Acute mesenteric ischemia (AMI) has a high mortality. Early diagnosis and treatment are very important. In our institution there is a therapeutic protocol that includes endovascular techniques (ET) in patients with AMI without peritoneal irritation at diagnosis. The aim of this study was to evaluate the use of ET in conjunction with conventional surgery in the management of potentially reversible IMA diagnosed by computed tomography (CT-angiography).
MethodsObservational, descriptive and retrospective study that evaluated the use of ET in patients with AMI (arterial origin) in two periods (before and after the application of a protocol that includes ET), between 2009 and 2013. All patients were diagnosed by a CT-angiography, as the diagnostic technique of choice, because of the clinical and analytical suspicion.
ResultsOur series included 73 patients with IMA diagnosed by CT-angiography (45: 2009–2011; 28: 2012–2013). Leukocytosis was common (82%), high lactate levels are less frequent (47% vs 53%). There were 49 patients with IMA without peritoneal irritation. In 51% bowel resection surgery was performed (44% survival); 18%: revascularization by ET (survival 67%); 31%: palliative treatment (0% survival). 33% of patients undergoing first-line RVI needed a surgical rescue (bowel resection). The overall mortality was 67% (2009–2011) vs 62% (2012–2013).
ConclusionsSince the protocol application, there is a higher indication of ET in patients with AMI without peritoneal irritation, showing a decreased mortality. With ET application, there is a higher survival in these patients. In our experience, the use of ET in cases of AMI without peritoneal irritation at diagnosis, may increase survival.
La isquemia mesentérica aguda (IMA) presenta una elevada mortalidad. El diagnóstico y el tratamiento precoces son claves. En nuestro centro aplicamos un protocolo terapéutico que incluye la radiología vascular intervencionista (RVI) en pacientes con IMA sin irritación peritoneal. El objetivo de este estudio fue evaluar el uso de la RVI conjuntamente con la cirugía convencional en el manejo de la IMA de intestino delgado potencialmente reversible diagnosticada mediante tomografía computarizada vascular (angio-TC).
MétodosEstudio observacional, retrospectivo y descriptivo, donde se valora el manejo diagnóstico y terapéutico de la IMA en 2 períodos (antes y después de la aplicación de un protocolo que incluye la RVI) entre 2009 y 2013. El diagnóstico de elección es mediante angio-TC, ante la sospecha clínico-analítica.
ResultadosNuestra serie incluye a 73 pacientes diagnosticados de IMA mediante angio-TC (45: 2009-2011; 28: 2012-2013). La leucocitosis es frecuente (82%), siendo menos frecuente la lactacidemia (47% vs. 53%). Hay 49 pacientes con IMA y exploración abdominal normal. En el 51% se realizó cirugía de resección intestinal (supervivencia 44%); 18%: revascularización mediante RVI (supervivencia 67%); 31%: tratamiento paliativo (supervivencia 0%). El 33% de los pacientes sometidos a RVI como primera línea precisaron de cirugía de rescate (resección intestinal). La mortalidad global es del 67% (2009-2011) vs. 62% (2012-2013).
ConclusionesDesde la aplicación del protocolo ha aumentado la indicación de RVI para tratar a pacientes sin irritación peritoneal, objetivando una disminución de la mortalidad global. En nuestra experiencia, la aplicación de RVI en casos de IMA sin irritación peritoneal al diagnóstico puede incrementar la supervivencia.
Acute mesenteric ischemia (AMI) is a rare entity, although it has a high mortality (above 50%).1 It increases in frequency in an aging population, as the frequency of cardiovascular risk factors also increases.
Reducing the mortality of AMI patients is fundamentally based on two pillars: early diagnosis and treatment.2 Vessel revascularisation is considered to be the treatment of choice when the ischemia is reversible, and it may be performed at an endovascular level by using vascular interventional radiology techniques (VIR) or conventional vascular surgery. For several years endovascular treatment has been described as the treatment of choice, if it is available, as it is less aggressive with similar morbidity and lower mortality than other treatments.3 The therapeutic arsenal which includes VIR is composed of mechanical or pharmacological fibrinolysis, balloon angioplasty (with or without a stent) and the intravenous perfusion of vasodilatadors.4–6
This study evaluates whether the use of VIR endovascular techniques improves the survival of patients with a potentially reversible mesenteric ischemia.
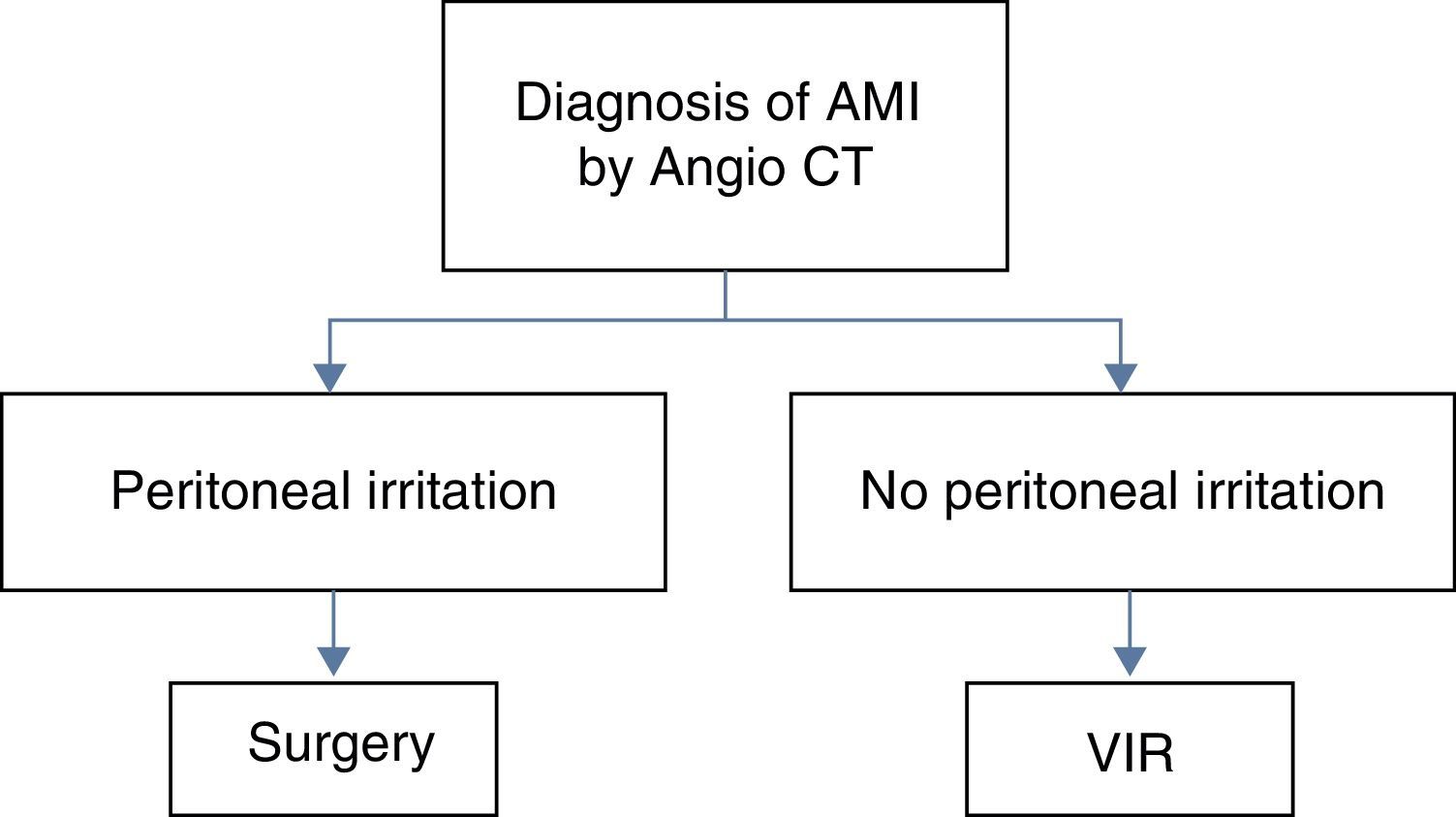
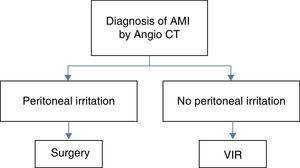
MethodsA diagnostic–therapeutic algorithm has been used in our hospital from January 2012, in which endovascular revascularisation using VIR techniques is indicated for all patients diagnosed with AMI of the small intestine (SI), following angiography using computerized tomography (CT-angiography), without any signs of peritoneal irritation in physical examination (a finding that can be correlated with non-transmural ischemia) (Fig. 1). The protocol applied distributes AMI patients into two groups, depending on the result of physical examination. If they have peritoneal irritation at diagnosis, surgery is the treatment of choice; if they do not present this, VIR is the technique used. In this group “technical success” is defined as meaning that treatment with VIR is sufficient (no rescue surgical treatment is needed); “therapeutic success” means that treatment using VIR is not sufficient and rescue surgery is necessary (intestinal resection), while “therapeutic failure” means that even using both treatments there is no successful outcome.
The clinical suspicion of AMI is based on abdominal pain that is out of proportion with the physical examination, patient history (vascular risk factors, prothrombotic states, etc.) and laboratory findings. The definitive diagnosis is by CT-angiography, which is the technique of choice for the diagnosis of this pathology.
VIR procedures include: embolectomy, balloon angioplasty±insertion of a stent, mechanical fibrinolysis, pharmacological fibrinolysis (with recombinant tissue plasminogen or rtPA activators) and vasodilatation (using nimodipine). We use prophylactic antibiotic treatment. The majority of cases require anticoagulation following the initial procedure, and depending on the etiology it may be prolonged.
We present an observational, retrospective and descriptive study in two periods, before and after the application of the protocol (January 2009–December 2011, and January 2012–December 2013). The results obtained in both periods are compared. All patients who presented small bowel AMI diagnosed by CT-angiography and who were admitted to our hospital are included.
The following variables were recorded: epidemiological data (age and sex), risk factors (atrial fibrillation, prothrombotic states and low output pathologies, etc.), such as AMI (arterial embolism, arterial thrombosis, venous thrombosis or non-occlusive mesenteric ischemia), clinical examination data at diagnosis (the presence or absence of signs of peritoneal irritation at diagnosis), laboratory data leucocytes (above 109/l), lactic acid (above 20mg/dl) and creatininase (CK) (above 195U/l), data from CT-angiography findings (the presence of mural hypocaptation, air in the intestinal wall or intestinal pneumatosis and portal air), initial treatment (surgery, endovascular treatment using VIR techniques or palliative measures), the results of the initial treatment (resolution of the condition, resolution using rescue surgery or “second look”, death) and the result at discharge (survival or death).
ResultsOur series includes a total of 73 patients with SI AMI diagnosed using CT-angiography from 2009 to 2013; 45 cases from 2009 to 2011; 28 cases from 2012 to 2013.
During the period 2009–2011 a total of 45 patients with SI AMI were treated. The average age of the group was 80 years old (range: 45–100). About 80% of these patients presented no signs of peritoneal irritation when examined. In laboratory tests 82% presented leukocytosis, 47% presented high level of lactic acid, and 33% had raised CK. Radiologically, 73% presented mural hypocaptation, 22% mural gas (intestinal pneumatosis) and 20% portal gas. Overall mortality in this group was 69% (Table 1). The etiological distribution of the series was: 15% embolic arterial AMI, 27% thrombotic arterial AMI, 2% thrombotic venous AMI and 56% non-occlusive AMI (NOAMI).
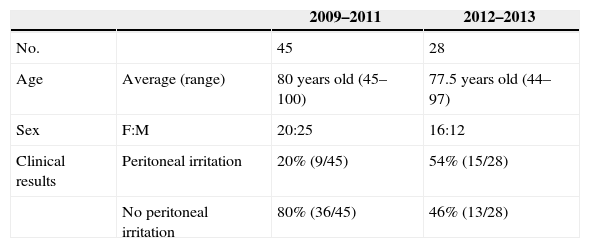
Distribution of the Epidemiological Data of Our Series, Distribution in Both Periods (2009–2011; 2012–2103).
| 2009–2011 | 2012–2013 | ||
|---|---|---|---|
| No. | 45 | 28 | |
| Age | Average (range) | 80 years old (45–100) | 77.5 years old (44–97) |
| Sex | F:M | 20:25 | 16:12 |
| Clinical results | Peritoneal irritation | 20% (9/45) | 54% (15/28) |
| No peritoneal irritation | 80% (36/45) | 46% (13/28) |
| Peritonism | No Peritonism | Peritonism | No Peritonism | ||
|---|---|---|---|---|---|
| Laboratory results | Leukocytosis | 89% | 80% | 80% | 84% |
| Raised lactate | 66% | 42% | 53% | 53% | |
| Raised CK* | 33% | 33% | 14% | 8% | |
| Angio CT | Mural hypocaptation | 78% | 72% | 87% | 92% |
| Intestinal pneumatosis | 33% | 19% | 47% | 39% | |
| Portal gas | 22% | 19% | 47% | 30% | |
| Mortality | Partial | 78% | 67% | 87% | 62% |
| Overall | 69% | 75% | |||
*CK: creatinines; F: female; M: male.
Of the nine patients who presented signs of peritoneal irritation in the initial examination, 56% received intestinal resection surgery as the first line of treatment, with a success rate of 40%. About 44% were offered symptomatic treatment due to advanced disease and/or a high level of comorbidity. About 78% of the patients died (Table 1).
Of the 36 patients who presented no signs of peritoneal irritation in the initial examination, 61% received intestinal resection surgery as the first line of treatment, 8% (three cases) received VIR revascularisation as the first line of treatment, and 31% were offered symptomatic treatment due to advanced disease and/or a high level of comorbidity. Of the three patients treated using VIR revascularisation, 67% (2/3) required resective rescue surgery (intestinal resection), with a total mortality rate of 33% (1/3). About 67% of these patients died (Table 1).
During the period 2012–2013 a total of 28 patients were treated for small intestine AMI. The average age of this group was 77.5 years old (range: 44–97). About 46% (13/28) presented no signs of peritoneal irritation. In laboratory tests, 82% presented leukocytosis, 53% raised levels of lactic acid, and 14% a high level of CK. Radiologically, 89% presented mural hypocaptation, 42% mural gas (intestinal pneumatosis) and 39% portal gas. Total mortality in this group was 75% (21/28) (Table 1). The etiological distribution of this series was: 32% embolic arterial AMI, 25% thrombotic arterial AMI, 4% thrombotic venous AMI and 36% NOAMI.
Of the 15 patients who presented signs of peritoneal irritation in the initial examination, 73% received intestinal resection surgery as the first line of treatment, with a success rate (resolution of the condition) of 18%, while 27% were offered symptomatic treatment due to advanced disease and/or a high level of comorbidity. About 87% of the patients died (Table 1).
Of the 13 patients who presented no signs of peritoneal irritation at their initial examination, 23% received intestinal resection surgery as the first line of treatment, 46% (six cases) were treated by VIR revascularisation as the first line of treatment, 31% (4/13) were offered symptomatic treatment due to advanced disease and a high level of comorbidity. Of the six patients who were treated by VIR revascularisation, 33% required resective rescue surgery (intestinal resection), with a total mortality in this group of 33%. About 62% of the patients died (Table 1).
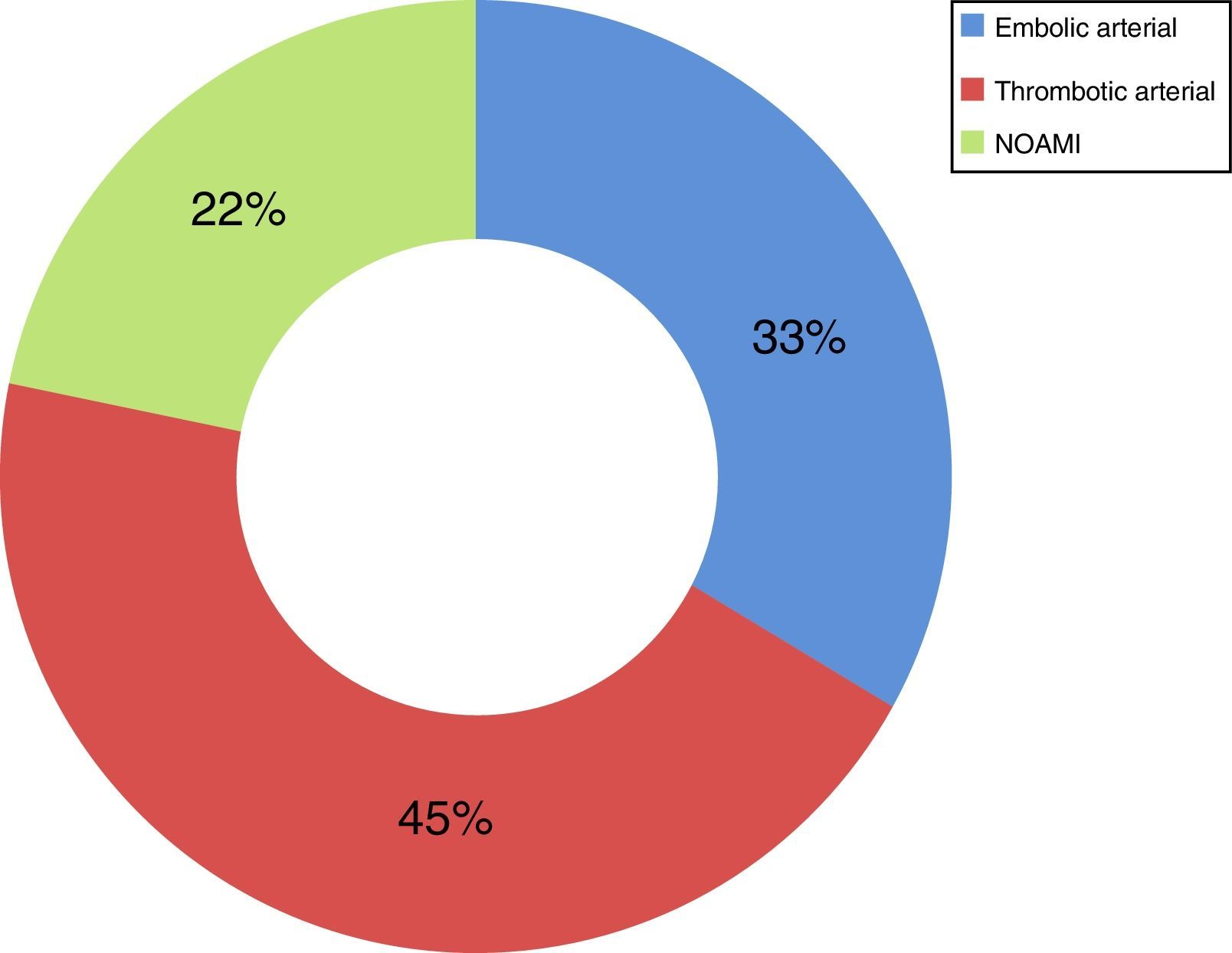
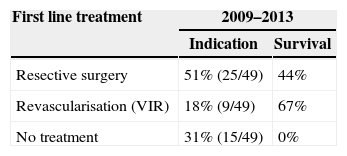
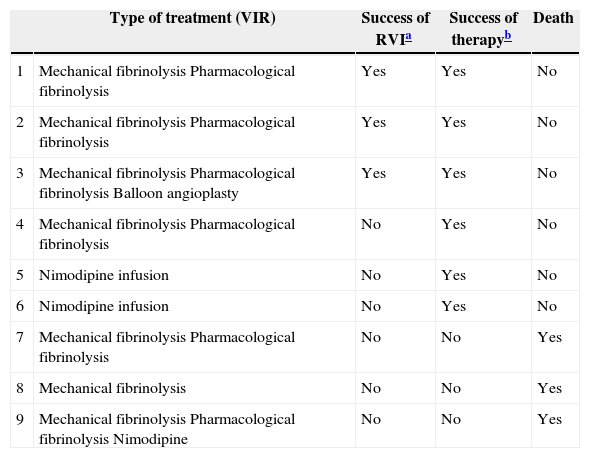
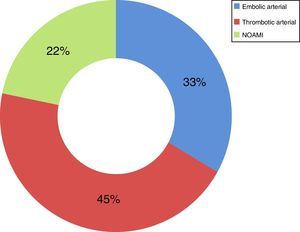
Now focussing on those patients in both periods of time (2009–2013) who presented no signs of peritoneal irritation in their physical examination (49 patients of the total number of 73), and analysing the treatment they received, 51% received intestinal resection as the first line of treatment, with a survival rate of 44%; 18% (nine cases) were treated using VIR revascularisation techniques, with a survival rate of 67%, and 31% were not offered any treatment due to the evolution of their condition and/or associated comorbidity, with a survival rate of 0% (Table 2). Of the patients who received VIR as the first line of treatment, 33.3% required surgery involving intestinal resection afterwards (Table 3). Analysing the nine patients who were treated using VIR, thrombotic arterial etiology was the most common (45%), followed by embolic arterial causes (33%) and non-occlusive AMI (NOAMI) (22%), with no cases of venous thrombotic etiology (Fig. 2).
Distribution in Both Periods (2009–2013) of the Patients Without Peritoneal Irritation in Physical Examination (49 Cases) According to Their First Line Treatment and Group Survival.
| First line treatment | 2009–2013 | |
|---|---|---|
| Indication | Survival | |
| Resective surgery | 51% (25/49) | 44% |
| Revascularisation (VIR) | 18% (9/49) | 67% |
| No treatment | 31% (15/49) | 0% |
Distribution in Both Periods (2009–2013) of the Patients Treated With VIR, the Type of Treatment Used and Results.
| Type of treatment (VIR) | Success of RVIa | Success of therapyb | Death | |
|---|---|---|---|---|
| 1 | Mechanical fibrinolysis Pharmacological fibrinolysis | Yes | Yes | No |
| 2 | Mechanical fibrinolysis Pharmacological fibrinolysis | Yes | Yes | No |
| 3 | Mechanical fibrinolysis Pharmacological fibrinolysis Balloon angioplasty | Yes | Yes | No |
| 4 | Mechanical fibrinolysis Pharmacological fibrinolysis | No | Yes | No |
| 5 | Nimodipine infusion | No | Yes | No |
| 6 | Nimodipine infusion | No | Yes | No |
| 7 | Mechanical fibrinolysis Pharmacological fibrinolysis | No | No | Yes |
| 8 | Mechanical fibrinolysis | No | No | Yes |
| 9 | Mechanical fibrinolysis Pharmacological fibrinolysis Nimodipine | No | No | Yes |
Results: success of VIR or “technical success”: 33.3% (3/9); therapeutic success (VIR+surgery): 67% (6/9); death or “therapeutic failure”: 33.3% (3/9).
VIR: vascular interventionist radiology.
The incidence of acute intestinal ischemia is low (1–2 cases/1000 hospital admissions/year),1,5,7,8 although its morbimortality is still high. The reasons for this outcome are delayed diagnosis and therefore delays in treatment, together with patient comorbidity.2,3,9 The overall mortality in our series is 71%, which is within the ranges described in the different series (50%–100%).1,4,10 We believe it is relevant to mention that in our series mortality was higher during the second period (2009–2011: 69% vs 2012–2013: 75%). A possible explanation for this is the fact that during the second period more patients with peritoneal irritation at diagnosis were seen (2012–2013: 54% vs 2009–2011: 20%).
The treatment of AMI using VIR was first described some years ago. The criteria for using this treatment are clear, and they are widely described in the literature. Those patients who present no signs of peritoneal irritation at diagnosis benefit the most from this treatment. Peritoneal irritation is an indirect sign of transmural intestinal ischemia, which is directly associated with the evolution and duration of the ischemia. In such cases revascularisation is not considered to be very effective, so that intestinal resection surgery is the treatment of choice.2–4,6 Our protocol defends the use of endovascular revascularisation (VIR) in those patients who present no peritoneal irritation at diagnosis. Following the implementation of the protocol the indication for treatment using VIR has increased, from 8% (2009–2011) to 46% (2012–2013). A slight reduction in mortality for those patients without signs of peritoneal irritation at diagnosis may be observed after increasing the indication for VIR after the protocol (2009–2011: mortality 67% vs 2012–2013: mortality 62%). One finding that may be surprising is the fact that following implementation of the protocol (the period 2012–2013) some patients without peritonitis are still not offered VIR as the first line of treatment (23% of patients who were treated using intestinal resection surgery and 31% of those who received symptomatic treatment). It should be pointed out that the treatment selected depended on the balance between the decision of the surgical–radiological team and the comorbidity of each patient, as there were exceptions in compliance with the protocol.
Several studies have suggested that survival improves with the use of VIR in comparison with conventional treatment. The morbidity of conventional treatment in AMI patients is considered to be very high, due to their advanced age and high comorbidity, and it may be less with endovascular revascularisation techniques.2,3,7 In fact, in comparison with conventional treatment VIR revascularisation is considered to achieve similar morbidity together with a reduction in mortality as well as a reduction in the possibility of the need for subsequent intestinal resection surgery.3,7,8 In our series an improvement in the survival of AMI patients without signs of peritoneal irritation at diagnosis was observed (survival: 67%) when they were treated using VIR, in comparison with those who received conventional surgery as the first line treatment (survival: 44%). Based on the definition of “therapeutic success” (VIR±intestinal resection), this group of patients includes those who were only treated with VIR (3/9) and those who also required rescue surgery (3/9), making up 67% of the group.
The cases in our series were analyzed conjointly, without differentiating between types of AMI, the vessel involved and the treatment depending on the etiology of the condition. The treatment of AMI by VIR differs depending on its etiology.4–6 There is no case of venous AMI in the patients treated using VIR, as they all have arterial etiology (thrombotic: 45%, embolic: 33%) or NOAMI (22%). There are no randomized studies that could be used for comparison. The group of Block et al.,11 in a retrospective study, shows similar mortality when endovascular treatment is compared to conventional therapy for patients with embolic occlusion of the upper mesenteric artery (33% vs 37%). This is lower in patients with thrombosis of the upper mesenteric artery who received endovascular treatment (56% vs 23%). The group of Jia et al.10 shows mortality at 30 days of 9.5% in those patients who received endovascular treatment due to thromboembolic occlusion of the superior mesenteric artery.
A data in our study that seems to be more disparate is the low number of patients without peritoneal irritation in the first period (2009–2011), at only 20%. This may be due to poor data recording, given that this is a retrospective study.
With respect to the laboratory data, the lack of sufficiently specific parameters for diagnosis is well-known. Neither raised lactic acid (which is usual in late stages) nor raised D-Dimer are specific enough.1 Leukocytosis normally occurs with an increase in younger forms, and it is usually the only element that appears in the early stages.12 In our series we found that leukocytosis (82%) due to raised neutrophils, and, less often, raised lactate, is the norm. Even so, we saw that raised lactic acid is more frequent in patients with peritoneal irritation than it is in those that do not present this (more developed disease). On the other hand, raised CK is present in fewer than half of the cases in our series (47%), and it is not described in the literature as a parameter which supports diagnosis. A CT-angiography is the “gold standard” for diagnosis, and it is considered to be a sufficiently specific (95%) and sensitive (94%) test.1 In our case, all of the patients were diagnosed using this imaging technique.
The most important limitation of our study is that it covers a short and retrospective series in which statistical validation is impossible, and where important data such as the time between the development of ischemia until the first treatment cannot be reliably included. This makes it impossible for us to know the real number of patients who could have benefited from an endovascular technique.
In spite of these limitations, we believe that usage of VIR when it is indicated (patients diagnosed AMI without peritoneal irritation at diagnosis) may increase their survival.
As a future line of research, it would be interesting to prepare a prospective registry of SI AMI cases that had been revascularised using VIR, and recording the risk factors associated with this pathology, the type of AMI, the duration of the ischemia, the treatment offered depending on its nature and procedural complications. As is described in the literature, in spite of therapeutic advances it is hard to increase the overall survival rate of these patients. Even so, it is possible to reduce mortality and the need for intestinal resection in those patients who have yet to present transmural ischemia at diagnosis, by using VIR revascularisation.
FinancingThis work has not received financing from any source.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Serracant Barrera A, Luna Aufroy A, Hidalgo Rosas JM, Cánovas Moreno G, Fortuño Andres JR, Falcó Fages J, et al. Isquemia mesentérica aguda: utilidad de las técnicas de revascularización endovascular. Cir Esp. 2015;93:567–572.