Currently, R1 resection is defined by the presence of tumor cells within <1 mm of the resection margin. The main aim of this study was to analyze the impact of positive margins (R1) on survival outcomes in pancreatic cancer.
MethodsWe performed a retrospective analysis with multivariate regression analysis of a prospective database from 2008 to 2017, which included resection margin status, expanded resection margin (R1 < 1 mm), vascular resection, lymphatic involvement, surgical complications, tumor differentiation grade and adjuvant treatment.
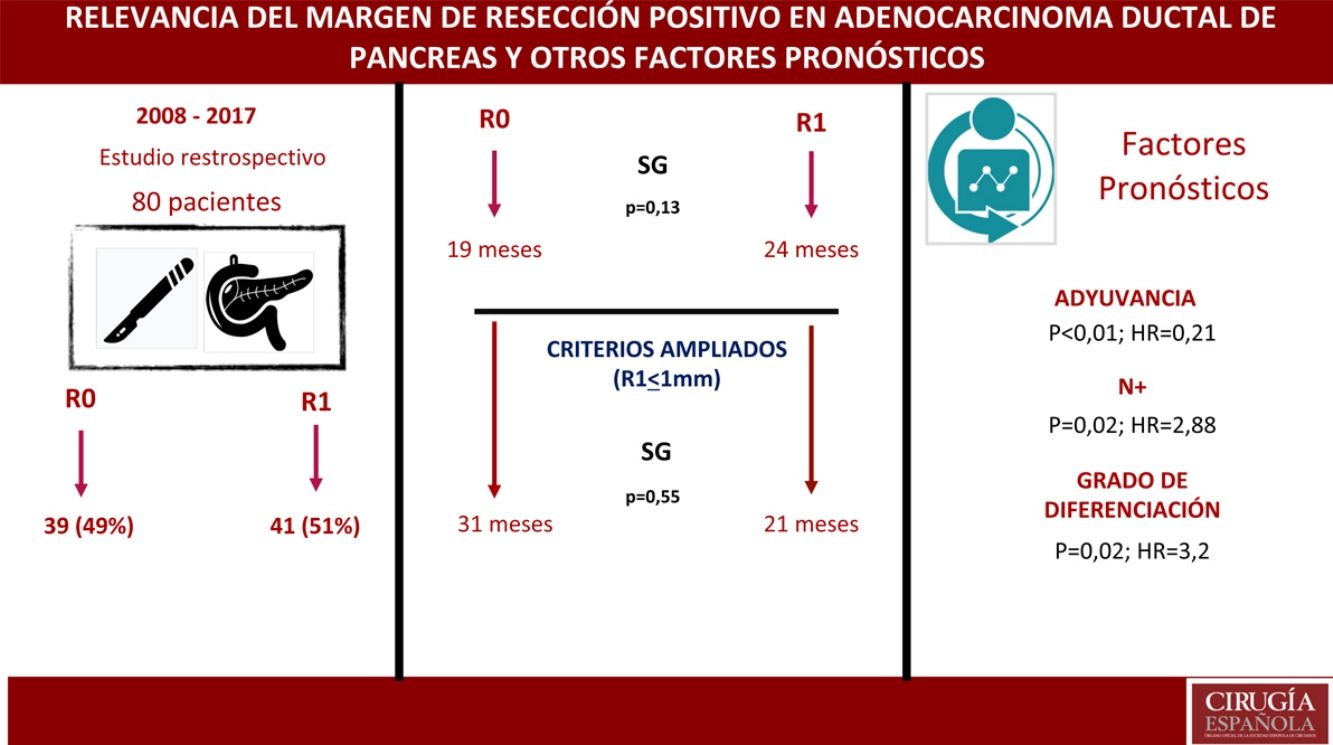
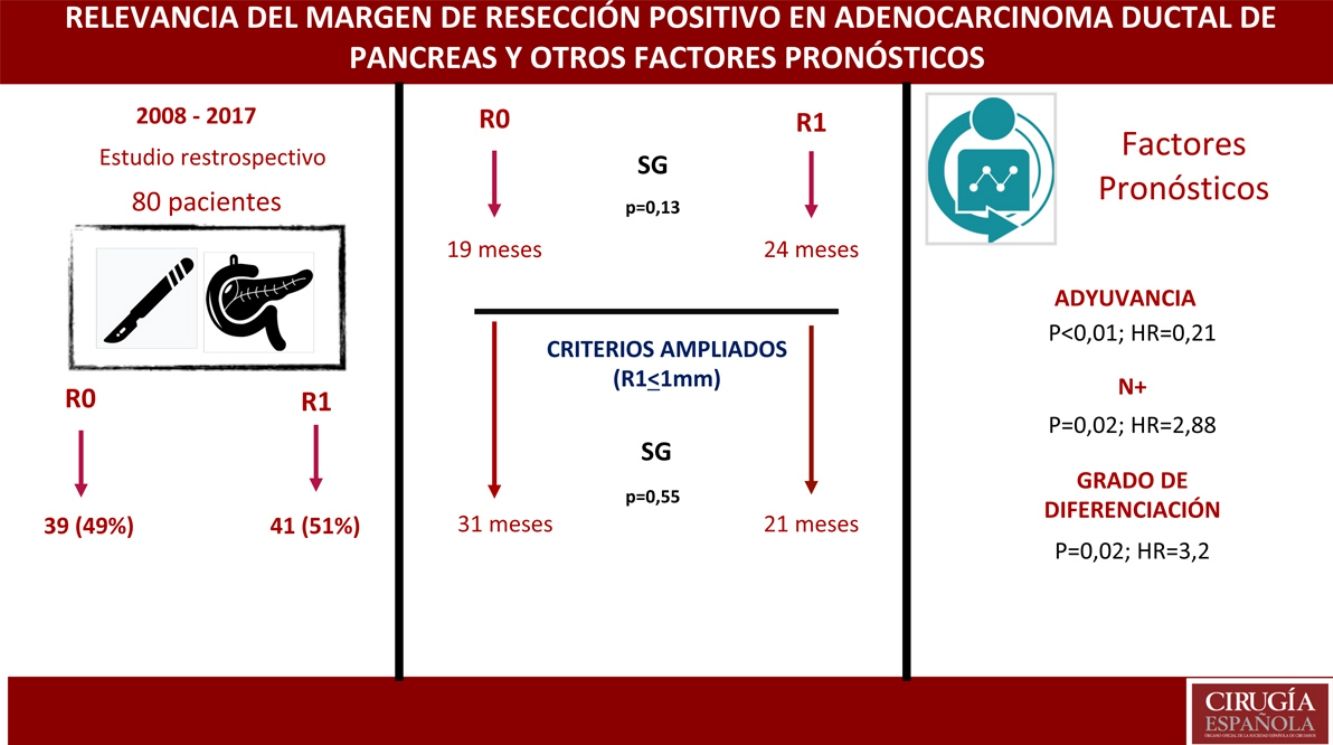
ResultsA total of 80 patients were analyzed: 42 (52%) R1; 38 (48%) R0. No differences were found in the composition of the two groups except for the vascular resection, which was more frequent in the R1 group: 12 (21%) vs 2 (3%). Overall survival in the R0 group was 19 months vs 24 months in the R1 group (P = .13). Wide R1 (R1 < 1 mm) had an overall survival of 21 months versus 31 months in wide R0 (P = .55). In the multivariate analysis, only lymph node involvement (P = .02, HR = 2.88), tumor differentiation (P = .02, HR = 3.2) and adjuvant therapy (P < .01; HR = 0.21) were found to be factors related to survival.
ConclusionR1 resection is not an independent risk factor. Lymph node involvement, differentiation grade and adjuvant treatment are prognostic factors. The benefit of expanding the resection margins should be demonstrated. More studies are needed to assess the impact of the resection margin.
Actualmente en cirugía del cáncer de páncreas se considera margen de resección afecto (R1) la presencia de celular tumorales a <1 mm del borde de resección. El objetivo principal del estudio es analizar el impacto del margen de resección en la supervivencia.
MétodosAnálisis restrospectivo con análisis de regresión multivariante de una base de datos prospectiva 2008–2017, donde se incluye el margen de resección, el margen de resección ampliado (R1 < 1 mm), la resección vascular, la afectación linfática, las complicaciones quirúrgicas, la diferenciación tumoral y el tratamiento adyuvante.
ResultadosUn total de 80 pacientes fueron analizados 42 (52%) R1 y 38 (48%) R0. No se encontraron diferencias en la composición de ambos grupos salvo en la resección vascular, que fue mayor en el grupo R1, 12 (21%) vs 2 (3%). La supervivencia en el grupo R0 fue de 19 meses vs. 24 meses en el grupo R1 (p = 0,13). El margen ampliado (R1 < 1 mm) tuvo una supervivencia de 21 meses vs. 31 meses en R0 ampliado (p = 0,55). En el análisis multivariante solo se encontraron la afectación ganglionar (p = 0,02; HR = 2,88), la diferenciación tumoral (p = 0,02; HR = 3,2) y la adyuvancia (p < 0,01; HR = 0,21) como factores pronósticos de supervivencia.
ConclusionesEn el estudio la resección R1 no supone un factor pronóstico. La afectación ganglionar, el grado de diferenciación y el tratamiento adyuvante son factores pronósticos. Debe demostrarse el beneficio de ampliar los márgenes de resección. Son necesarios más estudios para valorar el impacto del margen de resección.
Each year, there are more than 270,000 new diagnoses of pancreatic cancer worldwide, representing 10% of all gastrointestinal cancers.1 Pancreatic cancer is the fourth leading cause of cancer-related death in Western countries.2 Despite medical and technological advances, to date the median survival rate for pancreatic cancer is approximately 20 months, with a 5-year survival of around 20%.3 Surgery is a fundamental element to achieve improvements in long-term survival in these patients, with associated chemotherapy or radiochemotherapy. The possibility of a cure has been classically related to the removal of the tumor with surgical margins that are free of involvement,4 and tumor-free resection margins (R0) are considered a criterion for quality surgery.5 For some years now, the concept of resection margins has changed, and R1 affected margins are now considered any presence of tumor cellularity <1 mm from the surgical edge.6–10
Most studies currently accept as optimal a percentage of positive resection margins greater than 60%, although its prognostic value and influence on local and distance recurrence is the object of constant debate. The Hishinuma et al. study evaluated surgical results in the autopsies performed on 24 patients who died from non-cancer causes with a history of potentially curative pancreatic resection and free resection margins after diagnosis of pancreatic cancer; they found local recurrence of the disease in 75% of cases and distant disease in 50%.11
The main objective of this study is to establish the prognostic value of resection margins in adenocarcinoma of the head of the pancreas for overall survival (OS) and disease-free survival (DFS). The secondary objectives of the study is the determination of long-term survival prognostic factors and to determine the impact of restaging the resection margin.
MethodsThe study design is a retrospective analysis of data collected prospectively. Patient data were extracted from the pancreatic tumor database of a single hospital, selecting patients with a diagnosis of adenocarcinoma of the head of the pancreas treated surgically with pancreaticoduodenectomy (PD) between 2008 and 2017. All included patients were treated at the same tertiary hospital by the biliopancreatic surgery unit after assessment by the multidisciplinary committee.
The inclusion criteria for the study were: preoperative diagnosis of adenocarcinoma of the head of the pancreas, elective PD performed by surgeons of the specialized unit, macroscopic resection of the tumor (R0 and R1). None of the demographic parameters were exclusion criteria, nor were the performance of associated venous vascular resection or the choice of adjuvant or neoadjuvant therapy. The main exclusion criteria were the location of the tumor in the body or tail of the pancreas, tumor histology other than adenocarcinoma and incomplete macroscopic resection (R2).
Any type of involvement <1 mm from the resection edge was considered R1. R0 resection was the absence of tumor cellularity at least 1 mm from the edge of the surgical resection edge.6 Margins analyzed by pathologists included the posterior margin and SMA, medial and PV/SMV axis and pancreatic resection edge. Each margin was analyzed independently.10 Re-staging of the extended resection margin by more than 1 mm was carried out based on the pathology results and not performed ad hoc. All samples were analyzed by the pathology team specialized in the analysis of hepatobiliary and pancreatic tumors.
The TNM classification was conducted in accordance with the criteria established by the AJCC Cancer Staging Manual, 7th Edition.12 The cell differentiation of the tumor was established in three groups: well-differentiated (G1), moderately differentiated (G2) or poorly differentiated (G3).
Complications have been collected both globally and independently. The definition of pancreatic fistula used was from the modified ISGPS 2016 criteria.12,13
Patients were chosen with neoadjuvant and adjuvant treatment, which was determined by the criteria of the medical oncology service and approved by the multidisciplinary committee.
OS was established as the period between diagnosis and death. DFS was defined as the period between diagnosis and the presence of tumor recurrence, either distant or local.
The statistical analysis was calculated with SPSS Statistics® v20. Survival estimates were made using the Kaplan-Meier method, and the comparison between subgroups with the log-rank test. The multivariate regression analysis was conducted with the use of Cox proportional-hazards model. Chi-squared and Fisher’s tests were used for the analysis of categorical variables. P < .05 was considered statistically significant. Confidence intervals (CI) were at a 95% probability.
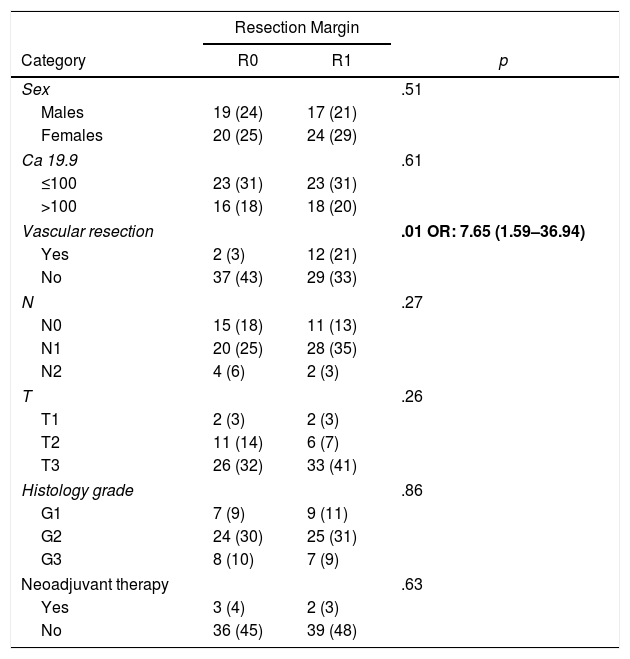
ResultsFrom January 2008 to January 2017, 80 patients were included: 41 patients (51%) with R1 resection (CI: 39.3–62.5) and 39 patients (49%) with R0 resection (CI: 35.5–58.8). The distribution of the characteristics studied between the two groups (R0 and R1) was homogeneous, except in the number of patients treated with vascular resection (Table 1): 2 patients (3%) in the R0 group and 12 (21%) in the R1 group (P = .01; OR = 7.65, CI: 1.69–36.94). Other parameters studied were sex: 19 men (24%) and 20 women (25%) with R0; 17 men (21%) and 24 women (30%) with R1; the presence of tumor marker Ca 19.9 above 100 U/mL, present in 15 patients (18%) with R0 resection and 17 with R1 resection (20%) (P = .61); 15 patients (18%) with N0 involvement and R0 resection and 11 with R1 resection (13%); in the N1 group, 20 patients (25%) with R0 and 28 with R1 (35%) and in the N2 group, 4 with R0 (6%) and 2 with R1 (3%). The distribution according to T stage was: T1, 2 patients (3%) in both the R0 and R1 groups; T2, 11 (14%) and 6 (7%) patients in the R0 and R1 groups, respectively; in T3, 26 (32%) and 33 (41%) patients in the R0 and R1 groups, respectively. The distribution according to histological grade was: in the G1 group, 7 (9%) and 9 (11%) patients in the R0 and R1 groups; in G2, 24 (30%) and 25 (31%) patients in R0 and R1; and in G3, 8 (10%) and 7 (9%) in R0 and R1, respectively. A total of 5 (7%) patients received neoadjuvant treatment: 3 in the R0 group (4%) and 2 patients in the R1 group (3%), with no statistical differences in the composition of the two groups (P = .63).
Patient Characteristics According to Resection Margin.
| Resection Margin | |||
|---|---|---|---|
| Category | R0 | R1 | p |
| Sex | .51 | ||
| Males | 19 (24) | 17 (21) | |
| Females | 20 (25) | 24 (29) | |
| Ca 19.9 | .61 | ||
| ≤100 | 23 (31) | 23 (31) | |
| >100 | 16 (18) | 18 (20) | |
| Vascular resection | .01 OR: 7.65 (1.59–36.94) | ||
| Yes | 2 (3) | 12 (21) | |
| No | 37 (43) | 29 (33) | |
| N | .27 | ||
| N0 | 15 (18) | 11 (13) | |
| N1 | 20 (25) | 28 (35) | |
| N2 | 4 (6) | 2 (3) | |
| T | .26 | ||
| T1 | 2 (3) | 2 (3) | |
| T2 | 11 (14) | 6 (7) | |
| T3 | 26 (32) | 33 (41) | |
| Histology grade | .86 | ||
| G1 | 7 (9) | 9 (11) | |
| G2 | 24 (30) | 25 (31) | |
| G3 | 8 (10) | 7 (9) | |
| Neoadjuvant therapy | .63 | ||
| Yes | 3 (4) | 2 (3) | |
| No | 36 (45) | 39 (48) | |
OR: odds ratio.
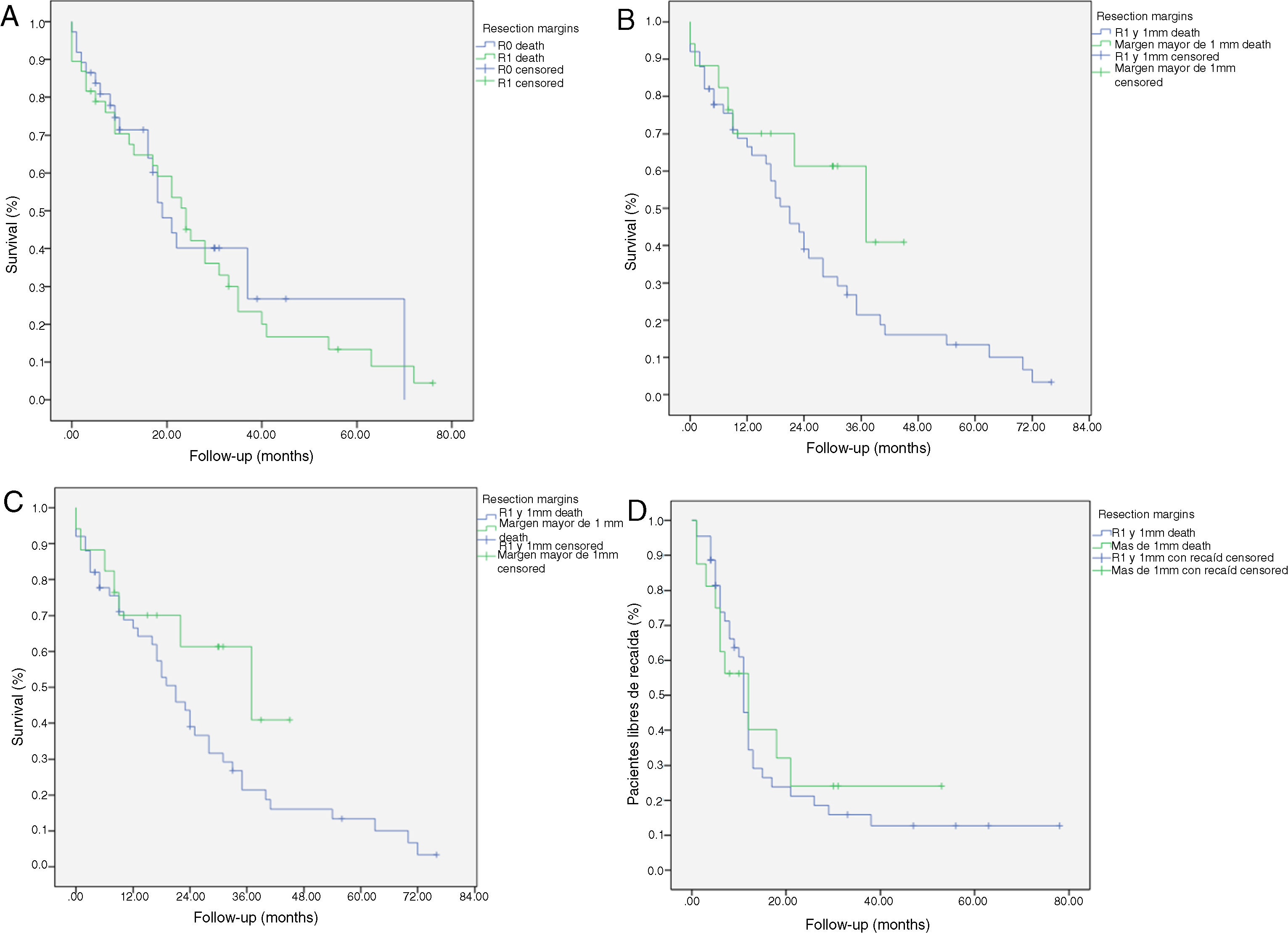
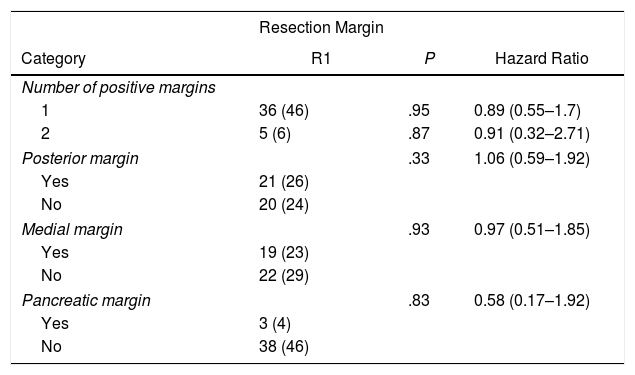
In the univariate analysis of OS and resection margins, there were 36 patients (46%) with a single margin that showed involvement (P = .95; HR = 0.89, CI: 0.55–1.70) and 5 patients (6%) with two affected resection margins (P = .87; HR = 0.91, CI: 0.32–2.71). R1 involvement according to the affected margins was 21 patients (26%) (P = .33) in the posterior margin, 19 (23%) in the medial margin (P = .93) and 3 (4%) in the pancreatic margin (P = .83), with no correlation being found between the affected margin and survival (Table 2).
Univariate Analysis of the Resection Margins and Overall Survival.
| Resection Margin | |||
|---|---|---|---|
| Category | R1 | P | Hazard Ratio |
| Number of positive margins | |||
| 1 | 36 (46) | .95 | 0.89 (0.55–1.7) |
| 2 | 5 (6) | .87 | 0.91 (0.32–2.71) |
| Posterior margin | .33 | 1.06 (0.59–1.92) | |
| Yes | 21 (26) | ||
| No | 20 (24) | ||
| Medial margin | .93 | 0.97 (0.51–1.85) | |
| Yes | 19 (23) | ||
| No | 22 (29) | ||
| Pancreatic margin | .83 | 0.58 (0.17–1.92) | |
| Yes | 3 (4) | ||
| No | 38 (46) | ||
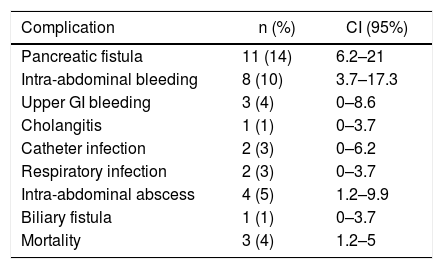
The surgical complications presented in the postoperative period of PD due to adenocarcinoma of the head of the pancreas in the series are shown in Table 3. The most frequent complications were: pancreatic fistula in 11 patients (14%) (CI: 6.2–21), intra-abdominal bleeding in 8 patients (10%) and intra-abdominal abscess in 4 patients (14%) (CI: 1.2–9.9). Three of the patients (4%) (CI: 1.2–5) died in the postoperative period and 32 (40%) had complications, 27 of which (33%) were ≥IIIA according to the Clavien classification.
Surgical Complications Associated With Pancreaticoduodenectomy.
| Complication | n (%) | CI (95%) |
|---|---|---|
| Pancreatic fistula | 11 (14) | 6.2–21 |
| Intra-abdominal bleeding | 8 (10) | 3.7–17.3 |
| Upper GI bleeding | 3 (4) | 0–8.6 |
| Cholangitis | 1 (1) | 0–3.7 |
| Catheter infection | 2 (3) | 0–6.2 |
| Respiratory infection | 2 (3) | 0–3.7 |
| Intra-abdominal abscess | 4 (5) | 1.2–9.9 |
| Biliary fistula | 1 (1) | 0–3.7 |
| Mortality | 3 (4) | 1.2–5 |
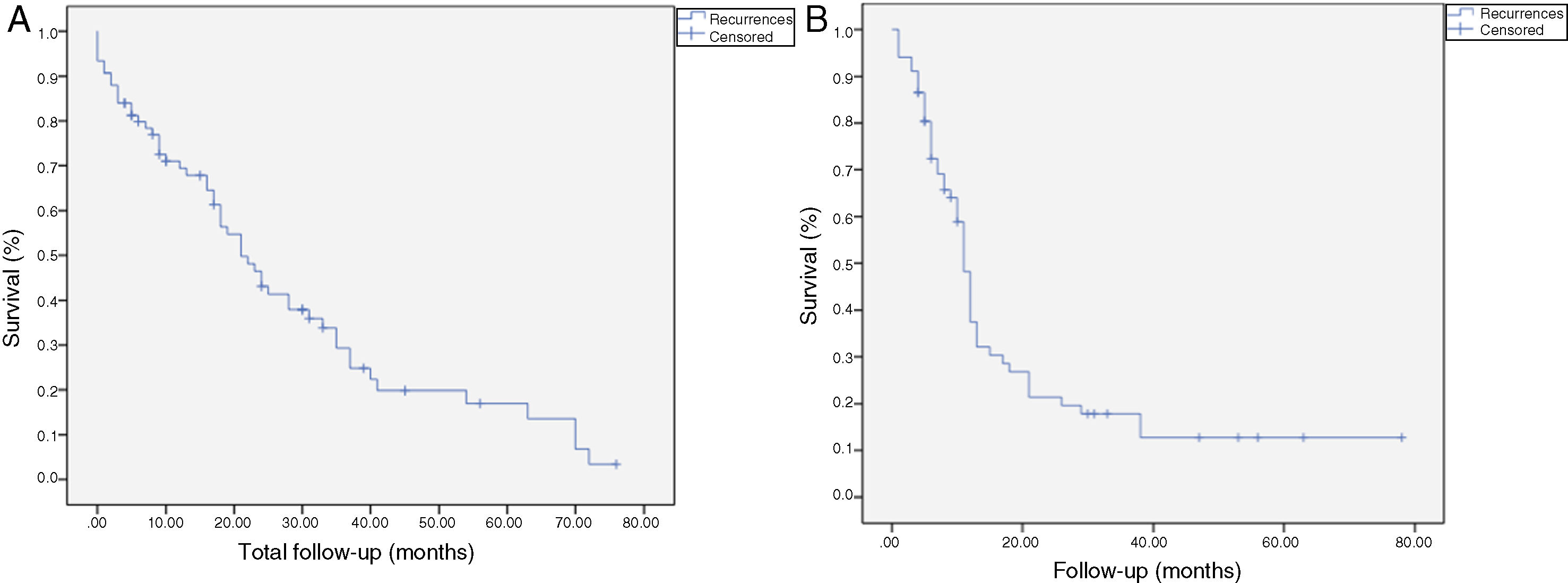
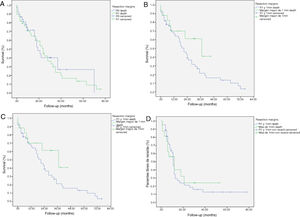
The median OS was 21 months (CI: 15.49–26.50) (Fig. 1); in the R0 group it was 19 months (CI: 14.4–23.6) and in the R1 group, 24 months (CI: 17.34–30.66), with no significant differences between the two groups found in the multivariate analysis (P = .13; HR = 1.09, CI: 0.75–1.90) (Fig. 2). The median DFS was 11 months (CI: 9.81–12.18) (Fig. 1). In R0 patients, median DFS was 12 months (CI: 10.12–13.87) compared to R1 patients, with a median DFS of 11 months (CI: 9.94–12.056), with no differences found between the two groups (P = .84; HR = 0.94, CI: 0.54–1.64).
Kaplan-Meier survival curves showing overall survival (OS) and disease-free survival (DFS) in patients with adenocarcinoma of the head of the pancreas after pancreaticoduodenectomy: A) OS in patients with R0 and R1 (median 37 months vs. 21 months) (P = .13); B) DFS in R0 and R1 patients (median 12 months vs. 11 months) (P = .84); C) OS in R0 patients expanded with a median of 37 months (10.04–63.96) vs. expanded R1 ≤ 1 mm with median of 21 months (14.65–27.34) (P = .55); D) DFS in patients with expanded R0 vs. expanded R1 (12 months vs. 11 months) (P = .73).
We have analyzed the impact of re-staging resection margins (Fig. 2) on OS and DFS, comparing expanded R0 patients >1 mm with expanded R1 patients ≤1 mm. The expanded R0 group included 23 patients (26.4%) (CI: 16.7–26.1), with a median OS of 37 months (CI: 10.04–63.96). The expanded R1 group included 57 patients (73.6%) (CI: 63.9–83.3), with a median OS of 21 months (CI: 14.65–27.34). OS was higher in the group with expanded resection margins (>1) than in the R1 group (≤1 mm), being 37 months vs. 21 months, respectively. However, statistical significance was not reached for OS (P = .55; HR = 0.55, CI: 0.24–1.24) or DFS (P = .73; HR = 0.89, CI: 0.45–1.76), with a median of 11 months in expanded R1 vs. 12 months in expanded R0.
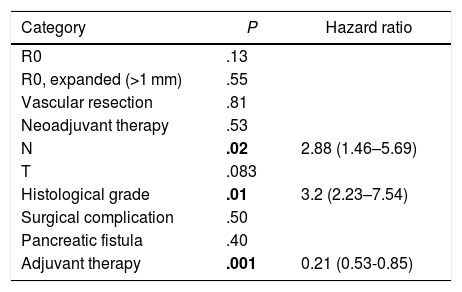
A multivariate analysis model was constructed (Table 4), including the variables for resection margin (R0 and R1 < 1 mm), the presence of vascular resection, neoadjuvant therapy, T and N staging, histological grade, presence of surgical complications, the presence of postoperative pancreatic fistula and adjuvant therapy. The observed risk analysis showed that both lymph node involvement (N) (HR = 2.88; CI: 1.46–5.69) and tumor histological grade (HR = 3.2; CI: 2.23–7.54) were associated with mortality due to adenocarcinoma of the head of the pancreas, while adjuvant treatment with chemotherapy is shown in the analysis as the only protective factor (HR = 0.21, CI: 0.53–0.85) that may decrease mortality in pancreatic cancer, according to this analysis. No correlation was found in the study between the resection margins and OS (P = .13) or with vascular resection (P = .81). Surgical complications were not shown to have an impact on survival, both in general (P = .53) and specifically pancreatic fistula (P = .4).
Multivariate Analysis for Overall Survival.
| Category | P | Hazard ratio |
|---|---|---|
| R0 | .13 | |
| R0, expanded (>1 mm) | .55 | |
| Vascular resection | .81 | |
| Neoadjuvant therapy | .53 | |
| N | .02 | 2.88 (1.46–5.69) |
| T | .083 | |
| Histological grade | .01 | 3.2 (2.23–7.54) |
| Surgical complication | .50 | |
| Pancreatic fistula | .40 | |
| Adjuvant therapy | .001 | 0.21 (0.53-0.85) |
The analysis of resection margins after PD should be done systematically and in accordance with current evidence.6,12–16 Incorrectly accepting a margin as free does not provide correct data for patient treatment.17–19 The most commonly affected margins are the posterior and medial margins,7,8,20 as in this study, at 26% and 23%, respectively.
Standardized pathology testing increases the percentage of R1 resections compared to previous criteria. Both the results by Esposito et al.,9 where an increase of 14%–76% was observed in R1 resections after adapting the criteria (R1 < 1 mm) compared to the previous ones (R1 with direct margin involvement), as well as Verbeke et al.,10 who also observed in their systematic review an increase of 18–85% in R1, have been instrumental in bringing about a global change in the analysis and interpretation of resection margins in pancreatic cancer surgery. Initially in European countries6 and in the last year in the American setting (AJCC),12,15 the same resection margin has been accepted more globally.
In a study done prior to the implementation of the current resection margin, Raut et al. studied in 360 patients (R1: 16.7%) the impact of the resection margin on survival and concluded that R0 does not affect survival. They identified the following prognostic factors: lymph node involvement, the presence of major complications and blood loss,21 and findings confirmed in the ESPAC-1 randomized controlled study, where differences were found between R0 and R1 (16 months vs. 10 months), although with a survival lower than in other studies in the R0 group.22 With the same resection margin criterion, Howard et al. concluded in a study of 226 patients (30% of which were R1) that R0 resection in these cases was a favorable prognostic factor for survival.5
Several studies have demonstrated that R0 resection following current criteria benefits long-term results. Hartwig et al. published a series of 1071 patients where R0 was found to have a benefit in survival compared to R1 (30.9 months vs. 19.7 months).23 These findings have been confirmed by other studies like Strobel et al., which demonstrated an improvement in survival in R0 with margin ≥1 mm compared to R1 < 1 mm and R1 of direct involvement.24 The study by Tummers et al. has provided similar results.25 Other studies have not found that difference in survival between R0 and R1 (<1 mm) to be significant.22–26 One of the most recent studies by ESPAC found better survival in patients with R0 and R1 < 1 mm with no direct margin involvement, with similar survival between both groups and significantly better than patients with direct involvement.27 These results are similar to those found in our study, where survival in patients with R0 (median 19 months [CI: 14.4–23.6]) was not greater than survival in patients with R1 (median 24 months [CI: 17.34–30.66]), while taking into consideration the obvious limitations derived from its retrospective nature with the consequent deterioration of scientific evidence. In the study, resection with involved margins (R1) was obtained in 42 patients (52.5%), which is compatible with the figures presented in the studies using the concept of R1 < 1 mm.6,9,22–24,28–30
Recently published studies have tried to assess the impact of expanding the resection margin distance.20,31 With 365 patients, Chang et al. concluded that expanding the resection margin to 1.5 mm significantly improves survival. Our study shows similar results in terms of improvement in the re-staging of the resection margin, comparing the long-term results among patients with a resection margin of 1 mm and >1 mm, with a median survival in the R0 group >1 mm of 37 months (CI: 10.04–63.96) vs. 21 months (CI: 14.65–27.34) in the 1 mm group. However, no statistically significant differences were found, probably related to the sample size.
There are important prognostic factors present in the majority of the studies, such as lymphatic involvement and the degree of tumor differentiation.5,21–23,31 Our multivariate analysis identified lymphatic involvement (P = .02; HR = 2.88, CI: 1.46–5.69) and degree of differentiation (P = .02; HR = 3.2, CI: 2.23–7.54) as factors for a poor prognosis, while adjuvant treatment improves prognosis (P < .01; HR = 0.21, CI: 0.53–0.85). Probably, both lymphatic involvement and the degree of tumor differentiation define the aggressiveness of the tumor and therefore present as factors for a poor prognosis.
Establishing an overall criterion for optimal resection margins is essential when administering treatments and designing future studies aimed at individualized patient treatment. The study from which adjuvant gemcitabine treatment was adopted showed only 17% R1,32 which is well below what is considered reasonable after standardized analysis of the surgical piece.9,10 In contrast, subsequent studies have used the R0 margin ≥1 mm when analyzing the results.33
This paper does not discuss the effect of neoadjuvant therapy on resection margins. Given the limited number of patients included (5; 7%), conclusions cannot be drawn. The role of neoadjuvant therapy will progressively acquire greater importance34,35 as studies show an improvement in R0 resection margins,36 although well-designed randomized controlled trials are necessary to accept this affirmation.
The results of this study should be analyzed while understanding its limitations, such as the retrospective nature and small sample size compared to other series published in this field.
In conclusion, this study has found no better long-term results in the group of patients with R0 resection margins in accordance with current standards, which was not identified as a prognostic factor in the multivariate analysis. It may be necessary to redefine the R0 concept, although this study, given its retrospective nature, cannot resolve this. Prospective studies with larger patient samples are necessary to improve the current evidence about resection margins. Both lymph node involvement and the degree of tumor differentiation are prognostic factors in adenocarcinoma of the head of the pancreas.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Ocaña J, Sanjuanbenito A, García A, Molina JM, Lisa E, Mendía E, et al. Relevancia del margen de resección positivo en adenocarcinoma ductal de páncreas y otros factores pronósticos. Cir Esp. 2020;98:85–91.