 years from the initial operation.18,19 The European Association for Cardio-Thoracic Surgery Database (EACTS CDB)20 reports a 7.8% mortality due to primary repair in neonates; whereas the Southern Thoracic Society reports 7.3%, better results being obtained when the repair is performed in infants between 3 and 12 months old.18
In the past 5 years, the policy in this highly specialised hospital has been to perform primary repair in under 2-year olds, as surgical mortality continues to be associated with age >2, as Murphy et al.21 report, due to chronic right ventricular hypertrophy, cyanosis and polycythaemia on cardiac structure and function.
Gatzoulis et al.22 report that right ventricular hypertrophy starts after birth, increases with age, and becomes irreversible towards the age of 4. This hypertrophy contributes to interstitial fibrosis and the need for extensive muscle resection, with a potential risk of ventricular arrhythmias and ventricular dysfunction.23
The presence of annular hypoplasia and pulmonary branches contraindicates primary repair, initial palliation being preferred with systemic-to-pulmonary shunt.23 Hypoplasia of the pulmonary branches reflects an absence of blood flow and systemic-to-pulmonary shunt is indicated to encourage growth.23,24
Vobecky et al.25 described their experience in 141 children palliated with modified Blalock-Taussig shunt, which they reported failed in 36 cases, with pulmonary arterioplasty during the repair of 10 cases, and 90% survival at 5 years.18,26
In our study there were 5 cases of palliation with modified Blalock-Taussig shunt, growth and no distortion of the branches were seen, 90% functionality at the time of the total repair, with one case of failure of the shunt, without influencing the outcomes of the subsequent surgery, which coincide with those of Fraser et al.11 and Pozzi et al.27
The criteria for immediate reoperation, described by Fraser et al.11 were: right ventricular/left ventricular pressure>two-thirds systemic pressure, residual interventricular communication with haemodynamic repercussions, residual obstruction of the right ventricular outflow tract, and severe tricuspid insufficiency.
Correction with transannular patch was performed in 80%, as reported by Seddio et al.8, 70% reported by Pigula et al.19, 54.2% by He et al.,15 41.5% by d’Udekem et al.,28 and in 35% by Mesquita et al.29 The European Association for Cardio-Thoracic Surgery,20 and van Dongen et al.30 report repair with transannular patch as the most frequently used technique (57% of their cases), while, in their series, Voges31 and Tirilomis et al.6 report its use in 27% of their cases.
During our series’ 75 months follow-up, pulmonary valve insufficiency was the most common cause of morbidity (61%). Yoo et al.32 report that patients corrected with restrictive physiology (mean residual gradient of right ventricular outflow tract 34±10mmHg) have smaller right ventricles, less prolonged QRS, reduced adverse affects of chronic pulmonary insufficiency, better tolerance to exercise, and a reduction in pulmonary valve replacement.29,31,33
Severe ventricular insufficiency can precede symptoms; these patients should be considered for pulmonary valve replacement before it becomes irreversible. The following are determinants for pulmonary valve replacement: deteriorated functional class, reduced tolerance to exercise; QRS prolongation>180ms observed on ECG, echocardiographic evidence of severe pulmonary insufficiency, dilatation and right ventricular dysfunction.6,17,29,33,34
Follow up of the patients at 30 years showed survival rates of over 91% at 5 years follow-up,8 and 89% from 10 to 15 years.9 When the patients did not require reoperation due to any cause, survival at 5 years was 70%8 and 96% at 20 years.9
In our institution, we showed a 100% survival rate at 10 years from the initial correction of tetralogy of Fallot with transannular patch, which suggests that primary repair with transannular patch can be undertaken safely and effectively.
Despite the long-term survival, and the excellent quality of life that these patients achieve after total correction of Fallot tetralogy, progressive right ventricular dysfunction does occur, therefore none of these patients can be considered to be cured, even asymptomatic patients, and all of them require continuous follow-up; only thus will it be possible to choose the optimal time for pulmonary valve replacement.
The goal of treatment should include: the prevention of long-term complications, and the reduced probability of early and late reoperation, and good neurological and functional development to enable a satisfactory quality of life.
ConclusionsFortunately, advances in medico-surgical strategies have ensured that the morbidity and mortality rates for children born with Fallot tetralogy are significantly lower in the current era compared to a few decades ago.
Conflict of interestsThe authors have no conflict of interests to declare.
To Dr. Alejandro González Ojeda for the time he has devoted to the final revision of this article.
Please cite this article as: Galicia-Tornell M, Reyes-López A, Ruíz-González S, Bolio-Cerdán A, González-Ojeda A, Fuentes-Orozco C. Tratamiento de la tetralogía de Fallot con parche transanular. Seguimiento a 6 años. Cirugía y Cirujanos. 2015;83:478–484.