Gastrointestinal bleeding (GB) is a potential complication after bariatric surgery and its frequency is around 2–4% according to the literature. The aim of this study is to present our experience with GB after bariatric surgery, its presentation and possible treatment options by means of an algorithm.
Patients and methodFrom January 2004 to December 2012, we performed 300 consecutive laparoscopic bariatric surgeries. A total of 280 patients underwent a laparoscopic Roux en Y gastric bypass with creation of a gastrojejunal anastomosis using a circular stapler type CEAA No 21 in 265 patients and with a linear stapler in 15 patients. Demographics, clinical presentation, diagnostic evaluation and treatment were reviewed. A total of 20 patients underwent a sleeve gastrectomy.
ResultsTwenty-seven cases (9%) developed GB. Diagnosis and therapeutic endoscopy was required in 13 patients. The onset of bleeding occurred between the 1st–6th postop days in 10 patients, and the origin was at the gastrojejunostomy staple-lines, and 3 patients had bleeding from an anastomotic ulcer 15–20 days after surgery. All other patients were managed non-operatively.
ConclusionConservative management of gastrointestinal bleeding is effective in most cases, but endoscopy with therapeutic intent should be considered in patients with severe or recurrent bleeding. Multidisciplinary postoperative follow-up is very important for early detention and treatment of this complication.
La hemorragia digestiva alta (HDA) es una potencial complicación tras la cirugía bariátrica, con una incidencia entre el 2 y el 4%. El objetivo de este estudio es presentar nuestra incidencia de HDA tras cirugía bariátrica, su forma de presentación y su manejo mediante un algoritmo terapéutico.
Material y métodoEstudio observacional prospectivo de una serie de 300 cirugías bariátricas por laparoscopia de manera consecutiva, desde enero del 2004 hasta diciembre del 2012. Se recogen datos demográficos, forma de presentación, diagnóstico y tratamiento de la HDA. En 280 pacientes se practicó bypass gástrico según la técnica de Wittgrove modificada, con anastomosis circular en 265 y anastomosis longitudinal en 15. En 20 pacientes se practicó gastrectomía vertical.
ResultadosAparecieron 27 casos (9%) de HDA tratados con: cirugía en un caso por inestabilidad hemodinámica; con gastroscopia diagnóstica-terapéutica en 13 casos (en 2 casos, 2 veces); en 10 de ellos, apareció de forma precoz (1–6 días) cuyo origen fue la línea de sutura de la anastomosis GY y en 3 de forma tardía, a los 15–20 días, siendo su origen una úlcera en la boca anastomótica. En el resto (13 pacientes), el manejo fue de forma conservadora.
ConclusionesAunque el manejo conservador de la HDA resuelve la mayoría de los casos, la clínica y la forma de presentación deben alertarnos, por lo que, en casos graves de sangrado, se requerirá de una endoscopia urgente. Es importante un equipo multidisciplinar y una comunicación estrecha entre cirujanos y endoscopistas para el manejo de esta seria complicación.
Obesity has become the epidemic of the 21st century; for this reason, the number of surgical procedures performed has grown exponentially. The laparoscopic Roux-en-Y gastric bypass has become the gold-standard surgical technique for the treatment of morbid obesity.1 According to the Fobi-Baltasar criteria that define a good treatment of obesity, it is accepted that the technique should be: safe (mortality <1% and morbidity <10%), reproducible, offer a good quality of life, require few follow-ups (<2%), have minimal side effects and be easily reversible.2–4
Early upper gastrointestinal bleeding (defined as that occurring within two weeks after bariatric surgery) has a variable incidence, between 0.6% and 4%, and is one of the most common complications associated with gastric bypass as compared with other techniques such as gastric sleeve or gastric banding, and its origin is generally found in the gastrojejunal anastomosis.1,5
It is not easy to determine the cause and progress of bleeding. Therefore, in certain cases, an endoscopy may be necessary to locate and treat its origin. The use of endoscopy in the immediate postoperative period is controversial and complex due to the risk of dehiscence or perforation of the anastomosis, with very limited and dissimilar published studies.5
The aim of this study is to present our incidence of upper gastrointestinal bleeding after surgery, its presentation and treatment in 300 patients who underwent laparoscopic bariatric surgery.
Material and MethodsIn the period between January 2004 and December 2012, 300 patients underwent bariatric surgery. Out of these patients, 123 were male and 177 were female, with a mean age of 49 years (25–60 range). The indications were BMI equal to or greater than 40kg/m2 or BMI of 35 if associated with major comorbidities, after being assessed by the endocrinology, psychiatry and gynaecology units. Some patients with BMI > 60 and severe disease were referred for placement of transient intragastric balloon. Following assessment of surgical risk by the anaesthetist, patients were operated by the same group of surgeons dedicated to bariatric surgery.
Regarding the surgical technique, 280 patients underwent a laparoscopic Roux-en-Y gastric bypass, using a modification of the Wittgrove's technique. For the creation of the gastric pouch, a horizontal section and two or three vertical ones were created using a blue load stapler and the area of the jejunal loop was divided using a vascular load stapler. An end-to-side gastrojejunal circular anastomosis with autosuture material type CEAA No. 21 was performed in 265 patients, and 15 patients underwent a longitudinal end-to-side anastomosis with linear mechanical suture using a 45-mm blue load stapler (3.5-mm staples) and closed with manual suture using a calibrated probe. The anastomosis was checked for air-tightness and afterwards two or three Hoffmeister-type stitches were performed on each side and in the centre of the anastomosis. The Y anastomosis was performed side-to-side with a 45-mm vascular load endostapler (2.8-mm staples) and the orifice was later closed manually.
Twenty patients underwent a vertical gastrectomy (tubular gastroplasty), sectioning the stomach with a blue load stapler.
Heparin (5000 subcutaneous units) was used as antithrombotic prophylaxis on the day before the procedure and on subsequent days. Intermittent pneumatic compression stockings were used during surgery and on the first postoperative day, with early mobilisation after their removal. A single 2-g dose of cefazolin was administered as routine antibiotic prophylaxis in the anaesthetic induction. In all cases, postoperative control was performed in the Intensive Care Unit within the first 24h after surgery. Daily monitoring of the procedure, from the medical as well as the nursing and nutritional point of views, was conducted in accordance with our clinical pathway.6
Patients were subject to follow-up through outpatient surgery consultations one month after surgery and then, periodically, every three months.
Follow-up was conducted prospectively. The following variables were gathered: sociodemographic data and personal history, BMI, type of surgical technique, upper gastrointestinal bleeding onset and form, performance of diagnostic-therapeutic endoscopy (type of haemostatic procedure), hospital stay, reoperation rates, and mortality and its causes. The statistical study is merely descriptive.
ResultsBetween January 2004 and April 2013, 300 patients underwent bariatric surgery in our Department of Surgery. All patients were monitored during the study period, with a mean follow-up of 89 months (range: 3–108 months). One patient died from massive pulmonary thromboembolism in the immediate postoperative period after reoperation for partial necrosis of gastric remnant.
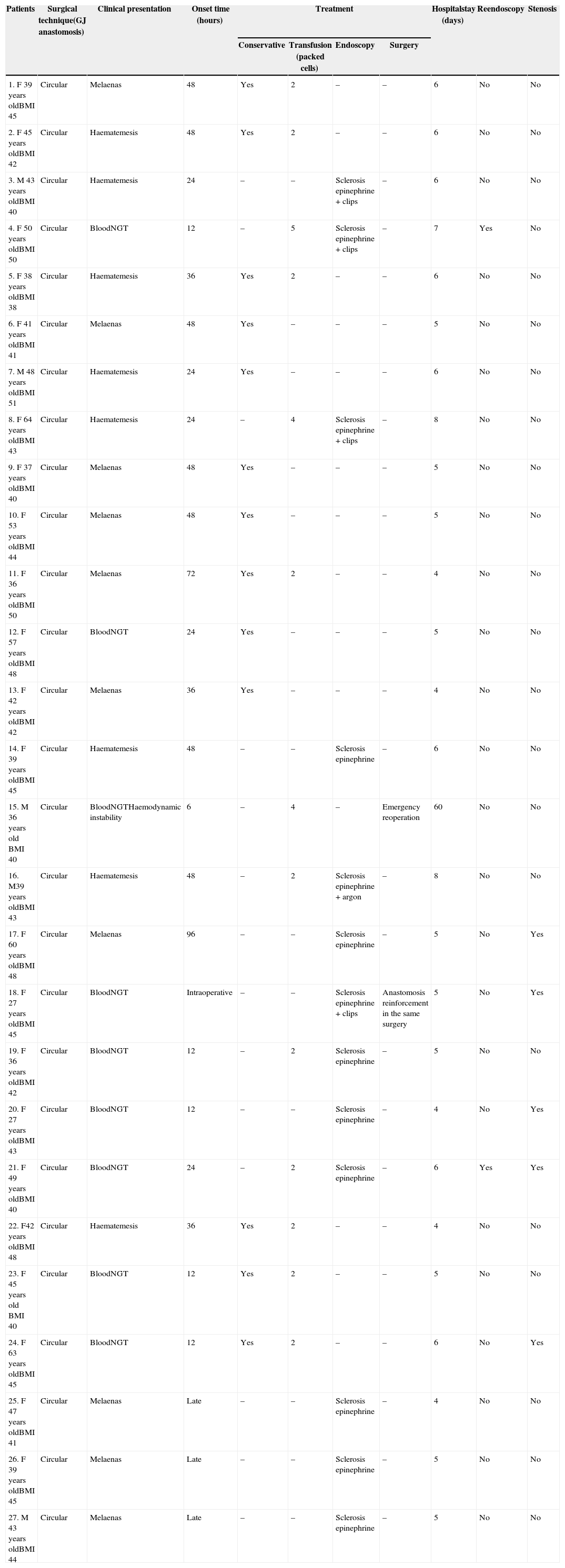
Upper gastrointestinal bleeding occurred in 27 cases (9%) (Table 1). Its presentation was early onset, between the 1st and the 6th day, in the form of haematemesis or blood loss through the nasogastric tube in 24 patients, and in 3 patients, late onset, between the 15th and 20th day, in the form of melaenas.
Clinical Presentation and Treatment of Gastrointestinal Bleeding After Bariatric Surgery.
| Patients | Surgical technique(GJ anastomosis) | Clinical presentation | Onset time (hours) | Treatment | Hospitalstay (days) | Reendoscopy | Stenosis | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Conservative | Transfusion (packed cells) | Endoscopy | Surgery | |||||||
| 1. F 39 years oldBMI 45 | Circular | Melaenas | 48 | Yes | 2 | – | – | 6 | No | No |
| 2. F 45 years oldBMI 42 | Circular | Haematemesis | 48 | Yes | 2 | – | – | 6 | No | No |
| 3. M 43 years oldBMI 40 | Circular | Haematemesis | 24 | – | – | Sclerosis epinephrine + clips | – | 6 | No | No |
| 4. F 50 years oldBMI 50 | Circular | BloodNGT | 12 | – | 5 | Sclerosis epinephrine + clips | – | 7 | Yes | No |
| 5. F 38 years oldBMI 38 | Circular | Haematemesis | 36 | Yes | 2 | – | – | 6 | No | No |
| 6. F 41 years oldBMI 41 | Circular | Melaenas | 48 | Yes | – | – | – | 5 | No | No |
| 7. M 48 years oldBMI 51 | Circular | Haematemesis | 24 | Yes | – | – | – | 6 | No | No |
| 8. F 64 years oldBMI 43 | Circular | Haematemesis | 24 | – | 4 | Sclerosis epinephrine + clips | – | 8 | No | No |
| 9. F 37 years oldBMI 40 | Circular | Melaenas | 48 | Yes | – | – | – | 5 | No | No |
| 10. F 53 years oldBMI 44 | Circular | Melaenas | 48 | Yes | – | – | – | 5 | No | No |
| 11. F 36 years oldBMI 50 | Circular | Melaenas | 72 | Yes | 2 | – | – | 4 | No | No |
| 12. F 57 years oldBMI 48 | Circular | BloodNGT | 24 | Yes | – | – | – | 5 | No | No |
| 13. F 42 years oldBMI 42 | Circular | Melaenas | 36 | Yes | – | – | – | 4 | No | No |
| 14. F 39 years oldBMI 45 | Circular | Haematemesis | 48 | – | – | Sclerosis epinephrine | – | 6 | No | No |
| 15. M 36 years old BMI 40 | Circular | BloodNGTHaemodynamic instability | 6 | – | 4 | – | Emergency reoperation | 60 | No | No |
| 16. M39 years oldBMI 43 | Circular | Haematemesis | 48 | – | 2 | Sclerosis epinephrine + argon | – | 8 | No | No |
| 17. F 60 years oldBMI 48 | Circular | Melaenas | 96 | – | – | Sclerosis epinephrine | – | 5 | No | Yes |
| 18. F 27 years oldBMI 45 | Circular | BloodNGT | Intraoperative | – | – | Sclerosis epinephrine + clips | Anastomosis reinforcement in the same surgery | 5 | No | Yes |
| 19. F 36 years oldBMI 42 | Circular | BloodNGT | 12 | – | 2 | Sclerosis epinephrine | – | 5 | No | No |
| 20. F 27 years oldBMI 43 | Circular | BloodNGT | 12 | – | – | Sclerosis epinephrine | – | 4 | No | Yes |
| 21. F 49 years oldBMI 40 | Circular | BloodNGT | 24 | – | 2 | Sclerosis epinephrine | – | 6 | Yes | Yes |
| 22. F42 years oldBMI 48 | Circular | Haematemesis | 36 | Yes | 2 | – | – | 4 | No | No |
| 23. F 45 years old BMI 40 | Circular | BloodNGT | 12 | Yes | 2 | – | – | 5 | No | No |
| 24. F 63 years oldBMI 45 | Circular | BloodNGT | 12 | Yes | 2 | – | – | 6 | No | Yes |
| 25. F 47 years oldBMI 41 | Circular | Melaenas | Late | – | – | Sclerosis epinephrine | – | 4 | No | No |
| 26. F 39 years oldBMI 45 | Circular | Melaenas | Late | – | – | Sclerosis epinephrine | – | 5 | No | No |
| 27. M 43 years oldBMI 44 | Circular | Melaenas | Late | – | – | Sclerosis epinephrine | – | 5 | No | No |
BMI: Body Mass Index (kg/m2); M: male; F: female; NGT: nasogastric tube.
Only one patient was treated with direct surgery, due to haemodynamic instability in the immediate postoperative period. Initially, the approach was laparoscopic, but conversion to laparotomy was required, where a new anastomosis was redone manually. This patient developed a torpid postoperative course, with prolonged admission to the Intensive Care Unit due to catheter sepsis. The remaining 26 patients did not require a new surgical procedure. Out of these, in 13 patients upper gastrointestinal bleeding (with early onset in 10 and late in 3) was significant enough to require diagnostic-therapeutic gastroscopy. The origin of the bleeding was found at the suture line of the GJ anastomosis, and in an anastomotic mouth ulcer in three cases. All endoscopies were performed with deep sedation without the need for endotracheal intubation, except when it was observed during the surgery. In this case, endoscopic management was performed simultaneously with direct laparoscopic visualisation and the suture was reinforced with loose silk stitches. In one patient, the cause of the bleeding could not be found after gastroscopy, so a double-balloon enteroscopy was performed and an ulcer was visualised in the excluded gastric remnant. All these patients underwent sclerosis with 2 cc of epinephrine 1/10000; also, in four cases endoclips were placed (Resolution Clip, Boston Scientific, Natick, MA, U.S.A.) and in one case, coagulation with argon was performed. Six patients needed packed red blood cell transfusion. Two of them suffered recurrent bleeding and required a new therapeutic gastroscopy, on the second and sixth days. In 13 patients, management was conservative and included observation, monitoring, analytical control and fluid therapy; the transfusion of packed red blood cells was necessary in 7 cases.
Regarding antithromboembolic treatment, heparin was initially withdrawn from 9 patients only, and was reinstated approximately 24h after the endoscopic procedure, if there were no signs of rebleeding or anaemia. Early mobilisation and ambulation was encouraged from the first postoperative day.
Oral tolerance in most cases was 24h after endoscopy. In one case of rebleeding, a new endoscopy was performed, but no findings were recorded. Therefore, an abdominal CT scan was indicated, which showed a subocclusive condition due to an intraluminal clot. This condition was resolved conservatively, but tolerance was delayed two more days. In 3 cases, tolerance began 48h after the test.
The mean length of hospital stay for these patients was 5.52 days, as compared with 4.1 days for patients who did not develop this complication.
Out of these cases, 5 patients (1.7%) developed GJ anastomotic stenosis. In the group that did not undergo diagnostic-therapeutic endoscopy, 15 cases of stenosis occurred (5.3%). In all these cases the anastomosis was circular.
In the subsequent outpatient follow-up, all patients were treated with heparin and esomeprazole 40mg/day for the first month. No control endoscopies were performed, except for one patient who had an episode of haematemesis with readmission and endoscopy two months after discharge, and was diagnosed with Mallory–Weiss syndrome, and remained on treatment with proton-pump inhibitors: another patient had persistent anaemia.
DiscussionEarly upper gastrointestinal bleeding after Roux-en-Y gastric bypass has an incidence that ranges between 0.6% and 4%. It is a major clinical and logistic problem that can lead to increased morbidity and potential reoperation.5
It usually occurs within the first 24–48h after surgery, although some cases may occur after a few days. It manifests as haematemesis, melaenas or fresh blood through the nasogastric tube. In severe cases, it occurs as tachycardia, hypotension and decreased haemoglobin.5,7
The mechanical suture line of the gastrojejunal anastomosis is usually considered the probable bleeding site and influences the technique used to perform the anastomosis. However, there are many sites where bleeding may originate, such as the jejunojejunal anastomosis or the gastric remnant. Studies in which open and laparoscopic techniques are compared have shown that gastrointestinal bleeding is more frequently associated with laparoscopic gastric bypass than with open surgery or other bariatric surgery techniques. This is attributed to the fact that manual suture or anastomosis reinforcement are more common in open surgery.1,7,8
Different staple-line bleeding prevention methods have been proposed. The use of endostaplers with shorter stapler height (2.5mm) is recommended as they provide greater tissue compression and, therefore, improved haemostasis. Other prevention methods would be reinforcement of the anastomosis or use of biological glue.7,8
The use of heparin and clopidogrel is considered a predisposing factor. Their use increases the risk of postoperative bleeding; therefore, discontinuation is recommended if gastrointestinal bleeding occurs. Obesity is an independent risk factor for venous thromboembolism. The risk of a thromboembolic event, including deep vein thrombosis or pulmonary thromboembolism, is increased in patients who have undergone bariatric surgery.1 Therefore, its administration as prophylactic treatment is recommended, in addition to pneumatic compression stockings and early ambulation, as preventive measures, as we do in accordance with our clinical pathway.6 After individualising each case, the withdrawal of antiplatelet therapy was considered in nine patients from our series, who also required blood transfusion. It was reintroduced as soon as possible if no signs of bleeding or anaemia existed, and it was maintained throughout the first month of the postoperative period.
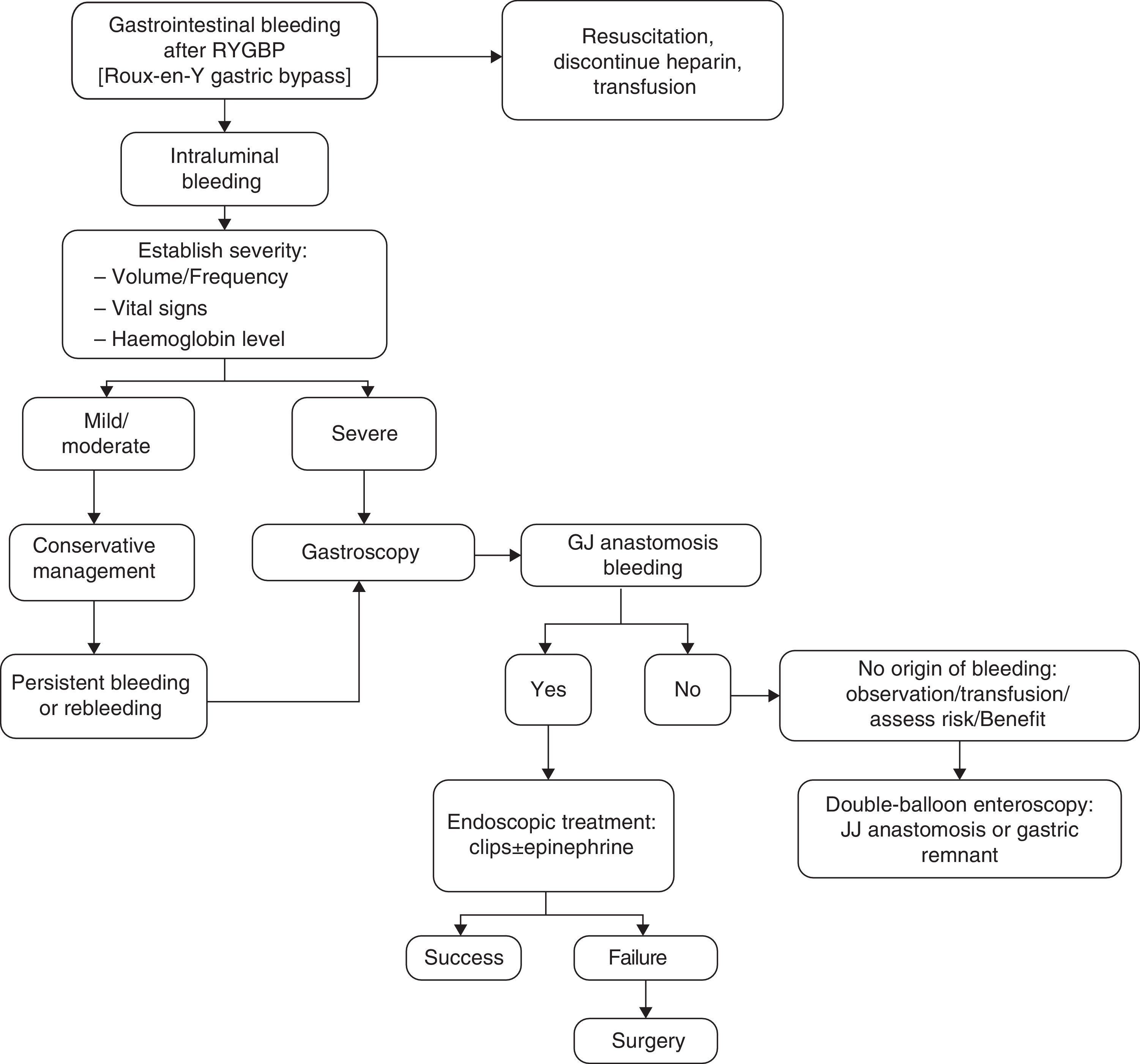
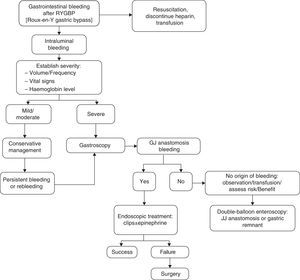
With regard to treatment, initial management consists of resuscitation measures, fluid therapy, blood transfusion, monitoring and determining the severity of bleeding, which depends on the clinical picture and presentation time. It is important to rule out extraluminal bleeding.1,5,9,10 Haematemesis or fresh blood per rectum suggest active bleeding. Hypotension, tachycardia, pallor and vasoconstriction require urgent reoperation. If bleeding occurs before six hours and is accompanied by a drop in haematocrit, reoperation will also be required. However, if bleeding occurs after 48h and does not present changes in the haematocrit, surgery will not be required.5
It is not easy to accurately determine the origin of the bleeding. Lack of adequate response to medical treatment requires performing a procedure that makes it possible to find the location and the cause of the bleeding site and plan for its treatment. Close and multidisciplinary postoperative follow-up is very important for the early detection and treatment of complications.6,11 An upper gastrointestinal endoscopy enables the performance of a diagnostic evaluation and treatment in cases of bleeding from the gastric pouch or gastrojejunal anastomosis and is used quite often in late postoperative bleeding. However, there is some controversy regarding its use in the immediate postoperative period because it is not exempt from risks, given the recent suture of the neo-gastric pouch, thereby increasing the risk of dehiscence and perforation. Most of the bleedings are mild and limited, and endoscopy should be considered when rebleeding occurs after conservative management, when there is haemodynamic instability or a drop in haemoglobin ≥2g. However, conservative treatment solves most acute bleeding, and the need for reoperation is rare.5,12 In those cases where the origin of bleeding is not found, but is suspected to be in the jejunostomy or excluded stomach, a double-balloon endoscopy may be performed, with the consensus of the team, in the operating room, taking extreme caution as it poses a greater risk of anastomotic dehiscence in the immediate postoperative period.13,14 In our series, a patient benefited from this technique, through which it was possible to locate the origin of bleeding in an ulcer in the excluded stomach.
Haemostasis was performed with epinephrine injection in all cases; in four of them, it was associated with the use of clips and in one with argon cauterisation. The rebleeding rate was 7.4% (two patients). One of the patients required urgent surgical treatment for haemodynamic instability. In the medium to long term, these haemostasis techniques may favour gastrojejunal stenosis, which occurred in five of our patients (1.7%), who later required endoscopic dilatation. Endoscopic sclerosis might be a risk factor for posterior stenosis as a consequence of the secondary inflammatory-scar reaction. This association has not been described in the literature and more studies would be needed to reach sound conclusions. In the group that did not undergo endoscopy, 15 patients had stenosis (5.3%). In all cases, a circular mechanical anastomosis was used.
In some studies, the use of endoclips is preferred, provided they are technically accessible, since unlike sclerosing injections or thermal coagulation, endoclips do not produce additional tissue damage and can be used concurrently for the treatment of anastomotic leaks or iatrogenic perforations and may reduce the risk of gastrojejunal anastomosis stenosis.15–19
We propose the algorithm shown in Fig. 1 for the management of gastrointestinal bleeding. An emergency gastroscopy should be recommended and performed by endoscopists with experience in haemostatic techniques, especially in the use of endoclips. Examination by endoscopy with minimal air insufflation during endoscopy, performed quickly and with caution, is recommended in these cases.5
The mean length of hospital stay of these patients was 5.52 days, slightly higher than that of patients who did not develop this complication (4.1 days).
Oral tolerance is not delayed when this type of complication occurs; it usually begins before the first 24h after the endoscopic procedure.
Regarding follow-up consultations, no control endoscopies are recommended systematically, but only in the case of persistent anaemia or suspected bleeding, as occurred in two of our cases. The use of proton-pump inhibitors is recommended during the first two months.
To conclude, upper gastrointestinal bleeding is a common complication after laparoscopic gastric bypass. Its most common origin is the gastrojejunal anastomosis. There are, however, many other sites where bleeding may originate in such cases. Therefore, an endoscopy makes it possible to perform a diagnostic evaluation and to establish treatment. Although expectant conservative management solves most cases, the clinical picture and presentation should alert us; thus, in severe cases of bleeding, an emergency endoscopy will be required. However, this procedure is not exempt from risks in the immediate postoperative period, given the recent suture of the neo-gastric pouch, or in the medium to long term, such as the gastrojejunal stenosis. Therefore, a multidisciplinary team and close communication among surgeons and endoscopists for the management of this serious complication are important.
FinancingArticle funded in part by the Fundación para la Formación e Investigación Sanitaria (FFIS) [Foundation for Healthcare Training and Research] of the Murcia Region, Spain, Group FFIS-008.
Conflicts of InterestThe authors declare that they do not to have any conflicts of interest.
Please cite this article as: García-García ML, Martín-Lorenzo JG, Torralba-Martínez JA, Lirón-Ruiz R, Miguel Perelló J, Flores Pastor B, et al. Endoscopia urgente por hemorragia digestiva tras cirugia bariátrica. Algoritmo terapéutico. Cir Esp. 2015;93:97–104.