Postoperative pancreatic fistula in distal pancreatectomy is one of the most important complications in this surgery and it is associated with high morbidity and mortality. Pancreatic fistula after distal pancreatectomy remains an unsolved problem and none preventive procedure has been shown effectively.
We present a new technique that combine pancreatic stent placement with round ligament autologous patch over pancreatic edge.
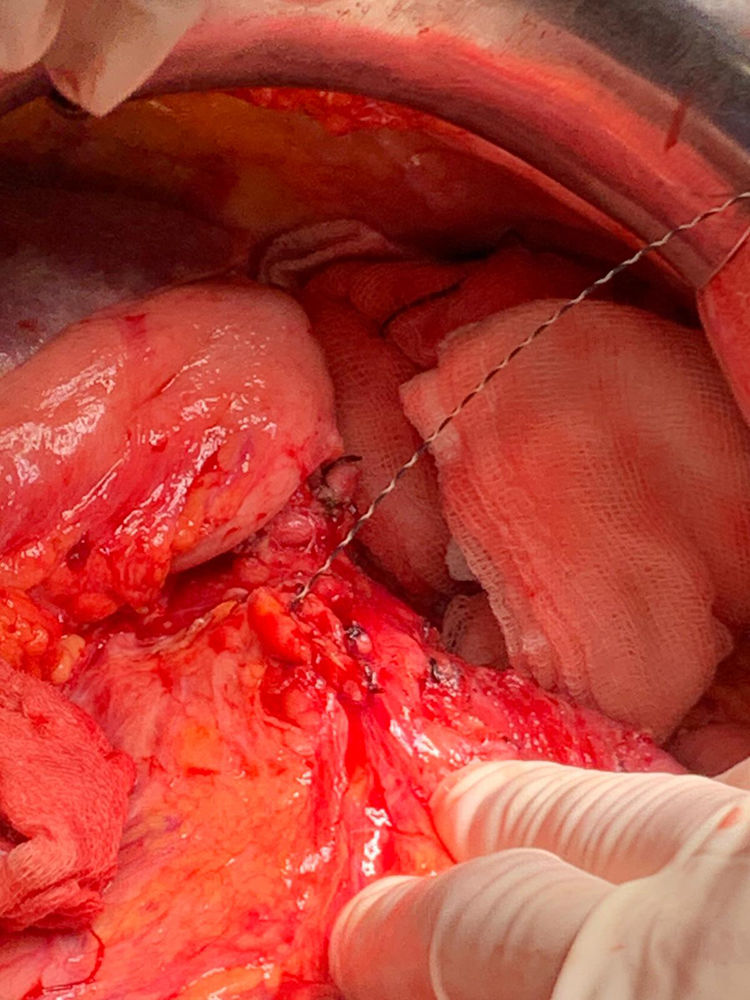
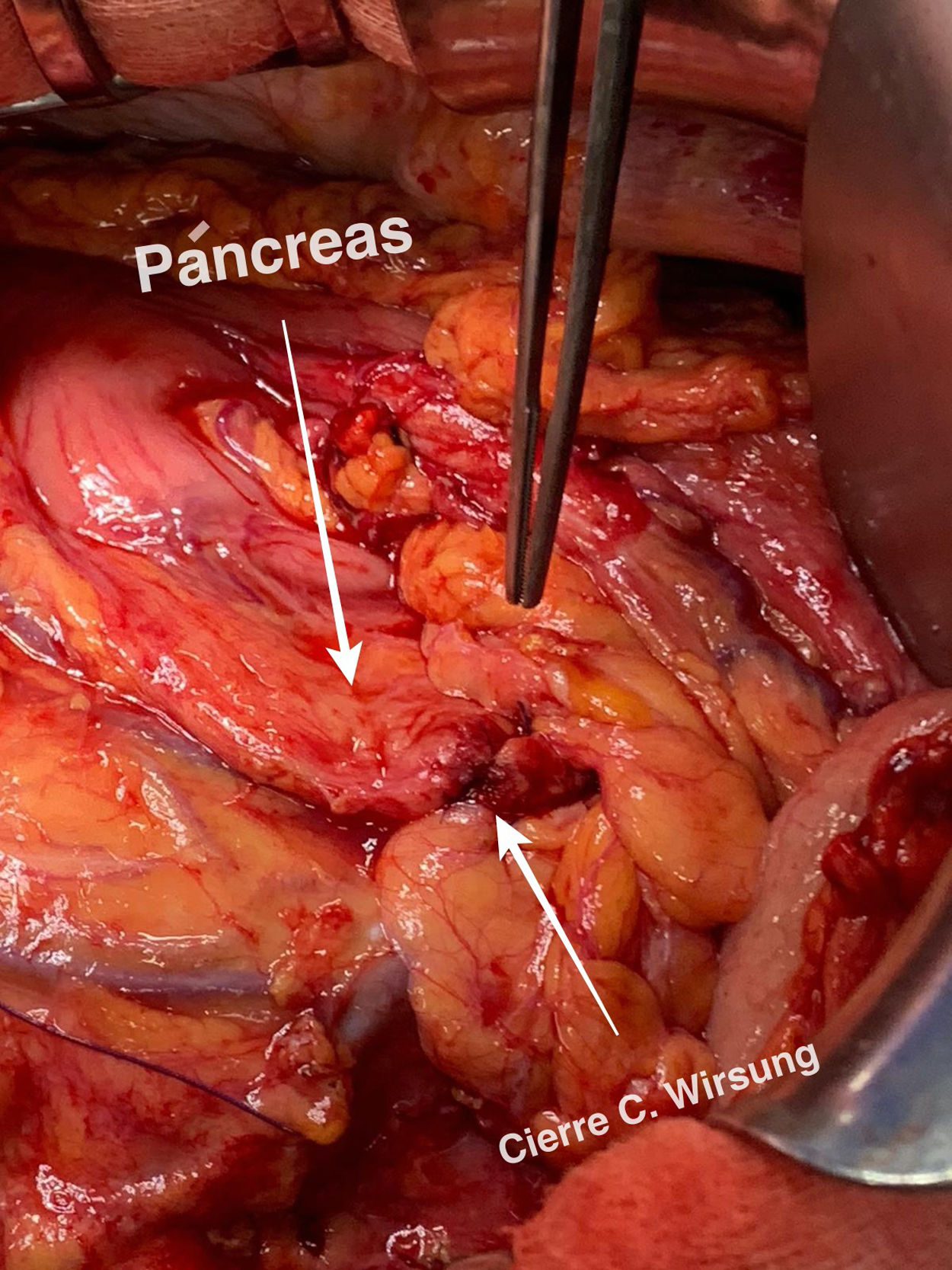
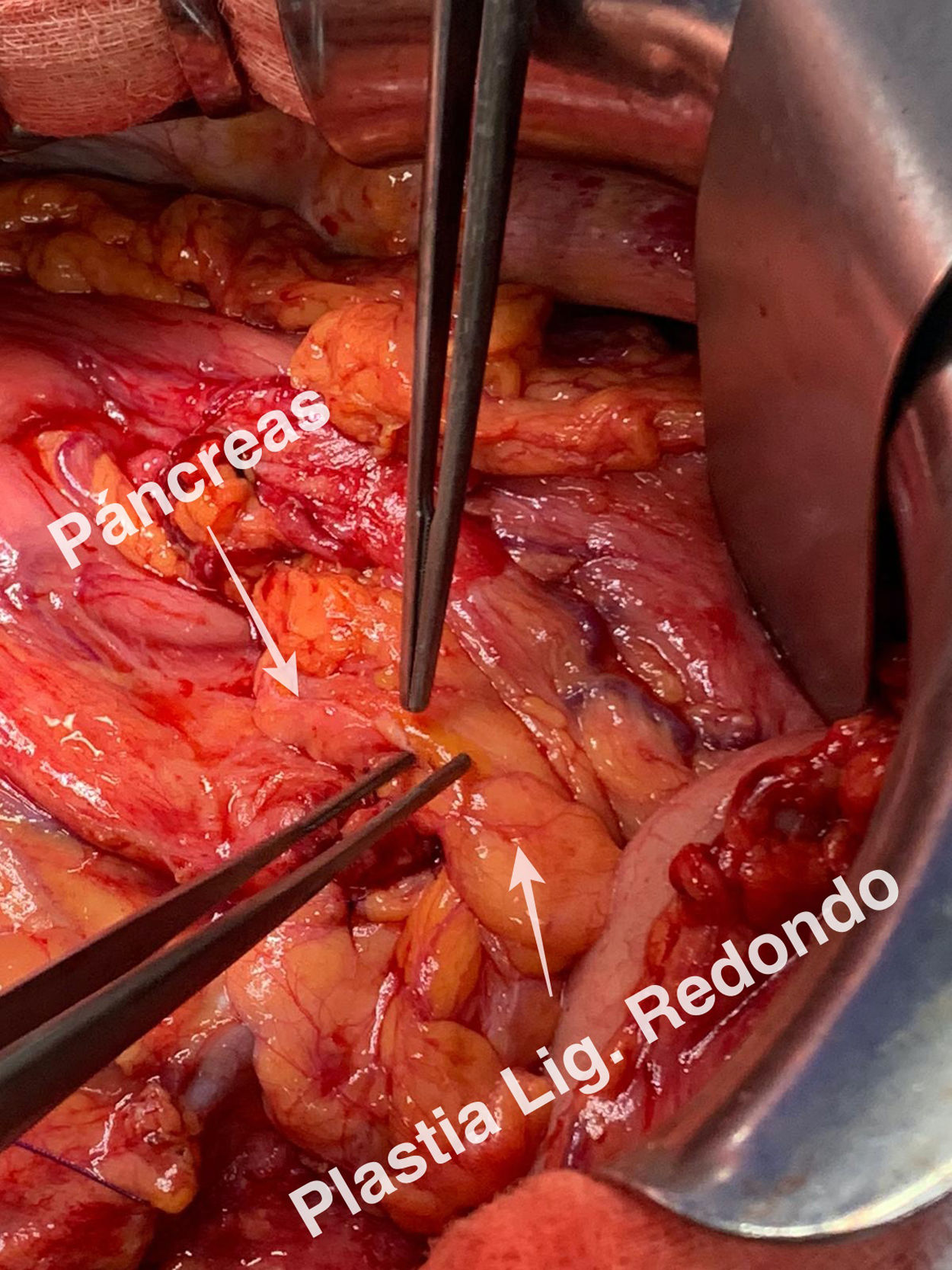
A guide is introduced through Wirsung duct prior to stent placement. After stent assessment, Wirsung duct is closed. Finally, falciform ligament autologous patch is placed over pancreatic edge. After 6–8 weeks, the stent is removed by oral endoscopy.
This technique introduces a new issue on the pancreatic fistula prevention.
La fístula pancreática tras la pancreatectomía distal supone una de las complicaciones más frecuentes y con mayor morbilidad asociada en este tipo de cirugía. Hasta el momento ningún método empleado se ha mostrado eficaz en reducir la fístula pancreática de forma contrastada.
Presentamos el desarrollo de una nueva técnica quirúrgica que combina la colocación del stent pancreático junto con la colocación de parche autólogo de ligamento redondo sobre el borde pancreático.
Tras la realización de la transección pancreática, se introduce de forma anterógrada la prótesis pancreática previo paso de una guía. Se comprueba la colocación de la prótesis y se cierra el conducto de Wirsung. Por último, se realiza una plastia autóloga de ligamento falciforme. A las 6–8 semanas se retira la prótesis vía endoscópica. La técnica ha sido empleada en 2 pacientes sin presentar fístula pancreática postoperatoria.
Esta técnica combinada pretende introducir un nuevo elemento en la prevención de la fístula pancreática mediante una técnica reproducible sin una dificultad técnica añadida.
Pancreatic fistula (PF) after distal pancreatectomy is one of the most frequent complications and presents the highest associated morbidity in this type of surgery. The International Study Group on Pancreatic Fistula (ISGPF) estimates its frequency at around 30%.1 The standardization of PF criteria by the ISGPF1 has helped homogenize the studies to be able to compare the results more reliably. Preoperative risk factors for developing PF are well known: male sex, elevated body mass index (BMI),2 age,3 texture of the pancreas,3,4 chronic pancreatitis of the remnant,5 and low serum albumin levels.6 Different techniques have been proposed to reduce the incidence of PF after distal pancreatectomy: use of fibrin glue, periampullary botulinum toxin, primary closure of the pancreatic stump, division with an endostapler, use of biological mesh, collagen matrix with fibrinogen and thrombin (Tachosil®), preoperative pancreatic surgical drain placement, autologous falciform ligament graft, as well as the use of perioperative octreotide and somatostatin analogues. However, none of these elements has consistently shown better results in terms of reducing PF in prospective randomized controlled trials (RCT).7,8
Surgical techniqueIndicationsWe present the development of a new surgical technique that combines the placement of a pancreatic stent together with the use of an autologous falciform ligament graft over the pancreatic edge.
The technique should be used in patients undergoing distal pancreatectomy with no anatomical alterations that prevent postoperative endoscopy. There are no absolute contraindications for performing the technique. Its use should be avoided in patients with acute or chronic pancreatitis of the pancreatic remnant.
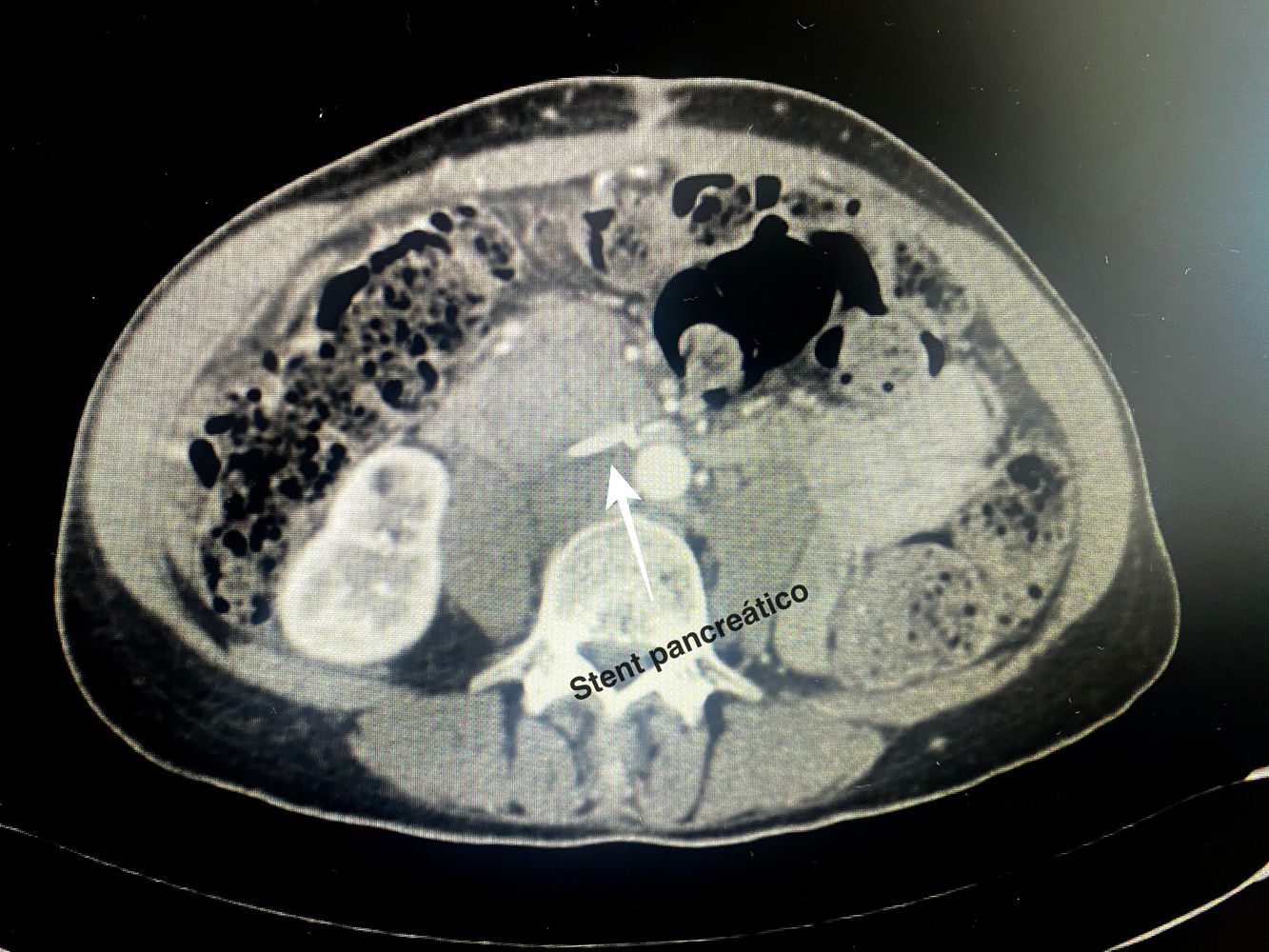
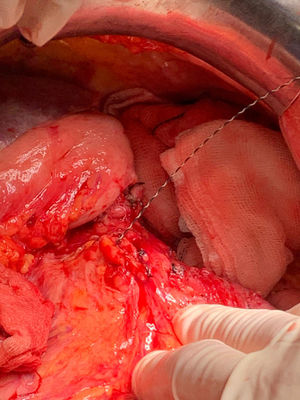
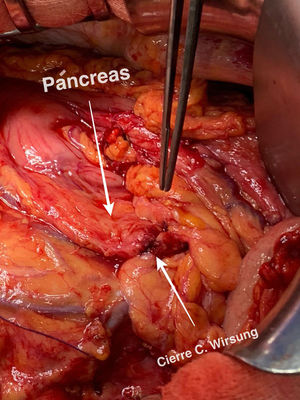
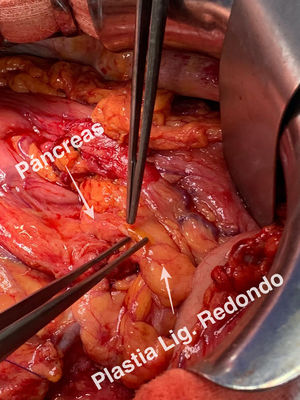
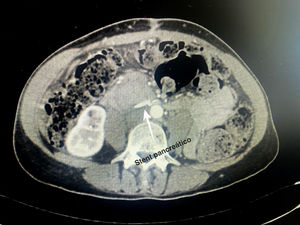
Technical detailsThe technique requires the use of a 13 cm 5 French pancreatic stent made of a long-lasting flexible polymer (Fig. 1), together with a 0.2-mm guidewire for its placement (Fig. 2). After performing the pancreatic transection with a cold scalpel, the guidewire is inserted into the Wirsung duct to confirm the permeability of the entire tract and the optimal passage to the duodenum. The size of the stent is adapted to the desired length, maintaining a distance of 0.5–1 cm from the edge of the division. After verifying its placement in the duodenum, the Wirsung duct was closed in the pancreatic remnant with slow-absorption sutures (Fig. 3). Finally, an autologous graft of the previously released falciform ligament was created over the closed stump (Fig. 4). The stent was removed endoscopically 6–8 weeks later, after verifying the presence of the stent with a radiological study (Fig. 5).
The technique has been performed in 2 patients to date, and neither has presented PF in the postoperative period. The first patient was a 49-year-old woman with a diagnosis of neuroendocrine tumor (low-grade pT3pN1) measuring 16 × 13 cm in the distal third of the pancreas. Surgical margins were free. The surgical drain was removed on the third day due to amylase levels lower than three times the serum value. She was discharged on the eighth postoperative day without complications.
The second patient was a 78-year-old woman with a diagnosis of acinar cell carcinoma (pT3 pN0) measuring 7 × 5 cm with free surgical margins located at the junction of the middle and distal thirds. She was discharged on the 9th postoperative day after removing the drain on the 5th day when amylase levels were lower than three times the serum value. The images are of this second case.
No somatostatin or perioperative analogs were used in either case. In both patients, the stents were removed endoscopically 6 weeks after discharge from hospital without associated complications after a radiological study (computed tomography [CT] scan and abdominal radiography, respectively).
DiscussionThe main surgical complication after distal pancreatectomy is PF, which has high associated morbidity and mortality rates.9 To date, no method used has proven to be effective in reducing PF in RCT with sufficient efficacy.7,8
A Cochrane review that included 19 RCT found a decrease in the incidence of PF with the use of somatostatin analogues,10 although it did not find differences in the subgroup of PF grades B and C. In this review, most of the studies analyzed pancreaticoduodenectomies. Later RCT analyzed the impact of other somatostatin analogues with different results.11,12 For the time being, the use of somatostatin analogues to reduce PF needs more solid evidence.
The use of fibrin-based biological glues has also been evaluated for the occlusion of the pancreatic duct in the prevention of PF. Despite the good initial results published in the first RCT,13 the use of these glues has not shown sufficient evidence in later RCT.14,15 The use of collagen matrix with fibrinogen/thrombin added to the closure of the pancreatic stump has been proposed as an effective method for reducing PF, although this has not been consistently demonstrated.16 The use of biological mesh accompanied by the closure of the pancreatic stump has been shown in an RCT and in different case-control studies to be effective in reducing PF,17–19 although this evidence has not been supported by other studies.20,21 The use of botulinum toxin in the sphincter of Oddi to reduce PF after distal pancreatectomy is currently under study22 after a phase I/II RCT showed it to be a safe, feasible technique with good clinical results.23
Over the years, there has been debate about the closure of the pancreatic stump by mechanical or manual suture. In a systematic review comparing both techniques, no significant differences were found, reporting a PF rate of 22% in the mechanical suture group and 31% in the manual suture group.24 The DISPACT25 RCT analyzing 352 patients found no differences between the two groups in the overall incidence of PF. This absence of difference was maintained in the subgroup of grades A and B PF with 20% and 21% of PF, respectively. A review after the publication of the DISPACT trial only found a lower rate of PF in the mechanical suture group compared to the manual suture group, which did not improve after adding a manual closure.26 Retrospective studies continued to support the use of mechanical suture,27,28 but more evidence is needed in new RCT with stronger evidence.
The use of a pancreatic stent prior to surgery has been proposed as an effective method for reducing PF after distal pancreatectomy. Several studies with case series29–31 and a systematic review that included one RCT and 3 case-control studies32 concluded that the placement of a preoperative pancreatic stent reduced the incidence of PF. After these promising results, a published RCT found no differences in the incidence of PF in the pancreatic prosthesis group compared to the standard technique, and the incidence of acute pancreatitis and intra-abdominal abscess was more frequent in the preoperative endoscopy group.33
Coverage of the residual pancreatic stump with a falciform ligament patch has been evaluated as a procedure to prevent PF. Hassenpflug et al34 demonstrated a significantly lower incidence of both grade B (7% vs 9%) and C (7% vs 21%) PF with the use of falciform ligament grafts after distal pancreatectomy. These results were not supported by the Carter et al study,15 although in this case biological glue was added to the ligament patch, without comparing the two techniques independently.
ReasoningNeither technique has been independently shown to be superior in reducing PF after distal pancreatectomy. This study describes a combination of intraoperatively placed pancreatic stents with coverage of the pancreatic stump using a falciform ligament graft together with closure of the main pancreatic duct. This combined technique aims to introduce a new element in the prevention of PF through a reproducible technique without any added technical difficulty. Subsequent comparative studies should be carried out to evaluate its efficacy.
Advantages and disadvantagesThe main advantage of the described technique is that it does not require complex surgical maneuvers that may cause greater patient morbidity. With the introduction of the antegrade pancreatic stent, manipulation of the papilla is reduced, along with a theoretical reduction in the possibilities of developing acute pancreatitis and complications derived from the endoscopic procedure, ensuring correct pancreatic drainage. If it is not expelled naturally, the stent can be removed endoscopically with little associated morbidity.
The drawback of the use of the technique is the need, in most cases, of a later endoscopy to remove the stent, with the complications that can occur, as well as the added radiation of the postoperative imaging study (radiograph or CT scan).
Conflict of interestsThere are no conflicts of interests to declare.
Please cite this article as: Ocaña J, Sanjuanbenito A, Lobo E, Fernández-Cebrián JM. Colocación intraoperatoria anterógrada de stent pancreático y plastia de ligamento redondo para prevenir la fístula pancreática tras la pancreatectomía distal. Cir Esp. 2021;99:374–378.