Pleurodesis is a common technique for treating the accumulation of air or liquid in the pleural space caused by pneumothorax or pleural effusion, it is based on the bounding of pleural layers through induced inflammatory lesions. There are several pleurodesis procedures.
ObjectivesTo test and describe the inflammatory effect of hyperthermia on the pleural and peritoneal mesothelia of rats, with the aim of testing the effectiveness of this process for inducing pleurodesis.
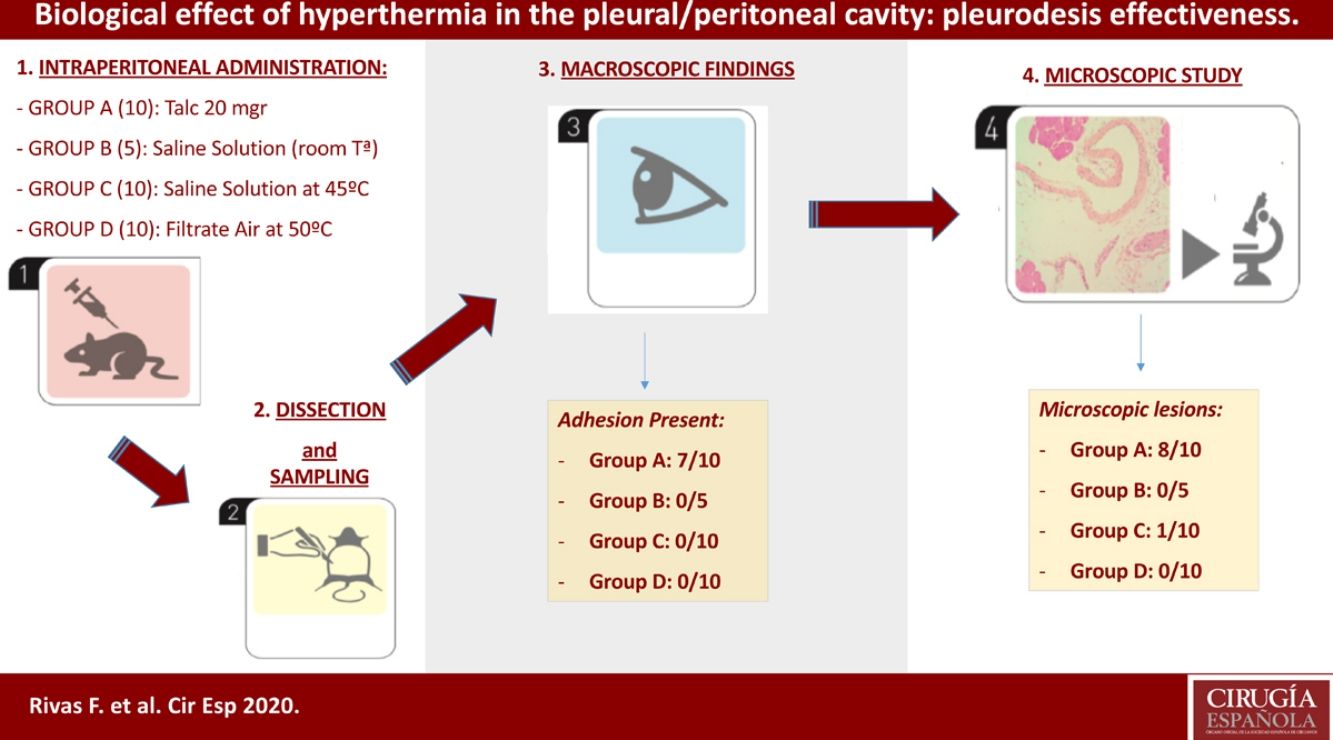
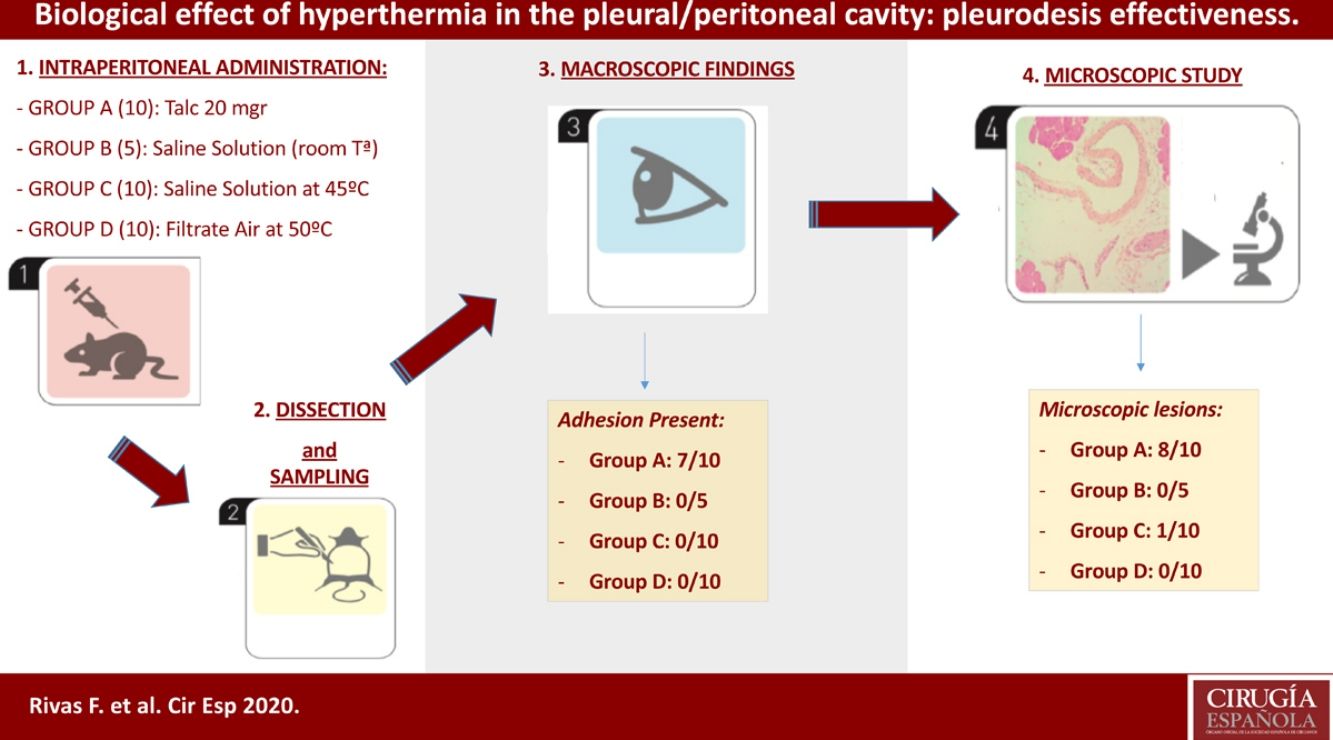
Methods35 Sprague-Dawley (male/female) rats were randomized into four treatment groups: Group A (Talc, 10 individuals); group B (control, 5 individuals); group C (hyperthermic isotonic saline, 10 individuals); and group D (filtrate air at 50°, 10 individuals). Inflammatory effect of hyperthermia was the primary outcome parameter.
ResultsIn the talc group, minimal adhesions between both pleural and peritoneal layers were observed in seven rats. Talc produced peritoneal mesothelium inflammation and fibrosis associated to foreign body giant cells in 80% (8/10) of the sample. Furthermore, clear evidence of a granulomatous foreign-body reaction was detected. No macroscopic and/or microscopic damage was registered in the remaining three groups (control, hyperthermic, and filtrate air).
ConclusionsTalc is an excellent method for producing pleuro-peritoneal inflammatory lesions. On the contrary, hyperthermia apparently does not induce the macroscopic and microscopic damage that is required for efficient pleurodesis. Therefore, hyperthermia should not be used for pleurodesis procedures.
La pleurodesis es una técnica común como tratamiento de la patología pleural, ya sea en el neumotórax o el derrame pleural; se basa en la delimitación de las capas pleurales mediante lesiones inflamatorias inducidas. Existen múltiples procedimientos para conseguir una sínfisis pleural.
ObjetivoEstudiar y describir el efecto inflamatorio de la hipertermia sobre el mesotelio pleural y peritoneal de ratas con el objetivo de valorar si presenta un efecto sinfisiante y por tanto útil para conseguir la pleurodesis.
MétodosTreinta y cinco ratas Sprague-Dawley (hembras/machos) fueron aleatorizadas en 4 grupos de tratamiento: grupoA (talco, 10 animales); grupoB (control, 5 animales); grupoC (suero salino hipertérmico: 10 animales), y grupoD (aire filtrado a 50°, 10 animales).
ResultadosEn el grupo de talco se evidenciaron adherencias entre las dos membranas pleurales y peritoneales en 7 animales. Se observó inflamación, fibrosis asociada a células gigantes de cuerpos extraño en el 80% (8/10) del mesotelio peritoneal. Paralelamente, se observó reacción granulomatosa a cuerpo extraño. No se registraron lesiones macroscópicas ni microscópicas en el resto de grupos estudiados (control, hipertermia y aire filtrado).
ConclusiónEl talco es un excelente método para producir inflamación a nivel del mesotelio pleural y peritoneal. Por el contrario, la hipertermia no parece inducir lesiones macroscópicas ni microscópicas requeridas para conseguir una pleurodesis eficaz.
Pleurodesis is the main treatment choice to prevent the accumulation of air or liquid in the pleural space caused by pneumothorax or pleural effusion. There are different pleurodesis procedures: chemical pleurodesis, mechanical pleurodesis or a combination of both. There are different chemical agents such as talc, polidocanol, tetracyclines, autologous blood, iodopovidone or hyperthermic saline solution with a greater or lesser effectiveness.1–7
Few studies have shown the efficacy of hyperthermia without chemotherapy as a sclerosing agent to treat malignant pleural effusion.7–9 Pneumothorax is defined as the collection of air in the pleural space between the lung and the chest wall. Primary pneumothoraces can arise in otherwise healthy people without any lung disease. In a recent epidemiological study, primary spontaneous pneumothorax (PSP) occurred in 7.4–18 cases (age-adjusted incidence) per 100,000 population each year in males, and 1.2–6 cases per 100,000 population each year in females. The exact pathogenesis of PSP is unknown.10 Secondary spontaneous pneumothorax has its origin on various diseases, including infectious processes, interstitial lung disease, connective tissue disease, and chronic obstructive pulmonary disease (COPD). Among the associated conditions, cystic fibrosis and COPD are the most commonly reported.11–13 Pleural effusion is a common complication of advanced cancers that may affect the performance status of patients. Pleural effusion can also exacerbate symptoms such as dyspnea and chest pain, which can be distressing for patients. These patients may undergo repeated interventions in search of relief from their symptoms.14
The aim of this paper is to study the changes in the pleural and/or peritoneal cavity after hyperthermia-induced pleurodesis (hyperthermic saline solution or filtrate hot air), testing the comparative capability of acute hyperthermia for producing pleurodesis in rats.
Materials and methodsAnimal groupsOur study included 35 male and female rats (Sprague-Dawley) that were 6–9 months old and weighed 400–900g. The Ethics Committee of our home institution (Universitat de Barcelona) approved the experimental procedures before. All animals were maintained in adequate cages and fed according to protocol. The animal laboratory at the Barcelona University housed the rat cages and was also the place where the operations took place. Our study was performed in the peritoneal cavity of the test-rats, instead of the pleural cavity, to avoid the risk of severe pneumothorax and mediastinal complications that are associated with the intrapleural administration of saline solution or hot air. However, talc was instilled in five animals to confirm the same lesions in both serous membranes. Although temperatures above 43° have been proved to be ineffective or only as effective as lower temperatures,15 we decided a hyperthermic treatment at 45° and 50°C because of the higher basal temperature of rats relative to humans.
The test-rats were assigned to the following groups:
Group A: composed of 10 animals that received an intraperitoneal administration of 20mg of talc- (Mg3Si4O10(OH)2) (Steritalc, Novatech, La Ciotat, France) in 1ml normal saline (extrapolated from the usual dose of 3–5g for a 70kg adult man). Five of these rats received also intrapleural dose at the same time, in order to confirm that intrapleural and intraperitoneal effects show no differences.
Group B: composed of 5 control animals that received an intraperitoneal administration of 100ml of 0.9% normal saline solution at 25° for 15min.
Group C: composed of 10 animals that received an intraperitoneal administration of 100ml of 0.9% normal saline solution at 45° for 15min.
Group D: composed of 10 animals that received an intraperitoneal administration of filtrate air at 50° for 15min, with 10ml instillations of normal saline every 5min.
Experimental protocolAll animals were anesthetized by isoflurane inhalation. Following the preparation of the operative field with povidone–iodine, five rats of group A received a 4-mm upper midline laparotomy (subxiphoid incision). Then, we administered 20mg of talc in 1ml of normal saline through a 16-gauge intravenous catheter, which was instilled in the left pleural cavity and in the peritoneal cavity. The remaining five rats underwent the same procedure but only in the peritoneal cavity.
The same procedure (B, 100ml of 0.9% normal saline solution; C, hyperthermic 0.9% normal saline solution; and D, filtrate hot air) was given for the other treatments groups. Body temperature was recorded in animals belonging to groups C and D using a rectal control. We rotated the animals with the purpose of distributing correctly the agents to the whole peritoneal space. Muscles and skin were sutured with a routine 2-layer continuous pattern using 2-0 Vicryl (Ethicon, Inc., Somerville, New Jersey). All animals received a subcutaneous dose of 0.2–0.3ml buprenorphine for three days. No surgery-related deaths or complications were recorded.
On the 21st day after the procedure all animals were euthanized by CO2 inhalation. We evaluated both the thoracic and abdominal cavities in five rats of group A. However, only the abdominal cavity was evaluated in the remaining rats. Macroscopic examinations of the pleural and peritoneal cavities were scored as 0 for no adhesions and 1 for adhesions present.
We collected representative tissue sections of the parietal pleura, parietal peritoneum, lung (visceral pleura), liver, brain and spleen for the microscopic analysis. The specimens were formalin-fixed and embedded in paraffin blocs. Using the standard methodology, we cut sections of 4–5μm thickness, staining the samples with hematoxylin-eosin (HE) and Masson's trichrome (MT), that stain green the fibrosis and collagen. Tissues were graded as 0 for the absence or 1 for the presence of inflammation and fibrosis.
Statistical analysisWe used SPSS v. 17.0 software package (SPSS Chicago, IL, USA) for the statistical analysis. We performed a descriptive univariate analysis, with the quantitative variables expressed as the means±standard deviations. The means of the continuous variables were then compared using a Kruskal–Wallis rank test. p values under 0.05 were considered statistically significant.
ResultsThe intrapleural administration of talc, saline solution or a filtrate hot air did not cause distress or other complications in any of the animals. We did not record significant differences in the mean weights among the four groups of animals (p>0.05).
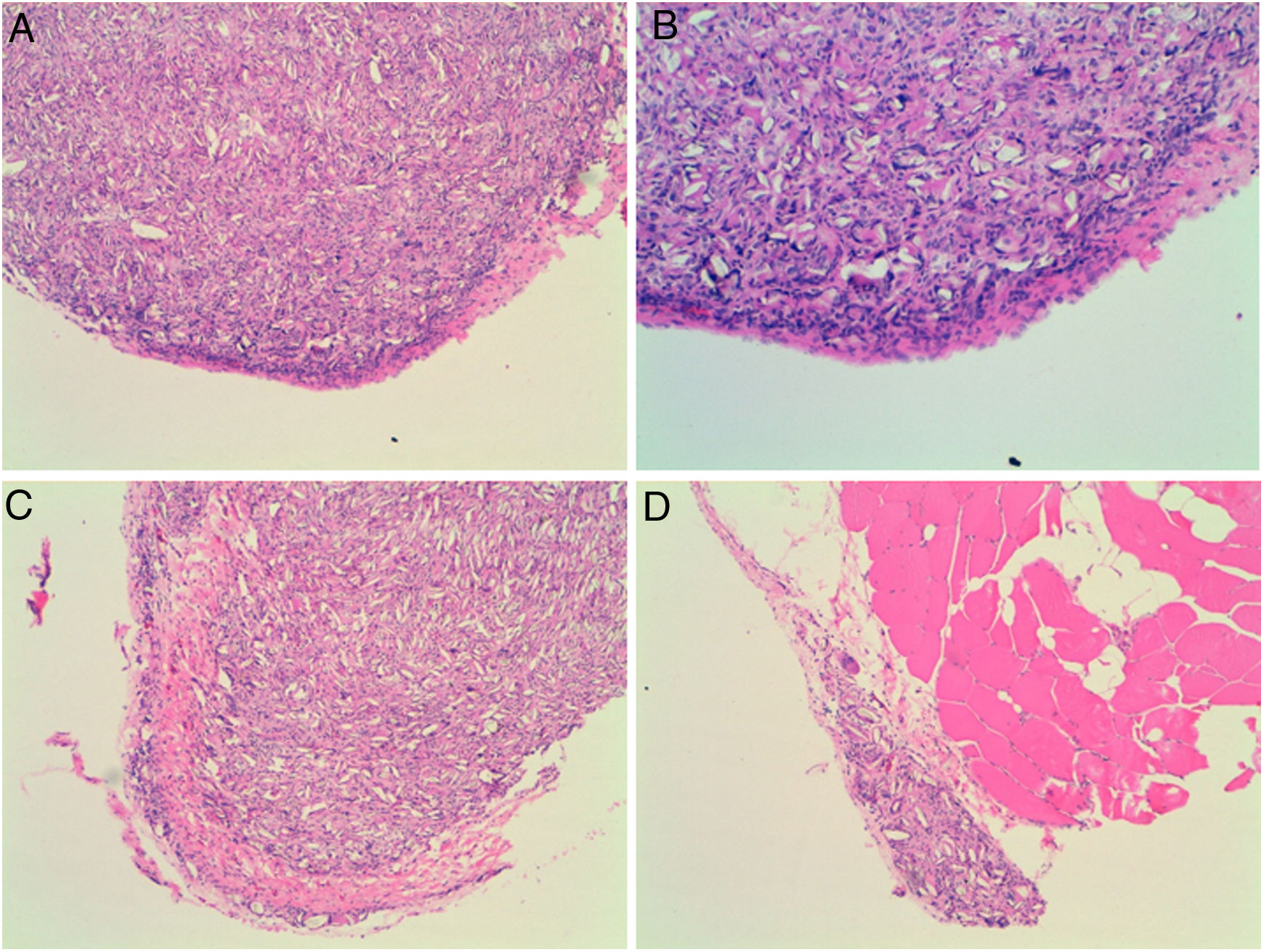
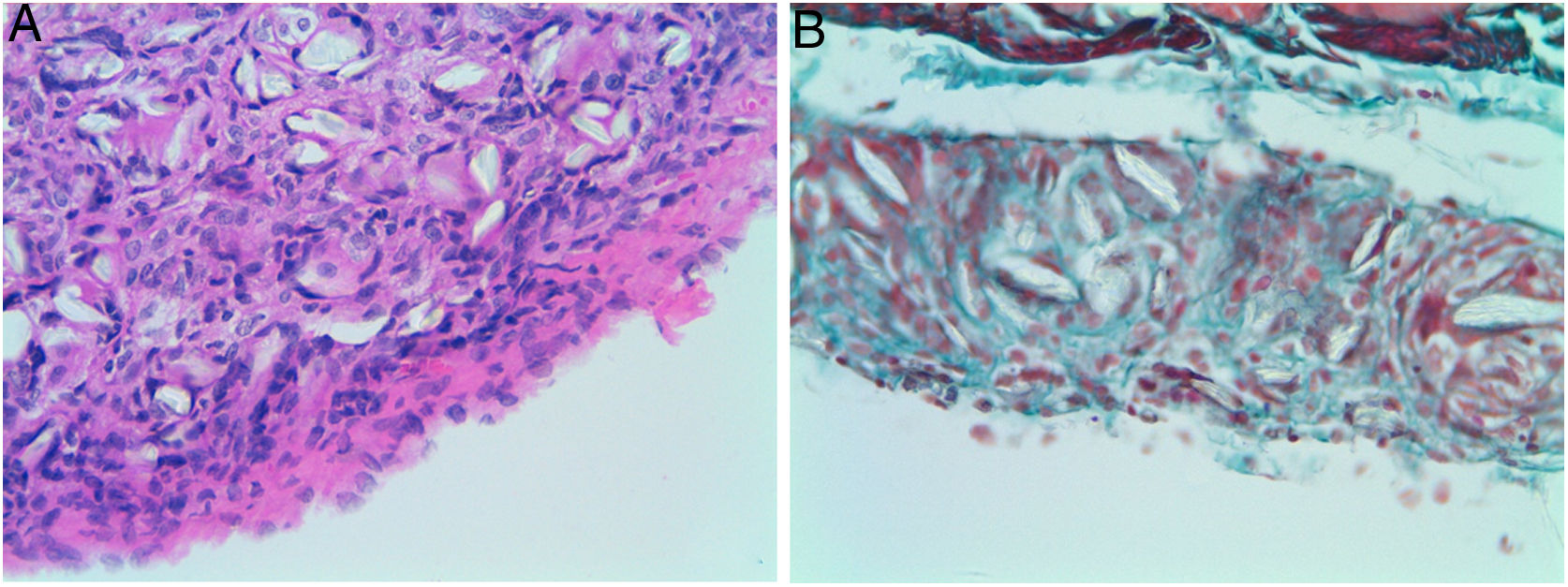
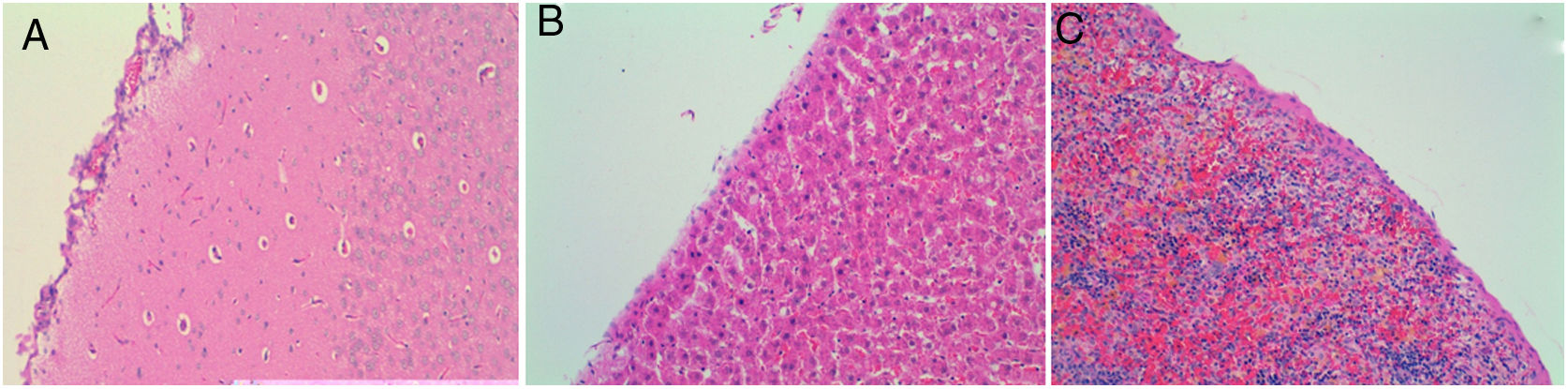
Four of the five rats (80%) of group A that received intraperitoneal and intrapleural administrations of talc showed few adhesions on the injection side between the pleural layers and also between the peritoneal layers. The microscopic study of these rats showed histological injuries in both the pleural and peritoneal mesothelium. There was a dense foreign body giant cell reaction with of granulomas that contained abundant birefringence talc crystals engulfed by giant cells. We also detected the presence of histiocites and some lymphocytes, as well as fibrosis of the submesothelial tissue. The microscopic results comparing pleural and peritoneal mesothelia showed similar effects and no significant differences (Figs. 1 and 2). No systemic distribution of talc was observed in the brain, spleen or liver (Fig. 3).
Microscopic findings on the parietal pleura and parietal peritoneum exposed to talc. Group A and B: parietal pleura and hematoxylin and eosin 100× and 200×. Group C and D: parietal peritoneum and hematoxylin and eosin 100× 200×. Signs of injuries, namely inflammation with foreign body granulomatous reaction (talc crystals), mesothelial proliferation and fibrosis, were observed in the pleural and peritoneal mesothelium.
Microscopic findings on the parietal pleura and parietal peritoneum after being exposed to talc. Group A: parietal pleura and hematoxylin and eosin × 400; Group B: parietal peritoneum and Masson's trichrome × 400. Multinucleated foreign body giant cells containing birefringent talc crystals with granulomas formation were observed in the submesothelium.
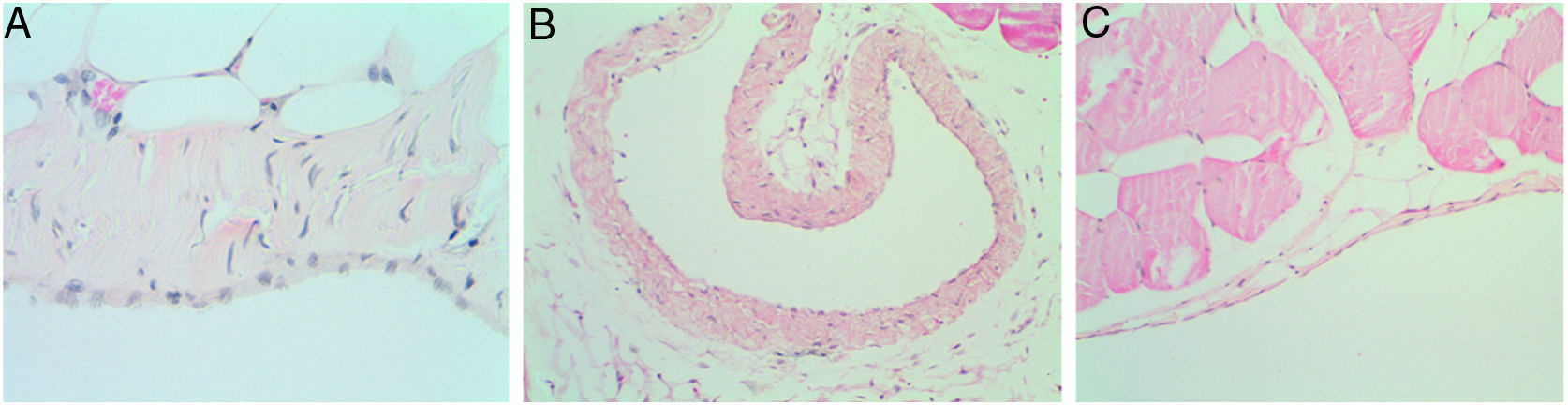
No adhesions (score: 0±0) were recorded in groups B, C, and D. Microscopically, we observed chronic lymphocytic inflammation in one rat of group C, without fibrosis or mesothelial proliferation.
There was no evidence of inflammatory response in groups D and B (Fig. 4, Table 1).
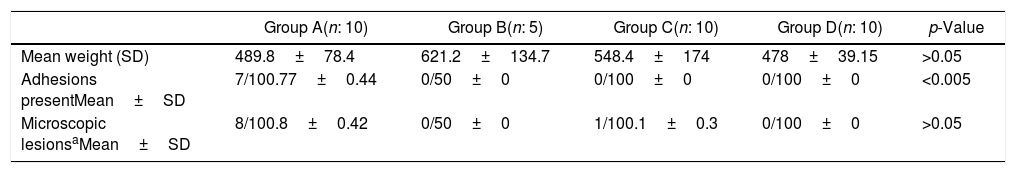
Microscopic and macroscopic scores.
| Group A(n: 10) | Group B(n: 5) | Group C(n: 10) | Group D(n: 10) | p-Value | |
|---|---|---|---|---|---|
| Mean weight (SD) | 489.8±78.4 | 621.2±134.7 | 548.4±174 | 478±39.15 | >0.05 |
| Adhesions presentMean±SD | 7/100.77±0.44 | 0/50±0 | 0/100±0 | 0/100±0 | <0.005 |
| Microscopic lesionsaMean±SD | 8/100.8±0.42 | 0/50±0 | 1/100.1±0.3 | 0/100±0 | >0.05 |
SD: Standard deviation. Microscopic lesions*: inflammation and fibrosis.
The mean weights of group C were 548.4±174 and 638.7±185, before and after the intraperitoneal administration of the hyperthermic saline solution respectively. The rectal temperature increased by an average of 0.6 degrees (presurgery: 37.9±0.5; postsurgery: 38.3±0.5). Similarly, the temperature also increased after the filtrate hot air was administered to rats of group D (presurgery: 37.4±0.7; postsurgery: 38.5±0.4).
DiscussionOur results indicate that acute hyperthermia does not induce macroscopic or microscopic lesions such as inflammation, fibrosis or proliferation in peritoneal space. However, Giovanella et al.22 suggested that hyperthermia is tumoricidal and induces high levels of proinflammatory proteins and cytokines, such as MIP-2, KC, RANTES and MCP-5. Also, hyperthermia may be chemo-attractant for polymorphonuclear leukocytes, monocytes and macrophages.16 Hyperthermia probably produces these changes acutely rather than chronically. Kimura et al.7 reported good results with hyper thermotherapy to treat malignant pleural effusion. More recently, Moon et al.8 studied the effect of hyperthermia by thoracoscopy to treat malignant pleural effusion with good results. In patients with malignant pleural effusion, the intrapleural administration of chemotherapeutic drugs seems to improve the survival rate.17,18 A combination of hyperthermia and chemotherapy has also produced good results in terms of survival rate and quality of life by reducing pleural effusion.19,20
Li et al.9 through an experimental study have confirmed that the therapeutic effect of hyperthermia without chemotherapy depends on time, temperature and depth. They observed that the response is greater in parietal pleural malignancies than in visceral malignancies. In our study the animals received normal saline solution at 45° for 15min, our opinion is that the perfusion time is an important factor to show pleural inflammation.
No clear surgical technique has emerged as a preferred treatment of choice after the long debate of choosing the ideal method for the management of recurrent pneumothorax and pleural effusion. Pleurodesis is the primary aim of the treatment. However, alternatives involve pleural abrasion and/or the intrapleural instillation of sclerosing agents in the pneumothorax, and also intrapleural instillation of sclerosing agents in pleural effusion. Several chemical agents have been used as sclerosing agents, including tetracycline, iodopovidone, silver nitrate, doxycycline, interleukins, chemotherapeutic drugs and talc.6 Both the British Thoracic Society and the American College of Chest Physicians have developed guidelines for treating pleural disease, but these guidelines are not uniformly followed because treatment protocols vary among institutions.21 Antunes et al.22 concluded that an ideal sclerosing agent should possess several essential qualities, including high molecular weight and chemical polarity, low regional clearance, rapid systemic clearance, and a steep dose-response curve. Also, the sclerosing agent needs to be well tolerated and produce minimal to no side effects. Among the known sclerosing agents, talc currently presents the best results,23,24 being the systemic dispersion of talc dependent on its type.5 The good results of talc pleurodesis were first described more than 20 years ago.25,26 However, the benefits of the procedure were later questioned due to possible side effects, although Shaw and Agarwal27 described talc as the most effective agent for pleurodesis, with thoracoscopic pleurodesis as their technique of choice.
ConclusionsThrough this experimental study, we can conclude that acute hyperthermia apparently does not induce fibrosis, proliferation or inflammation in the mesothelium, thus not producing the macroscopic and microscopic lesions that are requisite for pleurodesis. Because of that, it should not be considered to perform pleurodesis. However, talc is an excellent method for producing pleuro-peritoneal inflammatory lesions.
Authors’ contributionsRR generated the hypothesis. IE, CD, AU, AG, FR and RR designed the study. RP performed the pathological study. FR and RR wrote the manuscript.
Conflict of interestThe authors declare that they have no conflict of interest.
This study was supported by a Research Fund of Barcelona University (Project number: ACESB11/05).