There is controversy regarding the ideal pancreaticojejunostomy technique after pancreaticoduodenectomy. Many authors consider the external Wirsung stenting technique to be associated with a low incidence of fistula, morbidity and mortality. We analyse our experience with this technique.
Patients and methodsA retrospective analysis of the morbidity and mortality of a series of 80 consecutive patients who had been treated surgically over a 6.5-year period for pancreatic head or periampullary tumors, performing pancreaticoduodenectomy and pancreaticojejunostomy with external Wirsung duct stenting.
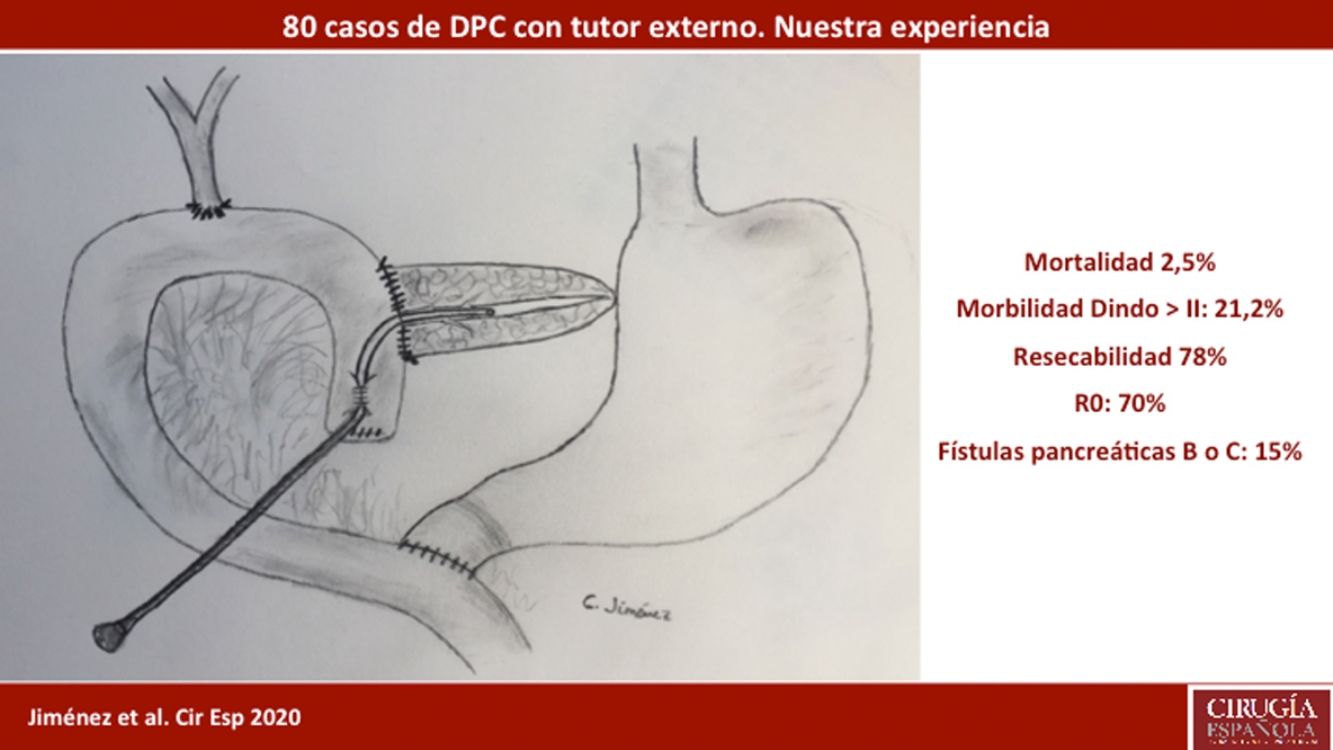
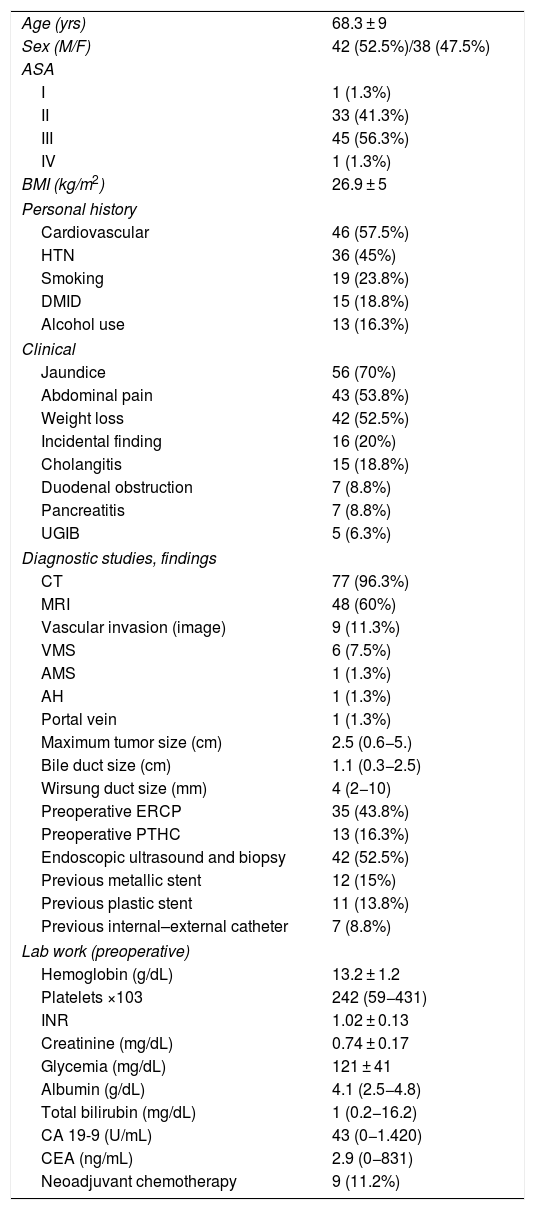
ResultsMean patient age was 68.3 ± 9 years, and the resectability rate was 78%. The texture of the pancreas was soft in 51.2% of patients and hard in 48.8%. Pylorus-preserving resection was performed in 43.8%. Adenocarcinoma was the most frequent tumor (68.8%), and R0 was confirmed in 70% of patients. Biochemical fistula was observed in 11.2%, pancreatic fistula grade B in 12.5% and C in 2.5%, whereas the abdominal reoperation rate was 10%. Median postoperative hospital stay was 16 days, and postoperative and 90-day mortality was 2.5%. Delayed gastric emptying was observed in 36.3% of patients, de novo diabetes in 12.5%, and exocrine insufficiency in 3. Patient survival rates after 1, 3 and 5 years were 80.2%, 53.6% and 19.2%, respectively.
ConclusionsAlthough our low rates of postoperative complications and mortality using external Wirsung duct stenting coincides with other more numerous recent series, it is necessary to perform a comparative analysis with other techniques, including more cases, to choose the best reconstruction technique after pancreaticoduodenectomy.
Existe controversia respecto a la técnica ideal de reconstrucción pancreático-yeyunal pos-resección duodeno-pancreática. La tutorización externa del Wirsung se ha considerado por muchos autores como una técnica con menor incidencia de fístulas y morbi-mortalidad. Analizamos nuestra experiencia con esta técnica.
Pacientes y métodosAnálisis retrospectivo de la morbi-mortalidad de una serie de 80 pacientes consecutivos intervenidos, durante 6,5 años, por tumores pancreáticos cefálicos o periampulares realizando resección y pancreático-yeyunostomía con tutorización externa del Wirsung.
ResultadosLa edad media de los pacientes fue 68,3 ± 9 años y la tasa de resecabilidad del 78%. La consistencia del páncreas era blanda en 51,2% de pacientes y dura en 48,8%. Se preservó el píloro en 43,8%. El tumor más frecuente fue el adenocarcinoma (68,8%) y se consiguió un R0 en 70%. La fístula bioquímica se presentó en 11,2%, la fístula pancreática grado B en 12,5% y la C en 2,5%, mientras que la tasa de reintervención abdominal fue del 10%. La mediana de estancia hospitalaria fue de 16 días y la mortalidad posoperatoria y a 90 días fue del 2,5%. La tasa de retraso del vaciamiento gástrico fue del 36,3%, diabetes de novo del 12,5% e insuficiencia exocrina del 30%. La supervivencia a 1, 3 y 5 años fue 80,2%, 53,6% y 19,2%.
ConclusionesAunque nuestras tasas de morbi-mortalidad con la tutorización externa del Wirsung son bajas, coincidiendo con series más amplias recientemente publicadas, se precisa un análisis comparativo con otras técnicas reconstructivas, con más casos, para elegir la mejor después de una duodenopancreatectomía cefálica.
Due to advances in surgical technique, perioperative management and centralization of pancreatic surgery in specialized hospitals with a large number of cases, the mortality rate associated with pancreaticoduodenectomy (PD) is less than 5%.1–6 However, according to recent series, post-PD morbidity rates continue to remain between 31% and 53%,4–7 mainly due to the incidence of pancreatic fistulae (PF) as more serious postoperative complications, which occur in 17.8%–34.9% of patients.5,6,8–13 When choosing a pancreatic diversion technique, no significant differences were observed in terms of the incidence of PF or global morbidity when comparing pancreaticojejunostomy with pancreaticogastrostomy.14–16
Complications can develop as a consequence of PF, such as abscesses, peritonitis, sepsis or intra-abdominal hemorrhage, which are associated with prolonged hospital stay and high mortality rates.17–19 The pathogenesis of PF is attributed to the filtration of pancreatic exocrine secretion through the pancreaticojejunal anastomosis. The most likely mechanism is self-digestion and destruction of perianastomotic tissue, which lead to dehiscence and leakage of bilioenteric content to the abdominal cavity.20
The objective of this descriptive and retrospective study is to analyze the results obtained in tumors of the head of the pancreas and periampullary tumors treated with PD and pancreaticojejunostomy using external Wirsung duct stenting.
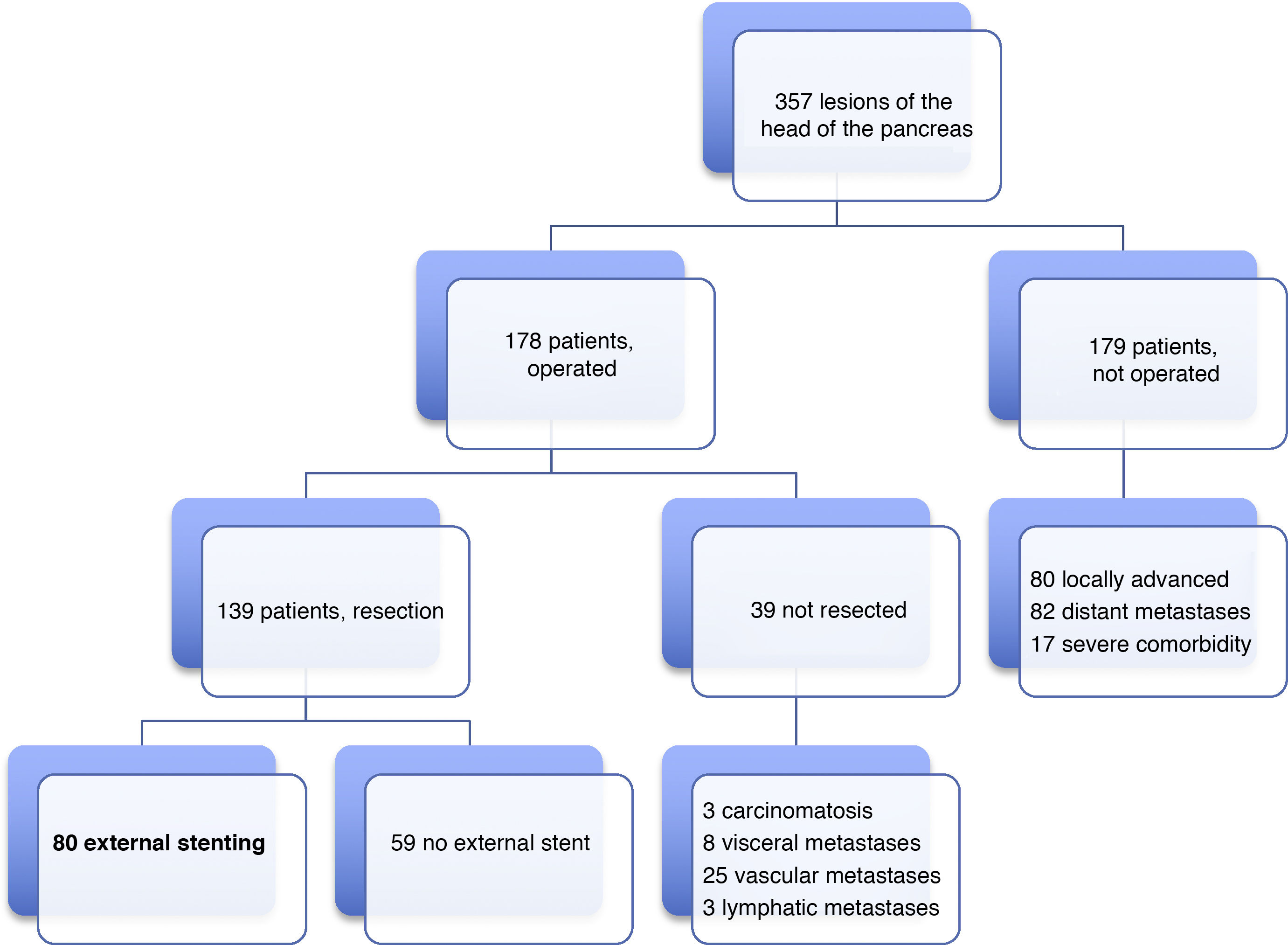
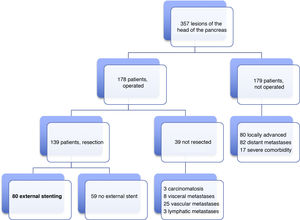
Patients and methodsIn our HBP Surgery Unit from January 19, 2012 to June 31, 2019, laparotomy was indicated with the intention of performing PD in 178 patients with lesions of the head of the pancreas, duodenum or periampullary region. PD was carried out in 139 (78%) patients, and end-to-side pancreaticojejunal reconstruction was performed with external stenting of the Wirsung duct in 80 patients. The 59 remaining resected patients were managed either with or without an internal stent. The present study analyzes a series of 80 consecutive patients operated on by four surgeons from our HBP Surgery Unit. The reconstruction technique with external stenting was freely chosen by the surgeons and systematically performed in all cases with indication for PD, without excluding any patient, even though there could be a potential risk of PF. The minimum follow-up of the series was seven months post-PD.
Preoperative diagnostic protocolSince the beginning of this series, there have been certain changes regarding the use of diagnostic tests. However, for diagnosis and staging, basically a computed tomography (CT) scan was ordered for almost all patients, complemented with magnetic resonance cholangiopancreatography (MRCP) and endoscopic ultrasound, as well as endoscopic retrograde cholangiopancreatography (ERCP) and percutaneous transhepatic cholangiography (PTHC) for biliary drainage. Currently, in general terms, the standardized protocol for pancreatic, periampullary and distal bile duct tumors consists of: thoracic-abdominal-pelvic CT and/or MRCP, endoscopic ultrasound and biopsy, as well as tumor markers (CEA and CA.19.9); PTHC and ERCP are used for bile duct drainage and diagnosis of periampullary lesions.
Surgical techniqueAll patients underwent supraumbilical midline laparotomy, cholecystectomy, division of the junction of the common hepatic duct with the common bile duct, and resection of the head of the pancreas and duodenum, which also included about 15 cm of the proximal jejunum. In malignant pathology, we conducted lymphadenectomy of the hepatoduodenal ligament, the common hepatic artery, and the right lateral branch of the superior mesenteric artery. When the tumor was distant from the duodenum, pyloric preservation was performed, or antrectomy if the tumor was close to the duodenum. In cases of tumor invasion of the portal vein or superior mesenteric vein, we performed segmental venous resection and end-to-end anastomosis, with no interposition graft.
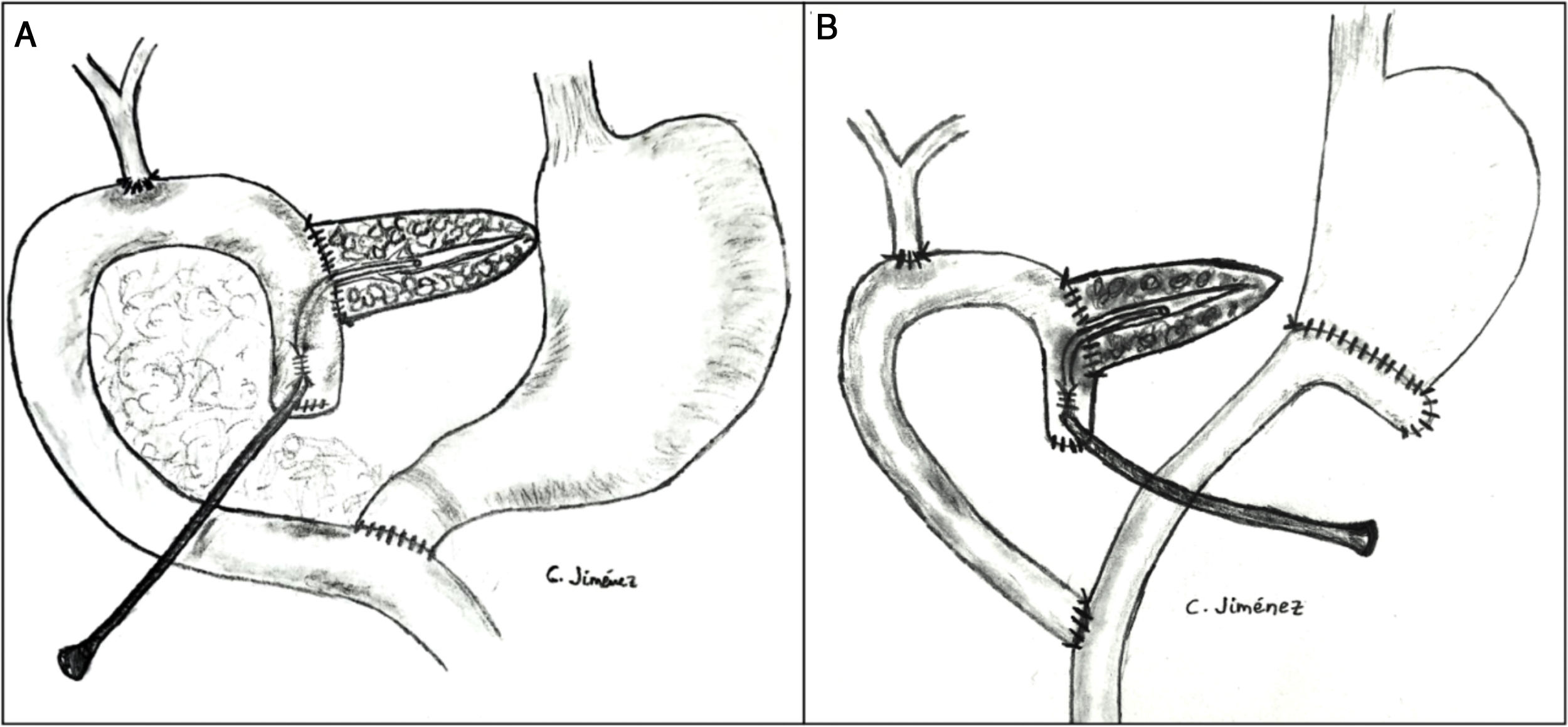
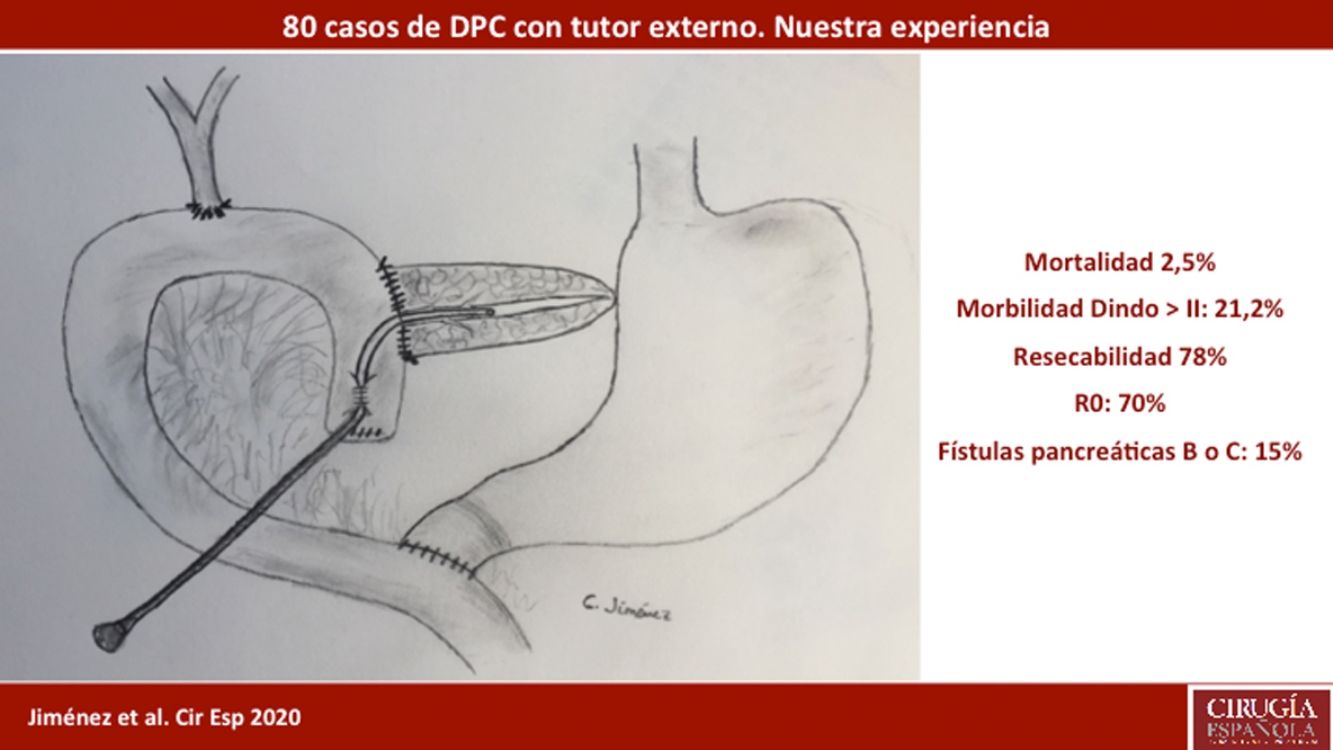
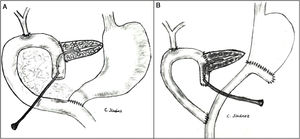
The pancreaticojejunostomy was performed in two planes: internal (suturing the Wirsung mucosa with the jejunal mucosa using 6–8 interrupted sutures of absorbable monofilament [5/6-0 polyglyconate]); and external (using interrupted polypropylene 3/4-0 sutures through the pancreatic parenchyma and 0.5 cm of the seromuscular layer of the jejunal loop, with no invagination of the pancreas into the jejunum). The end of the transanastomotic stent (polyvinyl cylindrical urethral catheter [Drenoplex-DICLISA®], with three distal holes measuring 2.0−390 mm, gauges 6 or 8, depending on the diameter of the Wirsung) was inserted about 4−5 cm in the Wirsung duct, passing into the jejunal lumen and then externalized through the ascending proximal afferent jejunal loop, affixing the external end of the stent according to the Witzel technique. The distal end of the catheter was externalized through the left anterior abdominal wall, where it was affixed with two 3/0 silk stitches (Fig. 1A). Once a PF was ruled out (amylase level <400 IU through the Penrose drainage orifice) and before discharge, the Wirsung stent was plugged until its extraction in the outpatient clinic 5–6 weeks after surgery.
Classic Child’s reconstruction (with a jejunal loop) was performed in 65 cases, and the two-loop technique was used in 15 cases. The hepaticojejunal anastomosis was created 10−15 cm from the pancreatic with 4/5-0 polyglyconate interrupted stitches. The duodenojejunal or gastrojejunal anastomosis was performed about 55−60 cm from the biliojejunal, in an end-to-side position and in two planes (internal with continuous 4/0 polyglyconate suture, and external with continuous polypropylene 4/0 suture). Reconstruction with two loops was the same as the previous one in terms of the pancreatic and biliary anastomosis, differing from the previous one in that, after antrectomy, an end-to-side gastrojejunal anastomosis was created and, 70 cm from this and the bilio-jejunal anastomoses, another end-to-side jejunojejunal anastomosis was performed, which was Y-shaped to avoid the reflux of pancreaticojejunal secretions towards the stomach (Fig. 1B).
The abdominal cavity was drained with two Penrose drains (one above and one below the pancreatic anastomosis), which were externalized on the right and left flanks, respectively.
Postoperative management and follow-upThe patients remained in the resuscitation unit for 24−48 h after surgery. Antibiotic prophylaxis was administered with 2 g of intravenous cefazolin. In patients with biliary stents, jaundice or cholangitis, iv treatment with piperacillin + tazobactam 4/0.5 was administered every six hours for five days.
The intra-abdominal drains were removed after days 5–6, always in the absence of fistula, hemorrhage or infection and with amylase levels <400 IU.
Pancreatic fistulae were classified according to the ISGPF update:21 grade A or biochemical fistulas (BF); grade B (require change in treatment or percutaneous drainage of collections); and grade C (patients present clinical instability; condition leads to organ failure and/or mortality, requiring drainage of collections or reoperations). Biliary fistulae were defined according to the Burkhart et al. criteria.22 Post-PD bleeding was classified according to the ISGPS23 definition and post-PD delayed gastric emptying (DGE) according to the Wente et al. criteria.24 Complications were registered according to the Clavien et al.25 classification. Reviews were carried out every month and then every three months.
Statistical analysisThe qualitative variables were expressed by absolute numbers and relative frequencies as a percentage. The quantitative variables with normal distribution were expressed by means and standard deviation; when the distribution was not normal, the median and percentiles 0 and 100 were used. Previously, the normality of the quantitative variables was studied using the Kolmogorov–Smirnov test. The relationship between quantitative variables was analyzed with Student’s t test in the case of normal distribution and, in the event of non-compliance with normality, the Mann–Whitney test was used. Patient survival was calculated according to the Kaplan–Meier actuarial method. A P value <.05 was considered statistically significant.
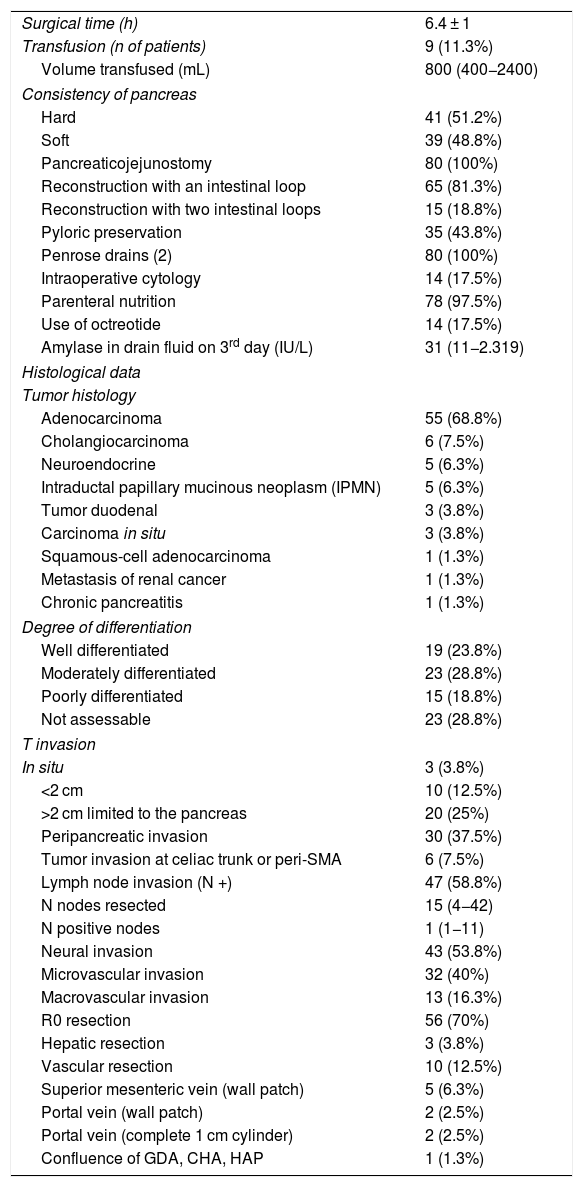
ResultsMean age was 68.3 ± 9 years, and 97.5% were ASA II–III. As for the symptoms, the presence of jaundice was observed in 56 (70%) patients, abdominal pain in 43 (53.8%), weight loss in 42 (52.5%), cholangitis in 15 (18.8%) and pancreatitis in seven (8.8%). The diagnostic tests and their findings, as well as preoperative lab work-up values, are shown in Table 1. Before surgery, a metal stent was placed in 12 (15%) patients, a plastic stent in 11 (13.8%) and an internal–external catheter in seven (8.8%). The global resectability rate among patients with surgical indication was 78%, and resection was ruled out for the reasons indicated in the flow chart (Fig. 2). Regarding the perioperative variables, nine (11.3%) patients were transfused with a median of 800 mL, finding a pancreas with a soft consistency in 41 (51.2%) and a hard consistency in 39 (48.8%). Pancreaticojejunostomy and abdominal drainage with 2 Penrose drains were performed in all patients, pyloric preservation in 35 (43.8%), treatment with parenteral nutrition in 78 (97.5%) and with octreotide in 14 (17.5%) patients with PF, basically grades B or C, in whom no improvement was observed. Octreotide prophylaxis was not used in any case. The most frequent tumor was adenocarcinoma (68.8%), followed by cholangiocarcinoma (7.5%), neuroendocrine tumor (6.3%), and intraductal papillary mucinous neoplasm (6.3%). With a median of 15 isolated lymph nodes in the resected specimens, metastases were detected in 47 (58.8%) patients. The degree of pancreatic, lymph node, neural and vascular invasion is shown in Table 2 with the rate of portal resection. R0 resection was achieved in 56 (70%) patients.
Characteristics and preoperative data.
| Age (yrs) | 68.3 ± 9 |
| Sex (M/F) | 42 (52.5%)/38 (47.5%) |
| ASA | |
| I | 1 (1.3%) |
| II | 33 (41.3%) |
| III | 45 (56.3%) |
| IV | 1 (1.3%) |
| BMI (kg/m2) | 26.9 ± 5 |
| Personal history | |
| Cardiovascular | 46 (57.5%) |
| HTN | 36 (45%) |
| Smoking | 19 (23.8%) |
| DMID | 15 (18.8%) |
| Alcohol use | 13 (16.3%) |
| Clinical | |
| Jaundice | 56 (70%) |
| Abdominal pain | 43 (53.8%) |
| Weight loss | 42 (52.5%) |
| Incidental finding | 16 (20%) |
| Cholangitis | 15 (18.8%) |
| Duodenal obstruction | 7 (8.8%) |
| Pancreatitis | 7 (8.8%) |
| UGIB | 5 (6.3%) |
| Diagnostic studies, findings | |
| CT | 77 (96.3%) |
| MRI | 48 (60%) |
| Vascular invasion (image) | 9 (11.3%) |
| VMS | 6 (7.5%) |
| AMS | 1 (1.3%) |
| AH | 1 (1.3%) |
| Portal vein | 1 (1.3%) |
| Maximum tumor size (cm) | 2.5 (0.6−5.) |
| Bile duct size (cm) | 1.1 (0.3−2.5) |
| Wirsung duct size (mm) | 4 (2−10) |
| Preoperative ERCP | 35 (43.8%) |
| Preoperative PTHC | 13 (16.3%) |
| Endoscopic ultrasound and biopsy | 42 (52.5%) |
| Previous metallic stent | 12 (15%) |
| Previous plastic stent | 11 (13.8%) |
| Previous internal–external catheter | 7 (8.8%) |
| Lab work (preoperative) | |
| Hemoglobin (g/dL) | 13.2 ± 1.2 |
| Platelets ×103 | 242 (59−431) |
| INR | 1.02 ± 0.13 |
| Creatinine (mg/dL) | 0.74 ± 0.17 |
| Glycemia (mg/dL) | 121 ± 41 |
| Albumin (g/dL) | 4.1 (2.5−4.8) |
| Total bilirubin (mg/dL) | 1 (0.2−16.2) |
| CA 19-9 (U/mL) | 43 (0−1.420) |
| CEA (ng/mL) | 2.9 (0−831) |
| Neoadjuvant chemotherapy | 9 (11.2%) |
ERCP: endoscopic retrograde cholangiopancreatography; CEA: carcinoembryonic antigen; PTHC: percutaneous transhepatic cholangiography; DMID: type 1 diabetes mellitus; UGIB: upper gastrointestinal bleeding; HTN: hypertension; BMI: body mass index; MRI: magnetic resonance imaging; CT: computed tomography.
Perioperative and histological data.
| Surgical time (h) | 6.4 ± 1 |
| Transfusion (n of patients) | 9 (11.3%) |
| Volume transfused (mL) | 800 (400−2400) |
| Consistency of pancreas | |
| Hard | 41 (51.2%) |
| Soft | 39 (48.8%) |
| Pancreaticojejunostomy | 80 (100%) |
| Reconstruction with an intestinal loop | 65 (81.3%) |
| Reconstruction with two intestinal loops | 15 (18.8%) |
| Pyloric preservation | 35 (43.8%) |
| Penrose drains (2) | 80 (100%) |
| Intraoperative cytology | 14 (17.5%) |
| Parenteral nutrition | 78 (97.5%) |
| Use of octreotide | 14 (17.5%) |
| Amylase in drain fluid on 3rd day (IU/L) | 31 (11−2.319) |
| Histological data | |
| Tumor histology | |
| Adenocarcinoma | 55 (68.8%) |
| Cholangiocarcinoma | 6 (7.5%) |
| Neuroendocrine | 5 (6.3%) |
| Intraductal papillary mucinous neoplasm (IPMN) | 5 (6.3%) |
| Tumor duodenal | 3 (3.8%) |
| Carcinoma in situ | 3 (3.8%) |
| Squamous-cell adenocarcinoma | 1 (1.3%) |
| Metastasis of renal cancer | 1 (1.3%) |
| Chronic pancreatitis | 1 (1.3%) |
| Degree of differentiation | |
| Well differentiated | 19 (23.8%) |
| Moderately differentiated | 23 (28.8%) |
| Poorly differentiated | 15 (18.8%) |
| Not assessable | 23 (28.8%) |
| T invasion | |
| In situ | 3 (3.8%) |
| <2 cm | 10 (12.5%) |
| >2 cm limited to the pancreas | 20 (25%) |
| Peripancreatic invasion | 30 (37.5%) |
| Tumor invasion at celiac trunk or peri-SMA | 6 (7.5%) |
| Lymph node invasion (N +) | 47 (58.8%) |
| N nodes resected | 15 (4−42) |
| N positive nodes | 1 (1−11) |
| Neural invasion | 43 (53.8%) |
| Microvascular invasion | 32 (40%) |
| Macrovascular invasion | 13 (16.3%) |
| R0 resection | 56 (70%) |
| Hepatic resection | 3 (3.8%) |
| Vascular resection | 10 (12.5%) |
| Superior mesenteric vein (wall patch) | 5 (6.3%) |
| Portal vein (wall patch) | 2 (2.5%) |
| Portal vein (complete 1 cm cylinder) | 2 (2.5%) |
| Confluence of GDA, CHA, HAP | 1 (1.3%) |
A minimal wedge resection of the liver (maximum 3 cm) was performed, finding a small metastasis in two patients (previously treated with neoadjuvant therapy) and a hemangioma in another.
Vascular resection for tumor invasion was performed in 10 patients: superior mesenteric vein in 5 (1-cm patch in all), portal vein in four (1-cm patch in two, and resection of a vein cylinder of approximately 1 cm in another two with end-to-end portal anastomosis) and resection in one patient of the confluence of the gastroduodenal artery, common hepatic artery and hepatic artery proper, with subsequent end-to-end anastomosis between the proper and common hepatic arteries. Four of these patients were reoperated for bleeding, but none developed PF.
Ten patients (12.5%) presented more than one complication. Among the postoperative complications, BF (previously called grade A PF) occurred in 9 patients (11.3%), grade B PF in 10 (12.5%), and grade C PF in 2 (2.5%). BF occurred in one (2.4%) case of hard-consistency pancreas, compared to 8 (20.5%) in soft-consistency pancreas (P = .02); while the rate of type B + C PF was 5 (12.2%) cases in hard-consistency pancreas versus 7 (17.9%) in soft consistency pancreas (P = .51).
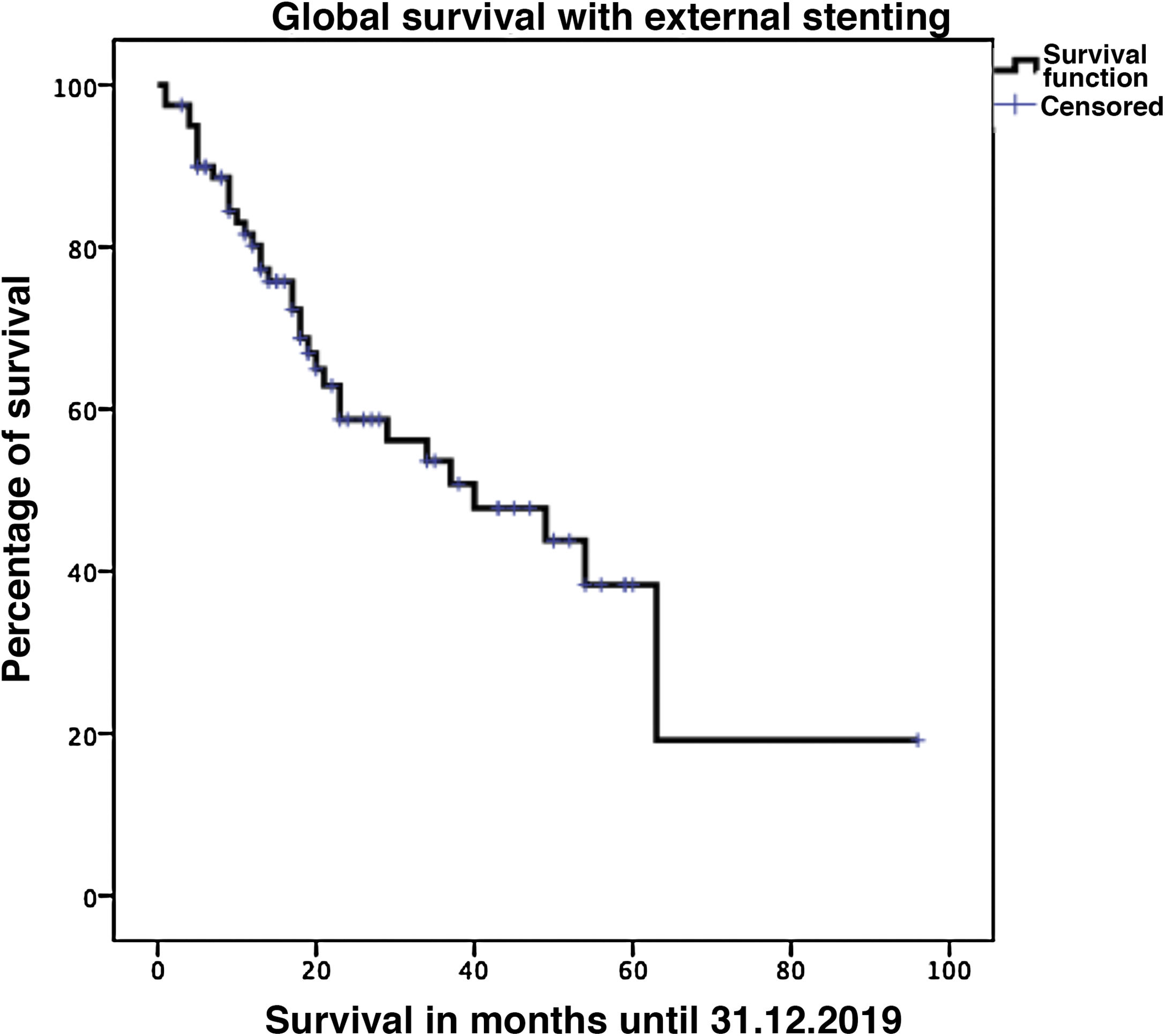
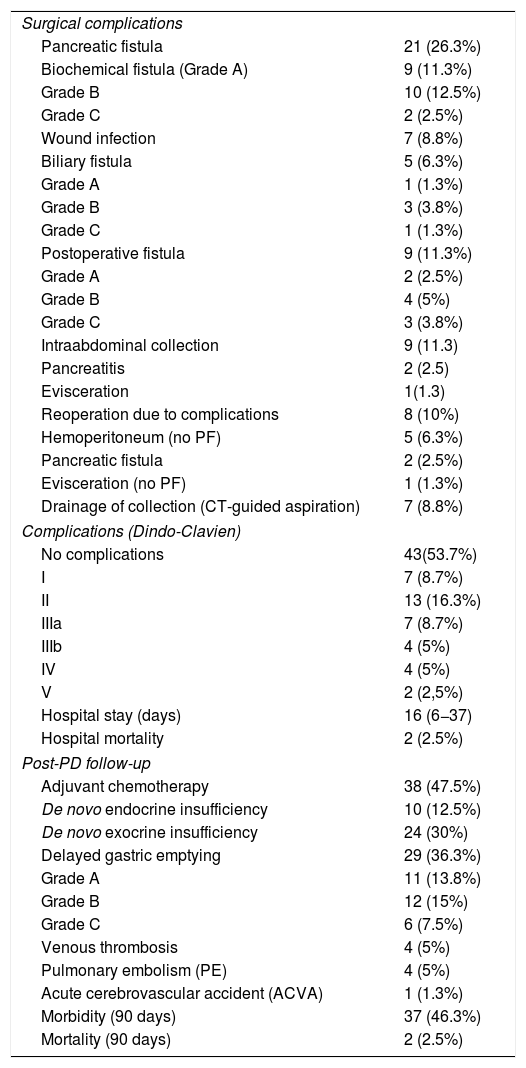
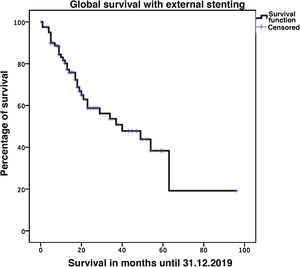
Biliary fistula was observed in 5 (6.3%) patients: grade A in 1, B in three, and C in one. Postoperative hemorrhage was presented by 9 (11.3%) patients: grade A in 2, B in 4, and C in 3. Surgical reoperation was indicated in 8 patients: 5 due to hemoperitoneum not related with PF, 2 for PF and one for evisceration, while 7 patients (8.8%) required drainage of an intra-abdominal collection by CT-guided aspiration. Median hospital stay was 16 days and hospital mortality 2.5% (2 patients). Neoadjuvant therapy was administered in 9 patients (11.2%) and adjuvant chemotherapy in 38 (47.5%); de novo exocrine failure was detected in 24 (30%) and de novo endocrine failure in 10 (12.5%). DGE was also diagnosed in 29 patients (36.3%). 90-day morbidity was 46.3% and mortality remained at 2.5%. Complications, defined by the Clavien classification,25 are also shown in Table 3. One-, 3- and 5-year patient survival rates were 80.2%, 53.6% and 19.2%, respectively (Fig. 3).
Postoperative morbidity, mortality and follow-up.
| Surgical complications | |
| Pancreatic fistula | 21 (26.3%) |
| Biochemical fistula (Grade A) | 9 (11.3%) |
| Grade B | 10 (12.5%) |
| Grade C | 2 (2.5%) |
| Wound infection | 7 (8.8%) |
| Biliary fistula | 5 (6.3%) |
| Grade A | 1 (1.3%) |
| Grade B | 3 (3.8%) |
| Grade C | 1 (1.3%) |
| Postoperative fistula | 9 (11.3%) |
| Grade A | 2 (2.5%) |
| Grade B | 4 (5%) |
| Grade C | 3 (3.8%) |
| Intraabdominal collection | 9 (11.3) |
| Pancreatitis | 2 (2.5) |
| Evisceration | 1(1.3) |
| Reoperation due to complications | 8 (10%) |
| Hemoperitoneum (no PF) | 5 (6.3%) |
| Pancreatic fistula | 2 (2.5%) |
| Evisceration (no PF) | 1 (1.3%) |
| Drainage of collection (CT-guided aspiration) | 7 (8.8%) |
| Complications (Dindo-Clavien) | |
| No complications | 43(53.7%) |
| I | 7 (8.7%) |
| II | 13 (16.3%) |
| IIIa | 7 (8.7%) |
| IIIb | 4 (5%) |
| IV | 4 (5%) |
| V | 2 (2,5%) |
| Hospital stay (days) | 16 (6−37) |
| Hospital mortality | 2 (2.5%) |
| Post-PD follow-up | |
| Adjuvant chemotherapy | 38 (47.5%) |
| De novo endocrine insufficiency | 10 (12.5%) |
| De novo exocrine insufficiency | 24 (30%) |
| Delayed gastric emptying | 29 (36.3%) |
| Grade A | 11 (13.8%) |
| Grade B | 12 (15%) |
| Grade C | 6 (7.5%) |
| Venous thrombosis | 4 (5%) |
| Pulmonary embolism (PE) | 4 (5%) |
| Acute cerebrovascular accident (ACVA) | 1 (1.3%) |
| Morbidity (90 days) | 37 (46.3%) |
| Mortality (90 days) | 2 (2.5%) |
PD: pancreaticoduodenectomy; PF: pancreatic fistulae; CT: computed tomography scan.
The disparity in the incidence of post-PD PF is so striking that significant differences have been found in randomized studies between different hospitals and surgeons.5 The rate of PF is different according to the definition adopted; for instance, according to the series by Winter et al.,8 the incidence of PF is 9.4% if the Johns Hopkins criteria are followed, compared to 24.4% if the ISGPF criteria are followed.26 The primitive definition of PF, based on the ISGPF,26 has been criticized by several authors12,27 based on the null clinical impact of grade A PF and moderate impact of grade B PF that will rarely require reoperation. Recently, the ISGPS has carried out a reclassification of PF, considering grade A types as BF, but not PF.21 According to this definition, our incidence of true PF (sum of B + C) is 15% (12 patients), and the incidence of the new BF (grade A) is 11.3% (9 patients). In a recent multicenter series of 4301 PD, the joint rate of grades B and C PF was 11.1% versus 8.1% BF. Also, when the incidence of grade C PF was compared between the utilization of internal or external stenting, the incidence was significantly higher with internal stents.28
Based on the published results, the use of stents is generally advocated over no stenting due to a lower overall rate of PF,29–32 while also observing a lower incidence of severe PF (types B and C) and postoperative morbidity.30
In a series comparing 2 of the 3 most commonly used techniques (no stent, internal or external stent), the rate of PF without stenting is reported between 1.4% and 40.9%; rates are between 6.1% and 47.7% with internal stents, and between 8% and 36.4% with external stents.4,5,8–11,13,33
In two meta-analyses that compared internal and external stents, the rate of PF was significantly lower in patients with an external stent,34,35 as were the overall morbidity and the rate of DGE.35 Another recent meta-analysis has also concluded that external stenting significantly reduces PF-related mortality.36 It has also been reported that, when patients with high risk of PF are selected (score 7–10) according to the criteria of Callery et al.,37 the best results are obtained in patients with external Wirsung stenting, pancreaticojejunostomy and intra-abdominal drainage, but not treated with octreotide.38
Our choice to use external Wirsung stenting over the past 6.5 years was based on the theoretical advantages of external over internal stenting due to the creation of a pancreatic-cutaneous fistula that diverts a large amount of pancreatic secretions out of the anastomosis, which allows for the anastomosis to heal and restores Wirsung patency. The external Wirsung stenting facilitates the ducto-mucosal anastomosis, can decompress the afferent loop, and improves the control of secretions in case of PF, while also making it possible to monitor the anastomosis radiologically.4,6,39–41 Internal stents have the added disadvantage of becoming easily detached and then migrating.
Grade B PF are characterized by presenting intra-abdominal collections that are usually drained by CT-guided aspiration, as in 7 (8.8%) of our patients. Intra-abdominal hemorrhage not related to PF can also be a reason for reoperation, which occurred in 5 (6.3%) of our patients.
Extremely serious post-PD grade C PF require reoperation; otherwise, these patients may develop multi-organ failure and die from this complication.21 In a French multicenter series of 680 patients with PD, the incidence of grade C PF was 5.3%, associated with a reoperation rate of 97% and a mortality rate of 25.7%.41 In another multicenter series that included patients with internal and external stenting, a significantly higher incidence of grade C PF was reported in the internal stent group, with a 2% 90-day mortality rate; 35% of mortality cases were grade C PF.28 The only twoº deceased patients in our series (2.5%) died due to abdominal sepsis after being reoperated for grade C PF. In reoperations for PF, the abscess is drained and, in case of pancreatic dehiscence or necrosis, it is recommended to disassemble the anastomosis and close the jejunal end, completing the procedure with a partial pancreatectomy and either leaving the tail of the pancreas tail, performing total pancreatectomy,41,42 or simply placing a drain tube at the resection margin of the pancreas.
Among the multiple risk factors for PF that have been described, the most frequently reported are: undilated Wirsung duct (<3 mm) and soft-textured pancreas,4–6,8–10,13,18,28,37,41 pancreatic steatosis,43,44 significant transfusion,18,41 PD due to duodenal or ampullary tumors,18,37,41 age >60 years,45 BMI > 25, no use of stent13 and prolonged surgery time (7.3 vs. 6.6 h).12
The consistency of the pancreas is a subjective parameter that is difficult to standardize, yet a soft texture has been correlated with Wirsung diameter <3 mm.12,13 According to a recent multicenter series,28 the hard-consistency pancreas rate is 46.4% versus 53.6% soft consistency, which are rates similar to ours (51.2% hard vs 48.8% soft). In our study, we have only shown a significantly higher incidence (P = .02) of BF in patients with soft pancreas versus hard pancreas, but the difference was not significant (P = .52) when comparing the rate of PF types B + C among patients with hard or soft pancreas.
Several strategies have been mentioned to prevent or reduce the incidence of PF, such as certain modifications to the technique,16 prophylaxis with octreotide,46 reinforcement of the anastomosis with fibrin sealant,47 placement of intraperitoneal drains,48 and internal transanastomotic stenting in either all patients9,10,49 or high-risk patients,50 although external stenting is the most frequently reported strategy to prevent PF,4,6,12,13,31,32,35,36,51,52 mainly in patients at high risk of PF.16
The placement of post-PD abdominal drains is a controversial issue. Thus, in a prospective randomized study (analyzing the use or not of Jackson-Pratt drains in PD and distal pancreatectomies), a significant increase in abscesses, intra-abdominal collections and fistulae was observed in the drain group.53 Another prospective, multicenter, randomized study shows that patients with moderate or severe risk of PF after PD seem to benefit from the use of abdominal drains, while PF drains can be avoided in one-third with low risk.48 A recent publication comparing the use of suction versus passive drains has concluded that 30-day mortality rates and overall complication rates are similar in both groups, although the authors did observe (with little evidence) that the use of suction drains versus passive drainage can slightly reduce hospital stay, and their early removal is recommended in patients with low risk of PF.54 We have opted for the use of Penrose drains to avoid problems of obstruction or suction of the suction drain on the anastomosis that can lead to the development of a PF.
Post-PD DGE is a common complication that occurs between 6% and 57% of cases,4,6,10,24 and we registered 36.3% (29 cases) in our experience. The pathogenesis is not clear, and it may appear both in PD with antrectomy and with pyloric preservation.24 Relevant PF (B or C) and intra-abdominal complications have been shown to be risk factors for the development of DGE.6,55
The limitation of this study is that it is a descriptive case series. However, out of all the patients with an indication for PD evaluated by the surgeons participating in this study, no single case was excluded from external stenting.
As a conclusion, in this preliminary series of post-PD external Wirsung duct stenting, we have observed a rate of complications consistent with that of other recently published larger series and a low mortality rate. Based on the results presented, our technique of choice is Wirsung stenting in all cases after PD. A comparative analysis with other techniques is needed to analyze the risk factors for PF and mortality, and thus be able to confirm with greater certainty the best post-PD reconstructive technique.
Authorship/collaboratorsCarlos Jiménez-Romero: study design; article composition; critical review and approval of the final review.
Laura Alonso Murillo: data collection; analysis and interpretation of the results.
Paula Rioja Conde: study design and data collection.
Alberto Marcacuzco Quinto: study design and data collection.
Oscar Caso Maestro: data collection; analysis and interpretation of the results.
Anisa Nutu: data collection.
Isabel Pérez Moreiras: data collection.
Iago Justo Alonso: critical review and approval of the final review; study design.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Jiménez Romero C, Alonso Murillo L, Rioja Conde P, Marcacuzco Quinto A, Caso Maestro Ó, Nutu A, et al. Duodenopancreatectomía cefálica y tutorización externa del conducto de Wirsung. Resultados de una serie de 80 casos consecutivos. Cir Esp. 2021;99:440–449.