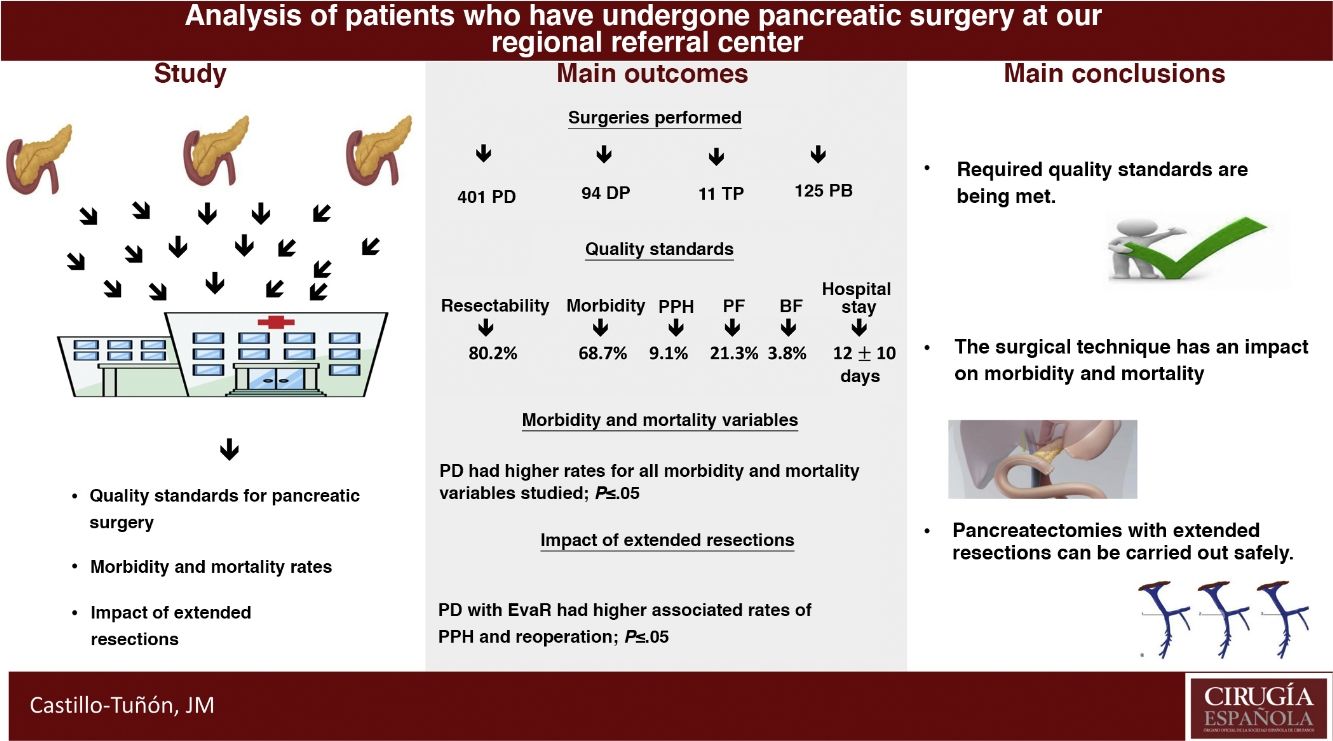
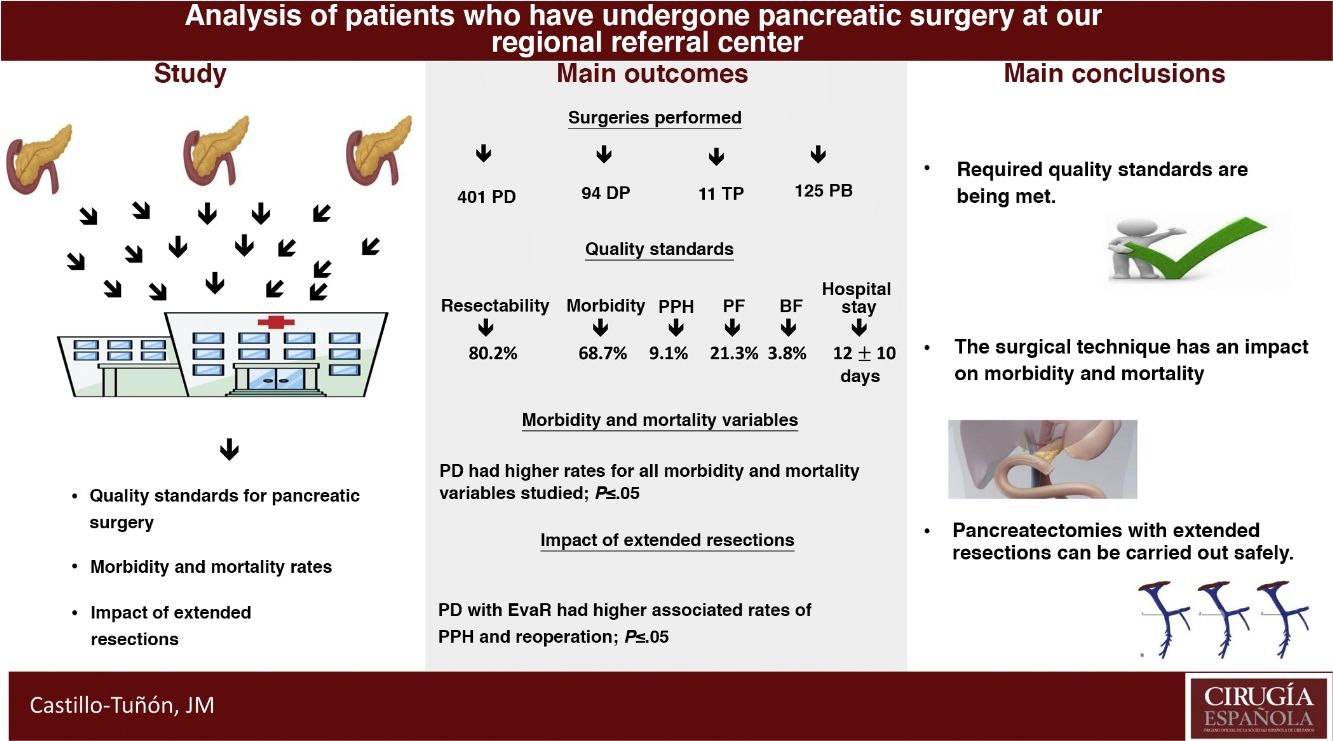
The main objective of this study is to determine whether our unit meets the quality standards required by the scientific community from the reference centers for pancreatic surgery in terms of peri-operative results. The secondary objectives are to compare the different pancreatic surgery techniques performed in terms of early post-operative morbidity and mortality and to analyze the impact of the resections added in these terms.
MethodDescriptive, retrospective and single-center study, corresponding to the period 2006−2019. The results obtained were compared with the proposed quality standards, by Bassi et al. and Sabater et al., required from the reference centers in pancreatic surgery. The sample was divided according to surgical technique and compared in terms of early post-operative morbidity and mortality, studying the impact of extended vascular and visceral resections. All patients undergoing pancreatic surgery in our unit due to pancreatic, malignant and benign pathology were included, since it was implemented as a reference center. Emergency procedures were excluded.
Results631 patients were analyzed. The values obtained in the quality standards are in range. The most frequent surgery was pancreaticoduodenectomy, which associated higher peri-operative morbidity and mortality rates (P ≤ .05). The extended vascular resections impacted the pancreaticoduodenectomy group, associating a longer mean stay (P = .01) and a higher rate of re-interventions (P = .02).
ConclusionsThe experience accumulated allows to meet the required quality standards, as well as perform extended resections to pancreatectomy with good results in terms of post-operative morbidity and mortality.
El objetivo principal de este estudio es determinar si la Unidad de Cirugía Hepato-Bilio-Pancreática y Trasplante Hepático del Hospital Universitario de Badajoz cumple los estándares de calidad exigidos por la comunidad científica a los centros de referencia de cirugía pancreática (CP) en términos de resultados perioperatorios. Los objetivos secundarios consisten en comparar las diferentes técnicas de CP realizadas en función de la morbimortalidad postoperatoria precoz y analizar el impacto de las resecciones extendidas en dichos términos.
MétodoEstudio descriptivo, retrospectivo y unicéntrico, correspondiente al periodo 2006−2019. Se compararon los resultados obtenidos con los estándares de calidad propuestos por Bassi et al. y Sabater et al., exigidos a los centros de referencia en cirugía pancreática. La muestra se dividió según técnica quirúrgica y se compararon en términos de morbimortalidad postoperatoria precoz, estudiando el impacto de las resecciones vasculares y viscerales extendidas. Se incluyeron todos los pacientes sometidos a cirugía pancreática en nuestra unidad por patología pancreática, maligna y benigna, desde que ésta se implementó como centro de referencia. Se excluyeron las realizadas de urgencia.
ResultadosSe analizaron 631 pacientes. Los valores obtenidos en los estándares de calidad se encuentran en rango. La cirugía más frecuente fue duodenopancreatectomía cefálica, la cual asoció mayor tasa de morbimortalidad perioperatoria (P ≤ ,05). Las resecciones vasculares añadidas impactaron en el grupo de duodenopancreatectomía cefálica asociando mayor estancia media (P = ,01) y mayor tasa de reinteEVRnción (P = ,02).
ConclusionesLa experiencia acumulada permite cumplir con los estándares de calidad exigidos, así como realizar resecciones extendidas a la pancreatectomía con buenos resultados en términos de morbimortalidad postoperatoria.
Although postoperative mortality is currently around 5% in high-volume pancreatic surgery (PS) centers1,2, morbidity continues to be high, reaching rates up to 60%3,4. Regardless of the general surgical complications associated with any procedure, PS presents specific complications: delayed gastric emptying (DGE), pancreatic fistula (PF) and post-pancreatectomy hemorrhage (PPH), with associated incidences of 19%–7%5, 2%–20%6 and 1%–8%7, respectively.
Sabater et al.8 established the required quality standards for oncological PS, proposing rates of resectability >58%, morbidity <73% and mortality <10%, with associated rates of biliary fistula (BF) <14%, PF< 29%, PPH< 21% and reoperation <20%, with a mean stay <21 days.
The three most common types of PS are pancreaticoduodenectomy (PD), distal pancreatectomy (DP), and total pancreatectomy (TP). The postoperative morbidity rate associated with PD is 50%–60%, with a mortality rate of 5%9; TP associates a morbidity of 59.3% and mortality 2.1%10; DP is the group with the lowest morbidity and mortality rates, at 18% and 0.6%11, respectively.
The main objective of this study is to determine whether the Hepato-Biliary-Pancreatic Surgery and Liver Transplantation Unit of the Hospital Universitario de Badajoz meets the quality standards required by the medical community for PS reference centers in terms of perioperative results. The secondary objectives are to compare the different PS techniques performed in terms of early postoperative morbidity and mortality rates and to analyze the impact of extended resections in these terms.
MethodsWe have conducted a descriptive, retrospective, single-center study from 2006 to 2019. The study included all patients who underwent surgery for pancreatic pathology, either malignant or benign, by our unit, which is a regional pancreatic surgery referral center. Emergency surgery was excluded.
Diagnosis and presurgical managementIn our hospital, pancreatic lesions are studied with imaging tests and laboratory studies. The first test ordered is a computed tomography (CT) scan. Magnetic resonance imaging (MRI) is used in cases of diagnostic doubt or CT contraindication, positron emission tomography (PET) is use for suspected distant metastasis, and endoscopic ultrasound if there is a need for biopsy or better characterization of the vascular involvement. Laboratory studies do not include specific pancreatic function parameters; complete blood count, general biochemistry and liver profile, coagulation and tumor markers are requested. In case of suspected neuroendocrine tumor (NET), specific tests are requested for each of them.
After imaging tests, the pancreatic ductal adenocarcinoma (PDAC) was classified according to MD Anderson Cancer Center criteria12 as resectable, borderline resectable, or unresectable. All PDAC in which surgery was indicated without neoadjuvant therapy were considered resectable; those in which neoadjuvant therapy was indicated were considered borderline resectable.
The resectability rate was calculated by whether patients had undergone laparotomy.
Our group indicates preoperative biliary drainage (PBD) in the following cases:
- •
Hyperbilirubinemia + biliary symptoms
- •
Hyperbilirubinemia + waiting time to surgery ≥15 days
- •
Hyperbilirubinemia + need for neoadjuvant treatment
Hyperbilirubinemia was established as a clinical situation in which the patient presents laboratory values of direct bilirubin greater than 0.3 mg/dL and/or total bilirubin greater than 1.2 mg/dL.
Neoadjuvant treatment was indicated in those cases in which the tumor was classified as borderline at diagnosis12,13. The scheme used was folfirinox ± radiotherapy (RT), or gemcitabine + paclitaxel ± RT (systemic therapy: capecitabine or 5-FU in continuous infusion) depending on patient status and evolution14. RT was administered after chemotherapy cycles (2–6), generally at 36 Gy in 2.4 Gy fractions.
Surgical technique and variables- •
PD15. We perform four different pancreatic anastomosis techniques:
In all of these, the use of a pancreatic stent was not standardized, and this was an intraoperative decision made by each surgeon. When stents were placed, they were carried out as a ‘lost’ stent20.
The sample was divided into three groups, according to surgical technique: PD, DP and TP.
Two types of extended resections were established:
- •
Extended visceral resection (EVR). Resection of an extra organ in the PD group, and splenectomy in the DP and TP groups.
- •
Extended vascular resection (EvaR). Resection of an arterial vascular structure (superior mesenteric artery, celiac trunk and/or hepatic artery) and/or venous structure (portal vein and/or superior mesenteric) in any of the groups.
The consistency of the pancreas was classified based on the macroscopic appearance and the feel of the pancreas as hard24 or soft25. On the other hand, the Wirsung size, which was measured using a surgical ruler, was classified as narrow if the diameter was <3 mm or wide if it was ≥3 mm26.
In our hospital, the laparoscopic approach is only indicated in cases of DP ± splenectomy, without added vascular or visceral involvement. Our experience is limited because this approach was added to our arsenal in 2017.
Morbidity and mortalityWe have studied the general rates of medical complications (MC) and surgical complications (SC), describing the most frequent, classifying them according to Clavien-Dindo27 and explaining their management. After this, the specific complications of pancreatic surgery were studied. We have used the definitions and concepts proposed by the International Study Group on Pancreatic Fistula (ISGPF) definition and by the International Study Group of Pancreatic Surgery (SGPS)28. The rates of SC, MC, PPH, reoperation and early mortality, as well as the mean hospital stay, have been calculated based on the resective surgeries. The calculation of the PF rate was only carried out in terms of overall PD and DP, while DGE was calculated in terms of total number of PD.
The mortality rate was calculated for the first 90 postoperative days. We also calculated the impact of extended resections on these morbidity and mortality variables.
Pathological protocolThe pathological protocol was based on the classification system proposed by the International Union Against Cancer29 and the study of surgical resection margins from the specimens of pancreatic adenocarcinomas in the Royal College study30.
Statistical studyThe statistical study was carried out with the IBM SPSS Statistics® V22.2 program. Nominal variables are expressed as number and percentages (%) and quantitative variables as median and interquartile range, in the corresponding units.
After confirming the normal distribution of the sample using the Shapiro Wilk test, the groups were established, and the morbidity and mortality rates were compared using the chi-squared test in the nominal variables, the Student’s t test in the continuous variables, when two groups were studied, as well as the ANOVA test when three groups were studied. Statistical significance was established with a P value ≤.05. The multivariate study was performed using logistic regression with those variables that presented P ≤ .2 in the univariate study. Statistical significance was maintained for a value of P ≤ .05 and the risk was estimated using risk coefficients B and EXP (B).
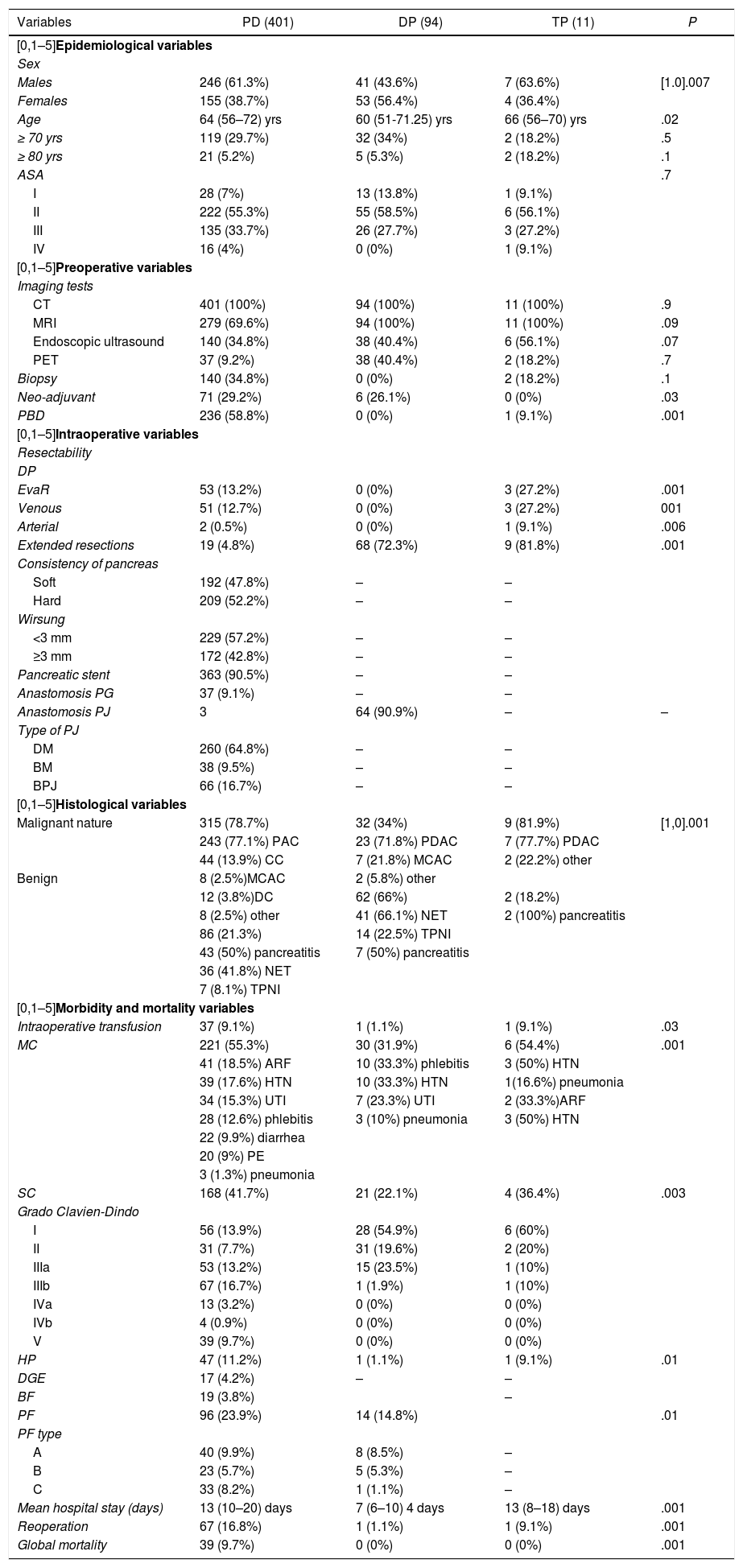
ResultsIn the study period, 631 PS were performed, with an associated resectability rate of 80.2%. The most frequent PS was PD, followed by DP and TP (Table 1).
Descriptive study by surgery group.
| Variables | PD (401) | DP (94) | TP (11) | P |
|---|---|---|---|---|
| [0,1–5]Epidemiological variables | ||||
| Sex | ||||
| Males | 246 (61.3%) | 41 (43.6%) | 7 (63.6%) | [1.0].007 |
| Females | 155 (38.7%) | 53 (56.4%) | 4 (36.4%) | |
| Age | 64 (56–72) yrs | 60 (51-71.25) yrs | 66 (56–70) yrs | .02 |
| ≥ 70 yrs | 119 (29.7%) | 32 (34%) | 2 (18.2%) | .5 |
| ≥ 80 yrs | 21 (5.2%) | 5 (5.3%) | 2 (18.2%) | .1 |
| ASA | .7 | |||
| I | 28 (7%) | 13 (13.8%) | 1 (9.1%) | |
| II | 222 (55.3%) | 55 (58.5%) | 6 (56.1%) | |
| III | 135 (33.7%) | 26 (27.7%) | 3 (27.2%) | |
| IV | 16 (4%) | 0 (0%) | 1 (9.1%) | |
| [0,1–5]Preoperative variables | ||||
| Imaging tests | ||||
| CT | 401 (100%) | 94 (100%) | 11 (100%) | .9 |
| MRI | 279 (69.6%) | 94 (100%) | 11 (100%) | .09 |
| Endoscopic ultrasound | 140 (34.8%) | 38 (40.4%) | 6 (56.1%) | .07 |
| PET | 37 (9.2%) | 38 (40.4%) | 2 (18.2%) | .7 |
| Biopsy | 140 (34.8%) | 0 (0%) | 2 (18.2%) | .1 |
| Neo-adjuvant | 71 (29.2%) | 6 (26.1%) | 0 (0%) | .03 |
| PBD | 236 (58.8%) | 0 (0%) | 1 (9.1%) | .001 |
| [0,1–5]Intraoperative variables | ||||
| Resectability | ||||
| DP | ||||
| EvaR | 53 (13.2%) | 0 (0%) | 3 (27.2%) | .001 |
| Venous | 51 (12.7%) | 0 (0%) | 3 (27.2%) | 001 |
| Arterial | 2 (0.5%) | 0 (0%) | 1 (9.1%) | .006 |
| Extended resections | 19 (4.8%) | 68 (72.3%) | 9 (81.8%) | .001 |
| Consistency of pancreas | ||||
| Soft | 192 (47.8%) | – | – | |
| Hard | 209 (52.2%) | – | – | |
| Wirsung | ||||
| <3 mm | 229 (57.2%) | – | – | |
| ≥3 mm | 172 (42.8%) | – | – | |
| Pancreatic stent | 363 (90.5%) | – | – | |
| Anastomosis PG | 37 (9.1%) | – | – | |
| Anastomosis PJ | 3 | 64 (90.9%) | – | – |
| Type of PJ | ||||
| DM | 260 (64.8%) | – | – | |
| BM | 38 (9.5%) | – | – | |
| BPJ | 66 (16.7%) | – | – | |
| [0,1–5]Histological variables | ||||
| Malignant nature | 315 (78.7%) | 32 (34%) | 9 (81.9%) | [1,0].001 |
| 243 (77.1%) PAC | 23 (71.8%) PDAC | 7 (77.7%) PDAC | ||
| 44 (13.9%) CC | 7 (21.8%) MCAC | 2 (22.2%) other | ||
| Benign | 8 (2.5%)MCAC | 2 (5.8%) other | ||
| 12 (3.8%)DC | 62 (66%) | 2 (18.2%) | ||
| 8 (2.5%) other | 41 (66.1%) NET | 2 (100%) pancreatitis | ||
| 86 (21.3%) | 14 (22.5%) TPNI | |||
| 43 (50%) pancreatitis | 7 (50%) pancreatitis | |||
| 36 (41.8%) NET | ||||
| 7 (8.1%) TPNI | ||||
| [0,1–5]Morbidity and mortality variables | ||||
| Intraoperative transfusion | 37 (9.1%) | 1 (1.1%) | 1 (9.1%) | .03 |
| MC | 221 (55.3%) | 30 (31.9%) | 6 (54.4%) | .001 |
| 41 (18.5%) ARF | 10 (33.3%) phlebitis | 3 (50%) HTN | ||
| 39 (17.6%) HTN | 10 (33.3%) HTN | 1(16.6%) pneumonia | ||
| 34 (15.3%) UTI | 7 (23.3%) UTI | 2 (33.3%)ARF | ||
| 28 (12.6%) phlebitis | 3 (10%) pneumonia | 3 (50%) HTN | ||
| 22 (9.9%) diarrhea | ||||
| 20 (9%) PE | ||||
| 3 (1.3%) pneumonia | ||||
| SC | 168 (41.7%) | 21 (22.1%) | 4 (36.4%) | .003 |
| Grado Clavien-Dindo | ||||
| I | 56 (13.9%) | 28 (54.9%) | 6 (60%) | |
| II | 31 (7.7%) | 31 (19.6%) | 2 (20%) | |
| IIIa | 53 (13.2%) | 15 (23.5%) | 1 (10%) | |
| IIIb | 67 (16.7%) | 1 (1.9%) | 1 (10%) | |
| IVa | 13 (3.2%) | 0 (0%) | 0 (0%) | |
| IVb | 4 (0.9%) | 0 (0%) | 0 (0%) | |
| V | 39 (9.7%) | 0 (0%) | 0 (0%) | |
| HP | 47 (11.2%) | 1 (1.1%) | 1 (9.1%) | .01 |
| DGE | 17 (4.2%) | – | – | |
| BF | 19 (3.8%) | – | ||
| PF | 96 (23.9%) | 14 (14.8%) | .01 | |
| PF type | ||||
| A | 40 (9.9%) | 8 (8.5%) | – | |
| B | 23 (5.7%) | 5 (5.3%) | – | |
| C | 33 (8.2%) | 1 (1.1%) | – | |
| Mean hospital stay (days) | 13 (10–20) days | 7 (6–10) 4 days | 13 (8–18) days | .001 |
| Reoperation | 67 (16.8%) | 1 (1.1%) | 1 (9.1%) | .001 |
| Global mortality | 39 (9.7%) | 0 (0%) | 0 (0%) | .001 |
Nominal variables expressed as number (n) and percentage (%).
Quantitative variables expressed as median and interquartile range.
PDAC: Pancreatic ductal adenocarcinoma; ASA: American Society Anesthesiology; BM: Blumgart; MCAC: mucinous cystadenocarcinoma; CC: cholangiocarcinoma; DC: duodenal cancer; MC: medical complication; SC: surgical complication; PBD: preoperative biliary drainage; DM: ductomucosa; PB: palliative bypass; PD: pancreaticoduodenectomy; BPJ: ‘binding’ pancreaticojejunostomy (BPJ); BF: biliary fistula; PF: pancreatic fistula; PPH: post-pancreatectomy hemorrhage; HTN: hypertension; ARF: acute respiratory failure; UTI: urinary tract infection; DP: distal pancreatectomy; PET: positron emission tomography; PG: pancreatogastrostomy; TP: total pancreatectomy; PJ: pancreatojejunostomy; MRI: magnetic resonance imaging; EvaR: extended vascular resection; EVR: extended visceral resection; DGE: delayed gastric emptying; CT: computed tomography; PE: pulmonary embolism; NET: neuroendocrine tumor; IPMN: intraductal papillary mucinous neoplasm.
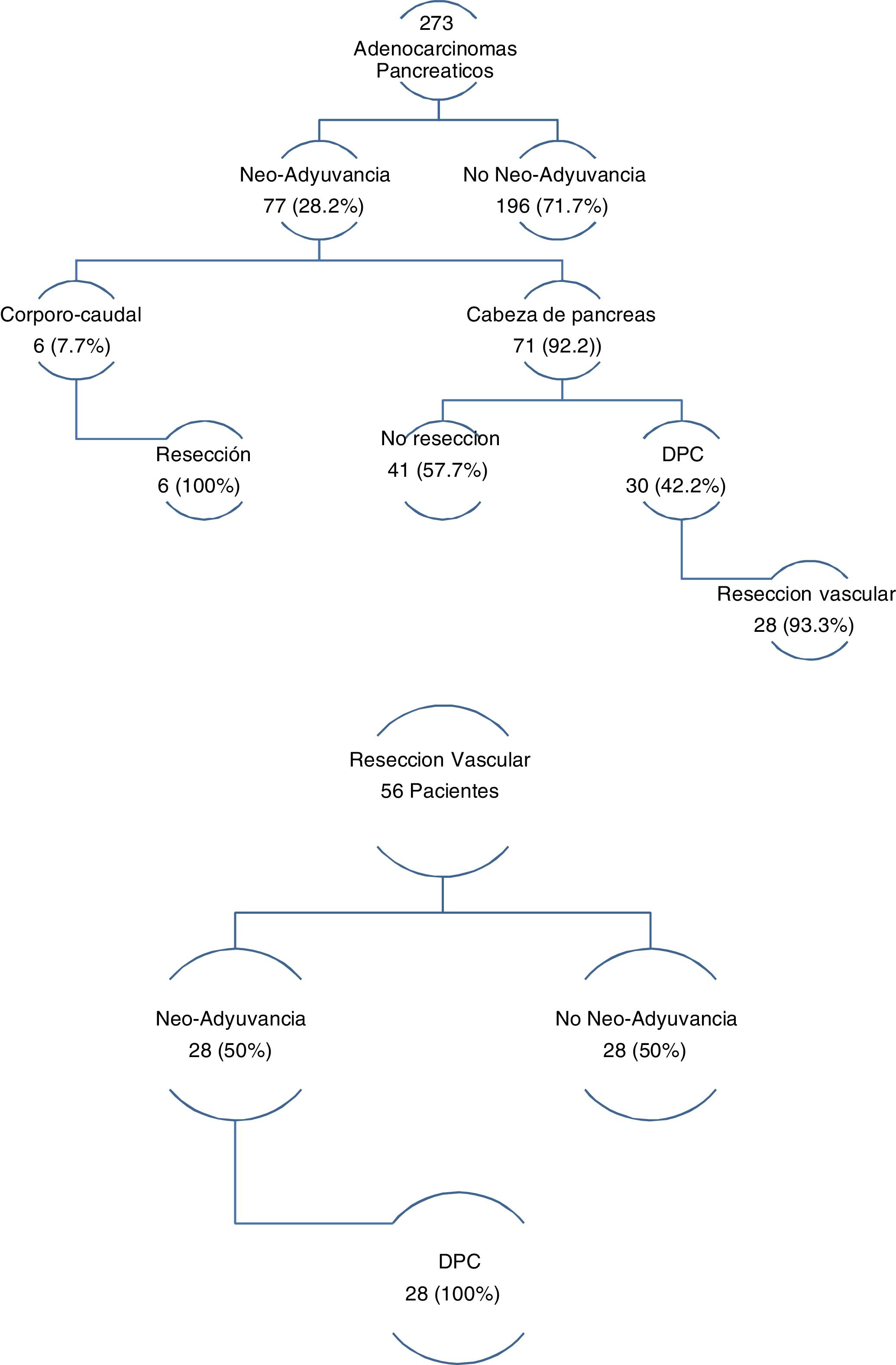
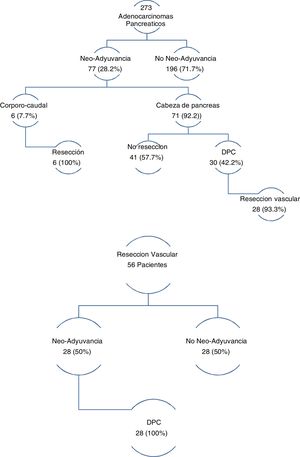
The three groups are homogeneous in terms of epidemiological and preoperative variables, except for patient sex (P = .007) and the rates of PBD (P = .03) and neoadjuvant therapy (P = .001), both of which were higher in the PD group (Table 1). Surgical salvage rates after neoadjuvant treatment were 100% in the DP group and 42.2% in the PD group (Fig. 1).
The most frequent malignant and benign pathologies were PDAC and NET, respectively. Most of the patients treated with PD presented surgical indication in the context of a malignant disease (P = .001) (Table 1).
The PD group presented a higher rate of general EvaR (P = .001), as well as higher rates of arterial (P = .006) and venous (P = .001) EvaR. Regarding EVR, the DP group had the highest rate (P = .001) (Table 1). The PD group rate was the lowest of the three, and the ascending colon was the most frequently resected organ.
In the group of patients with EvaR, the neoadjuvant rate was 50%, and surgical salvage after neoadjuvant treatment was 100% (Fig. 1).
PD presented the highest rates in the morbidity and mortality variables, with statistically significant differences for all (Table 1).
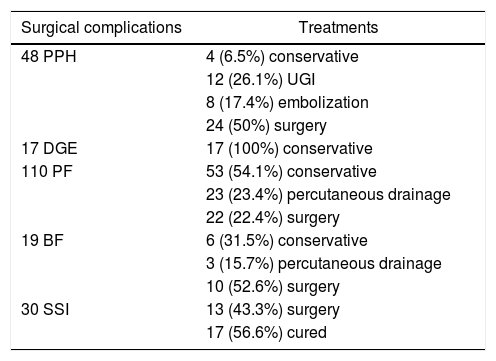
50% of the PPH and 22.4% of the PF required surgical treatment. As for DGE, treatment was conservative in all cases (Table 2).
Treatment of surgical complications.
| Surgical complications | Treatments |
|---|---|
| 48 PPH | 4 (6.5%) conservative |
| 12 (26.1%) UGI | |
| 8 (17.4%) embolization | |
| 24 (50%) surgery | |
| 17 DGE | 17 (100%) conservative |
| 110 PF | 53 (54.1%) conservative |
| 23 (23.4%) percutaneous drainage | |
| 22 (22.4%) surgery | |
| 19 BF | 6 (31.5%) conservative |
| 3 (15.7%) percutaneous drainage | |
| 10 (52.6%) surgery | |
| 30 SSI | 13 (43.3%) surgery |
| 17 (56.6%) cured |
UGI: upper GI endoscopy; BF: biliary fistula; PF: pancreatic fistula; PPH: post-pancreatectomy hemorrhage; SSI: surgical site infection; DGE: delayed gastric emptying.
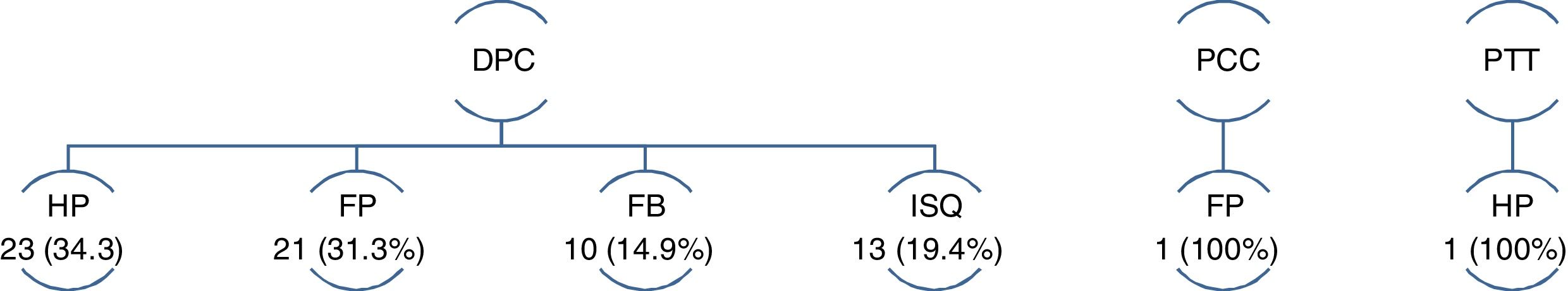
Out of the 69 reoperations, 97.1% correspond to the PD group (P = .001). Only one patient in the DP group and another in the TP group required reoperation, the first in the context of type C PF and the second for PPH (Fig. 2).
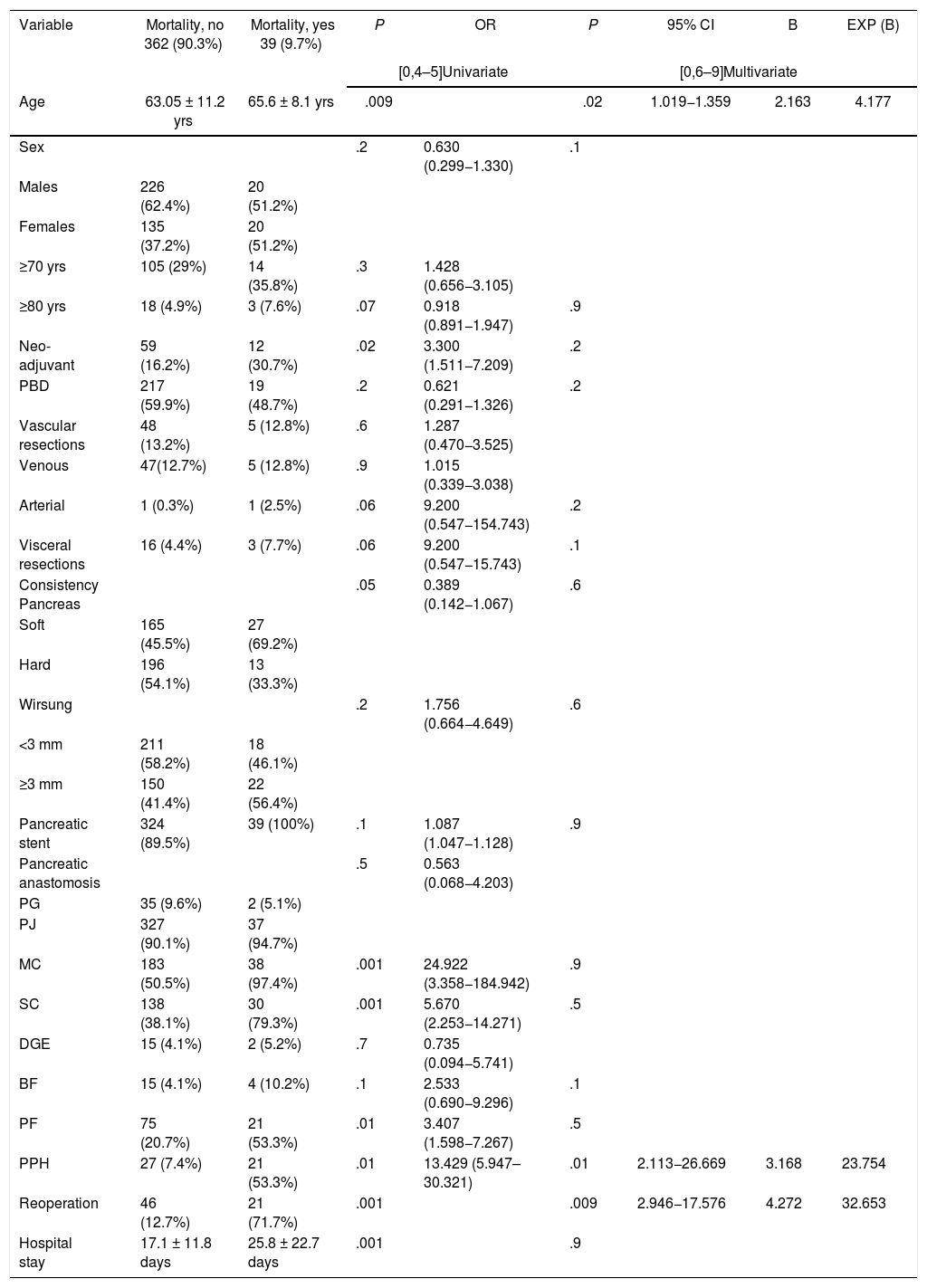
All mortality in the sample occurred in patients treated with PD (P = 0.001). The multivariate study showed that age (P = .02), reoperation (P = .01) and PPH (P = .009) were risk factors for mortality in the series studied (Table 3).
Uni- and multivariate study of the risk factors for mortality associated with pancreaticoduodenectomy.
| Variable | Mortality, no 362 (90.3%) | Mortality, yes 39 (9.7%) | P | OR | P | 95% CI | B | EXP (B) |
|---|---|---|---|---|---|---|---|---|
| [0,4–5]Univariate | [0,6–9]Multivariate | |||||||
| Age | 63.05 ± 11.2 yrs | 65.6 ± 8.1 yrs | .009 | .02 | 1.019−1.359 | 2.163 | 4.177 | |
| Sex | .2 | 0.630 (0.299−1.330) | .1 | |||||
| Males | 226 (62.4%) | 20 (51.2%) | ||||||
| Females | 135 (37.2%) | 20 (51.2%) | ||||||
| ≥70 yrs | 105 (29%) | 14 (35.8%) | .3 | 1.428 (0.656−3.105) | ||||
| ≥80 yrs | 18 (4.9%) | 3 (7.6%) | .07 | 0.918 (0.891−1.947) | .9 | |||
| Neo-adjuvant | 59 (16.2%) | 12 (30.7%) | .02 | 3.300 (1.511−7.209) | .2 | |||
| PBD | 217 (59.9%) | 19 (48.7%) | .2 | 0.621 (0.291−1.326) | .2 | |||
| Vascular resections | 48 (13.2%) | 5 (12.8%) | .6 | 1.287 (0.470−3.525) | ||||
| Venous | 47(12.7%) | 5 (12.8%) | .9 | 1.015 (0.339−3.038) | ||||
| Arterial | 1 (0.3%) | 1 (2.5%) | .06 | 9.200 (0.547−154.743) | .2 | |||
| Visceral resections | 16 (4.4%) | 3 (7.7%) | .06 | 9.200 (0.547−15.743) | .1 | |||
| Consistency Pancreas | .05 | 0.389 (0.142−1.067) | .6 | |||||
| Soft | 165 (45.5%) | 27 (69.2%) | ||||||
| Hard | 196 (54.1%) | 13 (33.3%) | ||||||
| Wirsung | .2 | 1.756 (0.664−4.649) | .6 | |||||
| <3 mm | 211 (58.2%) | 18 (46.1%) | ||||||
| ≥3 mm | 150 (41.4%) | 22 (56.4%) | ||||||
| Pancreatic stent | 324 (89.5%) | 39 (100%) | .1 | 1.087 (1.047−1.128) | .9 | |||
| Pancreatic anastomosis | .5 | 0.563 (0.068−4.203) | ||||||
| PG | 35 (9.6%) | 2 (5.1%) | ||||||
| PJ | 327 (90.1%) | 37 (94.7%) | ||||||
| MC | 183 (50.5%) | 38 (97.4%) | .001 | 24.922 (3.358−184.942) | .9 | |||
| SC | 138 (38.1%) | 30 (79.3%) | .001 | 5.670 (2.253−14.271) | .5 | |||
| DGE | 15 (4.1%) | 2 (5.2%) | .7 | 0.735 (0.094−5.741) | ||||
| BF | 15 (4.1%) | 4 (10.2%) | .1 | 2.533 (0.690−9.296) | .1 | |||
| PF | 75 (20.7%) | 21 (53.3%) | .01 | 3.407 (1.598−7.267) | .5 | |||
| PPH | 27 (7.4%) | 21 (53.3%) | .01 | 13.429 (5.947–30.321) | .01 | 2.113−26.669 | 3.168 | 23.754 |
| Reoperation | 46 (12.7%) | 21 (71.7%) | .001 | .009 | 2.946−17.576 | 4.272 | 32.653 | |
| Hospital stay | 17.1 ± 11.8 days | 25.8 ± 22.7 days | .001 | .9 | ||||
MC: medical complication; SC: surgical complication; PBD: preoperative biliary drainage; BF: biliary fistula; PF: pancreatic fistula; PPH: post-pancreatectomy hemorrhage; PG: pancreatogastrostomy; PJ: pancreatojejunostomy; DGE: delayed gastric emptying.
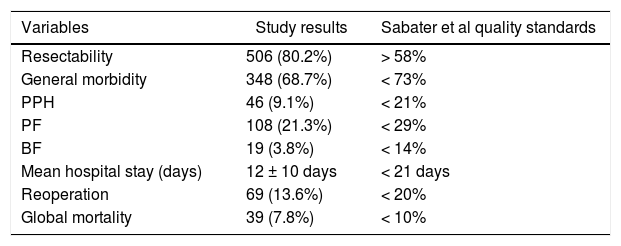
When we analyzed the global rates and comparing them with those required by the scientific community in the context of oncological PS, we verified that the values obtained were lower than those required in our country (Table 4).
Global values of the sample vs Sabater et al quality standards.
| Variables | Study results | Sabater et al quality standards |
|---|---|---|
| Resectability | 506 (80.2%) | > 58% |
| General morbidity | 348 (68.7%) | < 73% |
| PPH | 46 (9.1%) | < 21% |
| PF | 108 (21.3%) | < 29% |
| BF | 19 (3.8%) | < 14% |
| Mean hospital stay (days) | 12 ± 10 days | < 21 days |
| Reoperation | 69 (13.6%) | < 20% |
| Global mortality | 39 (7.8%) | < 10% |
Nominal variables are expressed as number (n) and percentage (%).
Quantitative variables are expressed as median and interquartile range.
BF: biliary fistula; PF: pancreatic fistula; PPH: post-pancreatectomy hemorrhage.
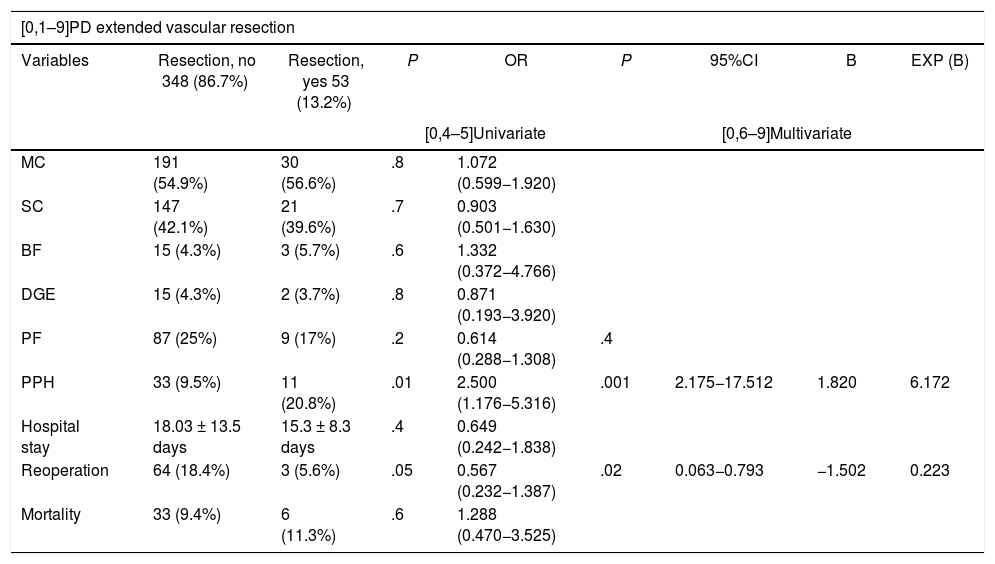
Furthermore, when we analyzed the effect on morbidity and mortality variables of extended resections in the different groups, we observed that EVR did not affect any of them (Table 5). Meanwhile, EvaR did affect the mean hospital stay (P = .01) and reoperation rate (P = .02) in the PD group (Table 5).
Uni- and multivariate study of the impact of extended resections a la duodeno-pancreatectomía cefálica on morbidity and mortality variables.
| [0,1–9]PD extended vascular resection | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Resection, no 348 (86.7%) | Resection, yes 53 (13.2%) | P | OR | P | 95%CI | B | EXP (B) |
| [0,4–5]Univariate | [0,6–9]Multivariate | |||||||
| MC | 191 (54.9%) | 30 (56.6%) | .8 | 1.072 (0.599−1.920) | ||||
| SC | 147 (42.1%) | 21 (39.6%) | .7 | 0.903 (0.501−1.630) | ||||
| BF | 15 (4.3%) | 3 (5.7%) | .6 | 1.332 (0.372−4.766) | ||||
| DGE | 15 (4.3%) | 2 (3.7%) | .8 | 0.871 (0.193−3.920) | ||||
| PF | 87 (25%) | 9 (17%) | .2 | 0.614 (0.288−1.308) | .4 | |||
| PPH | 33 (9.5%) | 11 (20.8%) | .01 | 2.500 (1.176−5.316) | .001 | 2.175−17.512 | 1.820 | 6.172 |
| Hospital stay | 18.03 ± 13.5 days | 15.3 ± 8.3 days | .4 | 0.649 (0.242−1.838) | ||||
| Reoperation | 64 (18.4%) | 3 (5.6%) | .05 | 0.567 (0.232−1.387) | .02 | 0.063−0.793 | −1.502 | 0.223 |
| Mortality | 33 (9.4%) | 6 (11.3%) | .6 | 1.288 (0.470−3.525) | ||||
| [0,1–9]PD extended visceral resection | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Resection, no 382 (95.2%) | Resection, yes 19 (4.8%) | P | OR | P | 95%CI | B | EXP (B) |
| [0,4–5]Univariate | [0,6–9]Multivariate | |||||||
| MC | 210 (55%) | 11 (56.6%) | .8 | 1.126 (0.443−2.862) | ||||
| SC | 159 (41.6%) | 9 (47.4%) | .6 | 1.270 (0.505−3.198) | ||||
| BF | 18 (4.7%) | 1 (5.3%) | .8 | 1.193 (0.150−9.468) | ||||
| DGE | 16 (4.2%) | 1 (5.3%) | .8 | 1.271 (0.160−10.121) | ||||
| PF | 91 (23.8%) | 5 (26.3%) | .8 | 1.142 (0.400−3.257) | ||||
| PPH | 44 (11.7%) | 1 (10.5%) | .3 | 0.438 (0.057−3.363) | ||||
| Estancia | 17.6 ± 13.1 days | 18.6 ± 12.7 days | .4 | 0.649 (0.242−1.838) | ||||
| Reintervention | 64 (16.7%) | 3 (15.8%) | .8 | 0.885 (0.250−3.126) | ||||
| Mortality | 36 (9.3%) | 3 (11.5%) | .6 | 1.429 (0.314−6.499) | ||||
| [0,1–9]DP extended visceral resection | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Resection, no 26 (27.7%) | Resection, yes 68 (72.3%) | P | OR | P | 95%CI | B | EXP (B) |
| [0,4–5]Univariate | [0,6–9]Multivariate | |||||||
| MC | 7 (26.9%) | 23 (33.8%) | .4 | 1.494 (0.551−4.050) | ||||
| SC | 3 (11.5%) | 18 (26.4%) | .07 | 3.167 (0.852−11.766) | .4 | |||
| PF | 1 (3.8%) | 13 (19.1%) | .09 | 5.107 (0.626−41.676) | .6 | |||
| PPH | 1 (3.8%) | 0 (0%) | .1 | 0.280 (0.202−1.387) | 1 | |||
| Stay | 7.6 ± 2.1days | 10.7 ± 11.4 days | .04 | 1.572 (1.234−2.573) | .2 | |||
| Reoperation | 1 (3.8%) | 0 (0%) | .1 | 0.280 (0.202−1.387) | .8 | |||
| Mortality | 0 (0%) | 0 (0%) | ||||||
| [0,1–9]TP extended vascular resection | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Resection, no 8 (72.8%) | Resection, yes 3 (27.2%) | P | OR | P | 95%CI | B | EXP (B) |
| [0,4–5]Univariate | [0,6–9]Multivariate | |||||||
| MC | 4 (50%) | 2 (66.6%) | .6 | 2.000(0.125−31.975) | ||||
| SC | 1 (12.5%) | 3 (100%) | .07 | 14.000 (0.579−338.778) | .2 | |||
| PPH | 1 (12.5%) | 0 (0%) | .5 | 0.875 (0.673−1.137) | ||||
| Stay | 12.2 ± 4.6 days | 21.5 ± 19.1 days | .001 | 2.357 (1.953−4.455) | .9 | |||
| Reoperation | 1 (12.5%) | 0 (0%) | .5 | 0.875 (0.673−1.137) | ||||
| Mortality | 0 (0%) | 0 (0%) | ||||||
| [0,1–9]TP extended visceral resection | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Resection, no 2 (18.2%) | Resection, yes 9 (81.8%) | P | OR | P | 95%CI | B | EXP (B) |
| [0,4–5]Univariate | [0,6–9]Multivariate | |||||||
| MC | 1 (50%) | 5 (55.6%) | .8 | 1.250 (0.058−26.869) | ||||
| SC | 1 (50%) | 3 (33.3%) | .4 | 0.286 (0.012−6.914) | ||||
| PPH | 0 (0%) | 1 (11.1%) | .6 | 1.125 (0.893−1.417) | ||||
| Hospital stay | 10.5 ± 4.9 days | 14.6 ± 8.6 days | .5 | 1.938 (1.695−3.848) | ||||
| Reoperation | 0 (0%) | 1 (11.1%) | .6 | 1.125 (0.893−1.417) | ||||
| Mortality | 0 (0%) | 0 (0%) | ||||||
MC: medical complication; SC: surgical complication; PD: pancreaticoduodenectomy; BF: biliary fistula; PF: pancreatic fistula; PPH: post-pancreatectomy hemorrhage; DP: distal pancreatectomy; TP: total pancreatectomy; DGE: delayed gastric emptying.
In 1999, Birkmeyer et al. reported post-PD mortality rates with the volumes of surgery at hospital centers, suggesting that the centralization of pancreatic surgery could impact patient survival31. Two years later, in 2001, the Institute of Medicine (IM) defined the concept of quality in the medical field, which is comprised of six elements: safety, effectiveness, timeliness, efficiency, centralization and equity32. Subsequently, in 2004, the Leapfrog group, with the aim to improve quality, proposed an evidence-based referral center system, and PS was identified as a group of procedures that would benefit from this system33. In this context, the relationship between PS results, the volume of the medical center and the experience of the surgeon was becoming established among the scientific community, thanks to various studies in this regard, including meta-analyses33–42. Little by little, the concept of a referral centers has become established in all healthcare spheres.
Currently, in order to earn PS accreditation, it is required to have performed 50 PS in three years, 30 of which must be PD, and the mortality rate must be less than 5%. A PS center is considered a high-volume unit if between 50 and 100 PS are carried out per year; very high volume centers perform more than 10043. Thus, and based on the concept proposed by Bassi et al., the demand increased and the main quality indicators of PS centers were published in 2016, which are divided into three groups: hospital, procedure, and results44, since mortality and volume of PS do not determine quality care per se. Sabater et al. went one step further and proposed quality standards required in Spain in the field of oncological PS8.
Our hospital complies with all the quality indicators proposed by Bassi et al.44 We have professionals from different specialties who exclusively work with pancreatic pathology, including interventional radiology and endoscopy, multidisciplinary committees, and a specific database with the required variables. Of all the indicators related to results, the latest to be developed in our unit is the ERA protocol45. If we focus on the required result-based standards, we present a higher mortality rate than that proposed in the definition of PS referral center (5% vs 7.8%)31. In terms of number of PS, we perform an average of 48.5 per year and have strictly complied with the standard number of surgeries of more than 50 since 201043. Focusing on the standards proposed by Sabater et al.8, which are specific for oncological PS, we definitely comply with all the requirements (Table 4).
The PD group had a higher associated rate of PBD (58.8% vs 0% vs 9.1%; P = .001). The overall rate for the sample was 37.5% and for post-drainage complications 22.3% (Table 1). It is a controversial procedure46–48, and there are studies that link PBD with higher rates of preoperative, early and late postoperative complications, DGE and surgical site infection (SSI)49. In 2018, a published meta-analysis concluded that PBD does not offer advantages in terms of postoperative complications or discomfort and that there is no evidence to determine the best technical option50.
PD also presented a higher neoadjuvant rate (29.2% vs 26.1% vs 0%; P = .03) (Table 1 and Fig. 1). By groups, the salvage rate was 42.2% in PD and 100% in DP. The overall rate for the sample was 28.2%, with a salvage rate of 46.7%. In PS, neoadjuvant treatment presented two main problems: lack of randomized phase III clinical trials, and applicability of RECIST criteria in the re-staging of borderline pancreatic cancer. Despite this, there are many meta-analyses and clinical guidelines that propose neoadjuvant treatment in pancreatic cancer with recommendations51–54. Regarding restaging, radiological response rates do not correspond to surgical salvage rates, which range from 69% to 93%55–57, higher than those published in this article. Laparoscopy has an important indication in the re-staging of borderline pancreatic cancer as it avoids morbidity that may delay the start of adjuvant treatment, while also increasing salvage rates.
We present an EvaR rate of 13.2% in the PD group and 27.2% in the TP group (Table 1). According to the results published in a systematic review from 201958, the venous resection rate ranges from 6.1% to 65.1%, with an impact on mortality, reoperation and PPH rates. On the other hand, arterial resections increase morbidity and mortality rates globally compared to standard resection, either with or without associated venous resection59,60. In expert hands, the associated mortality rate is 11.8% and morbidity 53.6%61. In our sample, EvaR had an impact on the mean hospital stay and reoperation rate of the PD group (Table 5).
As for EVR, we present rates of 4.8% in the PD group, 72.3% in DP and 81.8% in TP. A 2018 meta-analysis of 713 pancreatectomies with an EVR rate of 20% concluded that this type of resection does not associate statistically significant differences in terms of postoperative morbidity and mortality62. That same year, another study was published comparing DP with/without EVR, which also found no association between said resection and an increase in postoperative morbidity and mortality rates63. In our sample, EVR did not impact any morbidity and mortality variable in any of the groups studied (Table 5). The most frequently resected organs were the right colon in the PD group and the spleen in the DP and TP groups.
The PD group had the highest rates of postoperative morbidity and mortality, with statistical significance in all (Table 1). When we compared our results with those of the Spanish Lera-Tricas et al. group64, Clavien-Dindo grade iiib was the most frequent at a higher rate (16.7% vs 5.7%) and the PF rate was similar (23.9% vs 22.9%), at the expense of type A (9.9%), and a higher reoperation rate (16.8% vs 12.3%).
For its part, the DP group had the lowest rates of postoperative morbidity and mortality (Table 1). An article published in 2017 with 2026 DP reported reoperation and mortality rates of 5% and 0.6%, respectively, and a mean hospital stay of five days65. One year later, an article that included 157 DP presented morbidity rates of 18%, PF grades B/C of 8%, reoperation 3%, and early mortality 0.6%, as well as Clavien-Dindo grades III and IV as the most frequent66. All values were higher than those obtained in this article.
Lastly, the TP group presented an associated morbidity rate of 36.4%, a reoperation rate of 9.1%, and zero mortality (Table 1). These data are in range with what has been previously published67.
The centralization of pancreatic surgery in referral centers achieves excellent outcomes in terms of quality of care for patients with pancreatic surgical pathology. The accumulated experience from centralization allows for extended pancreatectomies to be performed safely and effectively. The design and development of accreditation programs in pancreatic surgery will further improve the results of these procedures.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Castillo Tuñón JM, Valle Rodas ME, Botello Martínez F, Rojas Holguín A, López Guerra D, Santos Naharro J, et al. Implementación de un centro de referencia regional en cirugía pancreática. Experiencia tras 631 procedimientos. Cir Esp. 2021;99:745–756.