Incisional hernia (IH) is common after open abdominal aortic aneurysm (AAA) repair. Recent studies reported incidence rates higher than previously stated. The aim of this study was to quantify the IH incidence after open AAA surgery. The secondary outcome was to identify the risk factors associated with the development of an IH.
MethodsRetrospective observational study of all consecutive patients who underwent an open repair of AAA, from January 2010 to June 2018, at our institution. Patients were free of abdominal wall hernias at the moment of inclusion in the study. Data were extracted from electronic records: baseline characteristics, surgical factors, and postoperative events. Computed tomography (CT) scans performed during follow-up were analyzed.
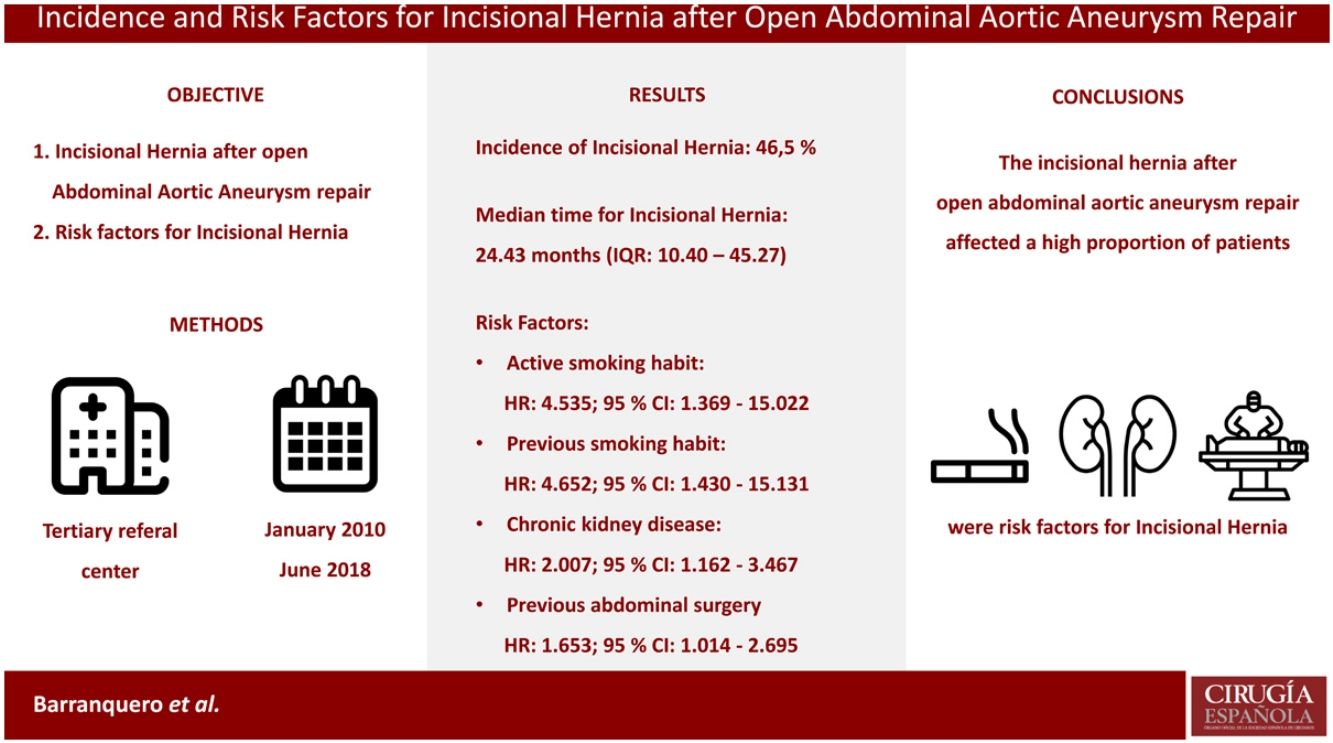
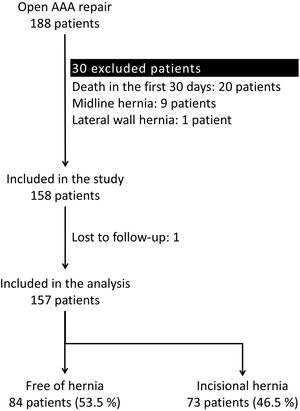
ResultsA total of 157 patients were analysed. The IH incidence after open repair of AAA was 46.5% (73 patients). The median time for IH development was 24.43 months (IQR: 10.40–45.27), while the median follow-up time was 37.20 months (IQR: 20.53–64.12). The risk factors linked to IH were: active (HR: 4.535; 95% CI: 1.369–15.022) or previous smoking habit (HR: 4.652; 95% CI: 1.430–15.131), chronic kidney disease (HR: 2.007; 95% CI: 1.162–3.467) and previous abdominal surgery (HR: 1.653; 95% CI: 1.014–2.695).
ConclusionThe incisional hernia after open abdominal aortic aneurysm repair affected a high proportion of the intervened patients. Previous abdominal surgery, chronic kidney disease, and smoking habit were independent factors for the development of an incisional hernia.
La hernia incisional (HI) tras la cirugía abierta del aneurisma de aorta abdominal (AAA) es común. Estudios recientes muestran incidencias superiores a las consideradas anteriormente. El objetivo es evaluar la incidencia de HI tras la cirugía abierta del AAA. El objetivo secundario fue evaluar los factores de riesgo de HI.
MétodosEstudio observacional retrospectivo de pacientes consecutivos sometidos a cirugía abierta del AAA de enero de 2010 a junio de 2018 en nuestro centro. Todos los pacientes estaban libres de hernias de pared abdominal en el momento de la cirugía. Se analizaron los datos de la historia clínica electrónica: características basales, factores quirúrgicos y eventos postoperatorios. Se analizaron también los estudios de tomografía computarizada durante el seguimiento.
ResultadosSe analizaron 157 pacientes. La incidencia de HI tras la cirugía abierta del AAA fue del 46,5% (73 pacientes). La mediana de tiempo para el desarrollo de HI fue de 24,43 meses (RIC 10,40-45,27), con una mediana de seguimiento de 37,20 meses (RIC 20,53-64,12). Los factores de riesgo asociados fueron: tabaquismo activo (HR 4,535; IC 95% 1,369-15,022) o hábito tabáquico previo (HR 4,652; IC 95% 1,430-15,131), enfermedad renal crónica (HR 2,007; IC 95% 1,162-3,467) y cirugía abdominal previa (HR 1,653; IC 95% 1,014-2,695).
ConclusionesLa HI tras la cirugía abierta del AAA afectó a un gran número de pacientes intervenidos. La cirugía abdominal previa, la enfermedad renal crónica y el hábito tabáquico fueron factores de riesgo independientes de HI.
Abdominal aortic aneurysm (AAA) repair is recommended when the aneurysm diameter exceeds 5.5cm in the male or 5cm in female patients.1 Even in the era of endovascular aortic repair, there is still a considerable proportion of open surgical repair.2
The IH incidence after a midline incision is estimated to be 12.8% (range: 0–35.6%).3 However, patients who undergo an open repair of AAA face an increased risk of IH.3 The IH incidence after open surgery of AAA ranges from 11.3% to 37.2%4–6 but might be up to 54.05%.7 More recent studies report higher IH rates with the use of radiological imaging techniques for diagnosis.8
Risk factors for AAA and abdominal wall hernias are similar: smoking habit, high body mass index, or chronic obstructive pulmonary disease.9–11 AAA and abdominal hernias share pathogenic mechanisms, with increased degradation of collagen type I due to increased metalloproteinase activity and an augmented synthesis of disorganized collagen type III.12
IH decreases patients’ quality of life, causes chronic pain, and may result in potential complications: hernia incarceration or bowel strangulation.13 Population studies show that after an open AAA surgery only 10.4% had an IH repaired.14 In this context, an optimal abdominal wall closure technique is recommended to reduce the IH incidence.15
ObjectiveThe primary aim was to quantify the IH rate after open AAA surgery, defined according to the European Hernia Society (EHS)16 as “any abdominal wall gap with or without a bulge in the area of a postoperative scar perceptible or palpable by clinical examination or imaging”. The secondary outcome was to identify the risk factors associated with IH.
MethodsThis article was written according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies.17
Study designSingle institution retrospective observational study.
Setting and participantsPatients included were all consecutive patients undergoing an open repair of AAA, from January 1, 2010 to June 1, 2018, at our institution. Our hospital is a tertiary referral center that belongs to the Spanish National Health Service. It is an 800-bed facility and attends a population of 597000 people. The Vascular Surgery Department is composed of nine surgeons, all performing aortic surgery. The main surgeon in each surgery was responsible for the abdominal wall closure.
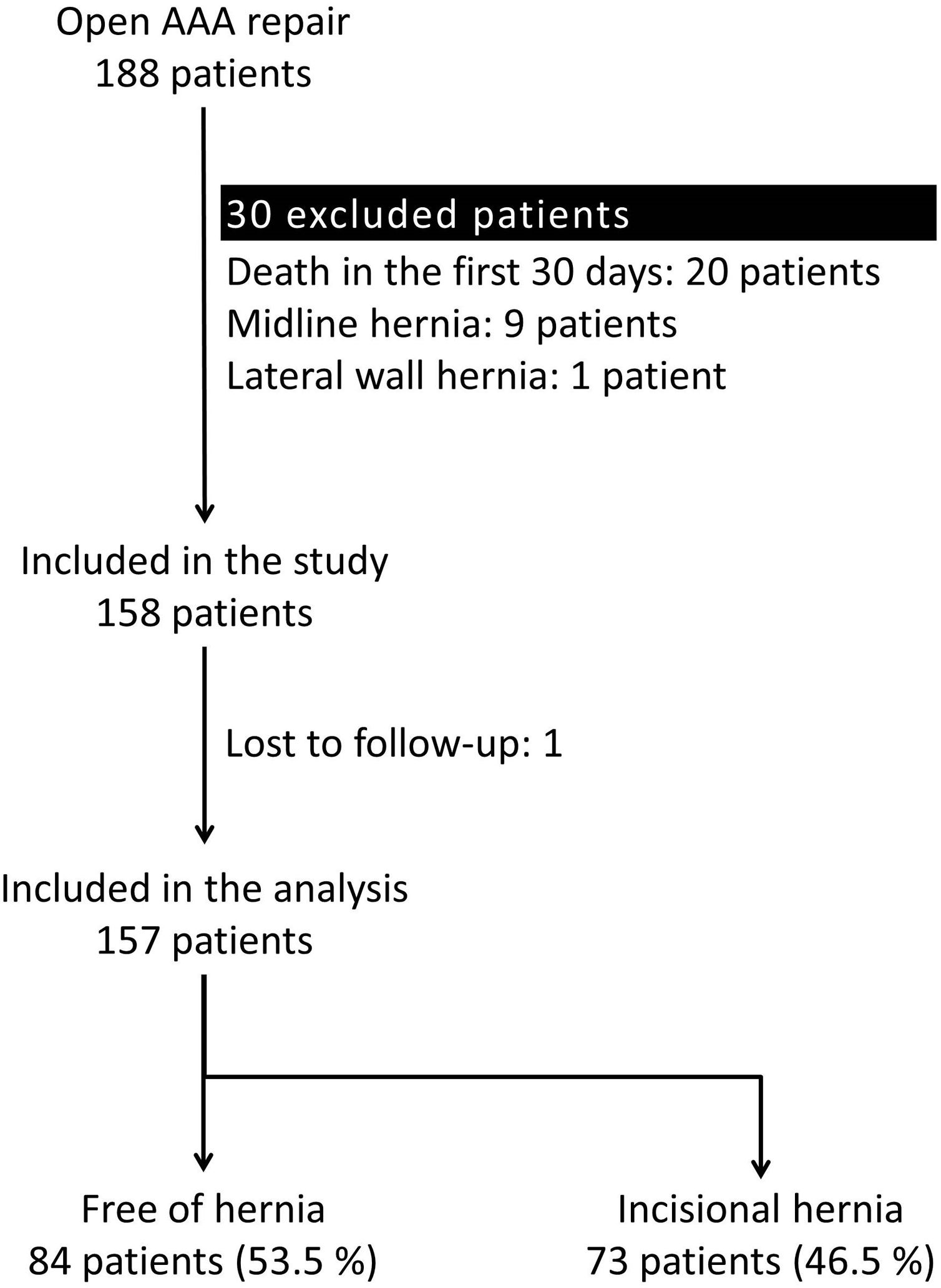
Excluded patients were those deceased within 30 days after surgery, those lost to follow-up, or those with midline or lateral abdominal wall hernias prior to the AAA repair. Therefore, all patients were free of hernias at the moment of inclusion in the study.
The design of the study was approved by the institutional Ethics Committee for Clinical Investigation (025-20 resolution). Permission was obtained to withdraw the informed consent.
Follow-upPatients were followed up with an annual visit with physical examination and abdominal ultrasound examination. A CT angiography was requested in selected cases to check the prosthesis and evaluate other possible aneurysms of different locations.
During this period, the clinical examinations noted in the electronic medical records, and the CT scans done to the patients for other causes were reviewed. The first description of IH in the medical records, or the first CT scan diagnosing an IH was considered the date of IH formation.
VariablesData were extracted from the electronic medical records and the CT scans stored in the institution. Preoperative data analysed were: age at AAA surgery, sex, body mass index, patients’ comorbidities, personal history of abdominal surgery or hernia, ASA category, and maximum AAA diameter. Intraoperative variables analysed were: timing of surgery, abdominal incision, aortic exposure, type of aortic bypass, and suture used for the closure of the abdominal incision. Postoperative variables were the length of hospital stay, hemoglobin and white blood cell (WBC) count 48hours after surgery, and postoperative complications according to Clavien-Dindo classification.18
Study sizePrevious articles quantified the IH rate after open repair of AAA from 11.3% to 37.2%.4–6 Therefore, a minimum of 100 participants was required in order to include at least 10 events of interest (IH) to analyze the risk factors in a univariate analysis.
Statistical analysisCategorical variables were described as numbers and percentages. Quantitative variables with normal distribution were described with mean and standard deviation (sd); and those with non-normal distribution, with median and interquartile range (IQR). The distribution was determined through the Shapiro–Wilk test.
Survival analysis was applied to evaluate the risk factors for IH. A Kaplan–Meier curve was used for the graphical depiction of the IH development. Log-rank test was used for dichotomous categorical variables and Cox regression for polytomous categorical and quantitative variables. Variables were described with Hazard Ratio (HR) and 95% confidence interval (95% CI). Firstly, a univariate analysis of all the variables explored was used. Next, a multivariate analysis with Cox regression was conducted using the variables that showed a higher statistical signification in univariate analysis. p<0.05 was considered statistically significant.
The analysis was performed with IBM® SPSS Statistics 23®.
ResultsParticipantsA total of 188 patients underwent an open repair of AAA from January 1, 2010, to June 1, 2018, at our institution. Thirty-one patients were excluded applying the exclusion criteria. Therefore, 157 patients were analyzed (Fig. 1).
Descriptive dataBaseline characteristics are shown in Table 1. The majority of the patients were male (96.2%), with a mean age of 70.49 years (sd: 7.23). Cardiovascular risk factors were common, and 89.2% demonstrated an active or previous history of tobacco exposition.
Baseline characteristics.
| n | % | |
|---|---|---|
| Median | IQR | |
| Age (years)a | 70.49 | 7.23 |
| Sex (male:female) | 151:6 | 96.2:3.8 |
| BMI (kg/m2) | 26.75 | 23.99–30.08 |
| Hypertension | 113 | 72% |
| Dyslipidemia | 106 | 67.5% |
| Diabetes mellitus | 35 | 22.3% |
| Smoking habit | ||
| Non-smoker | 17 | 10.8% |
| Active | 54 | 34.4% |
| Quitted | 86 | 54.8% |
| Ischemic heart disease | 28 | 17.8% |
| COPD | 36 | 22.9% |
| Chronic kidney disease | 29 | 18.5% |
| eGFR<45ml/min | ||
| Previous abdominal surgery | 47 | 29.9% |
| History of prior hernia | 42 | 26.8% |
| Aneurysm maximum diameter (cm) | 5.6 | 5.3–6.4 |
| ASA classification | ||
| II | 31 | 19.7% |
| III | 85 | 54.1% |
| IV | 17 | 10.8% |
| Unknown | 24 | 15.3% |
BMI: body mass index.
eGFR: estimated glomerular filtration rate.
Surgical variables are listed in Table 2. Most patients underwent an elective repair of the AAA (92.4%), through a midline incision (88.5%) with transabdominal exposure of the abdominal aorta (84.1%). The most usual AAA repair was an aorto-aortic bypass (59.2%). The most common suture material for the abdominal wall closure was polydioxanone monofilament (82.2%). No prophylactic meshes in the abdominal wall closure were used.
Surgical factors.
| n | % | |
|---|---|---|
| Timing of surgery | ||
| Elective | 145 | 92.4% |
| Urgent | 12 | 7.6% |
| Incision | ||
| Midline | 139 | 88.5% |
| Pararectal | 17 | 10.8% |
| Lumbotomy | 1 | 0.6% |
| Aortic exposure | ||
| Transabdominal | 132 | 84.1% |
| Retroperitoneal | 25 | 15.9% |
| Aortic bypass | ||
| Aorto-aortic | 93 | 59.2% |
| Aorto bi-iliac | 46 | 29.3% |
| Aorto bi-femoral | 10 | 6.4% |
| Aorto-iliac and femoral | 5 | 3.2% |
| Aorto-uni-iliac | 3 | 1.9% |
| Suture | ||
| Polydioxanone monofilament (PDS®) | 129 | 82.2% |
| Polypropylene monofilament (Prolene®) | 9 | 5.7% |
| Polyglactin multifilament (Vicryl®) | 16 | 10.2% |
| Unknown | 3 | 1.9% |
The postoperative course was uneventful for 59.3% of the patients, with a median postoperative stay of 7 days (IQR: 5.50–10). The 10.8% of the patients suffered major complications according to Clavien-Dindo classification (Table 3).
Postoperative course.
| n | % | |
|---|---|---|
| Median | IQR | |
| Length of hospital stay (days) | 7 | 5.50–10 |
| Haemoglobin (g/dl) 48h after surgery | 10.3 | 9.05–11.40 |
| WBC count (WBC/μl) 48h after surgery | 10800 | 8500–13300 |
| Surgical site infection | 5 | 3.2% |
| Reintervention | 9 | 5.7% |
| Complications according to Clavien-Dindo classification | ||
| I | 7 | 4.5% |
| II | 39 | 24.8% |
| III | 9 | 5.7% |
| IV | 8 | 5.1% |
| V | 1 | 0.6% |
WBC count: white blood cell count.
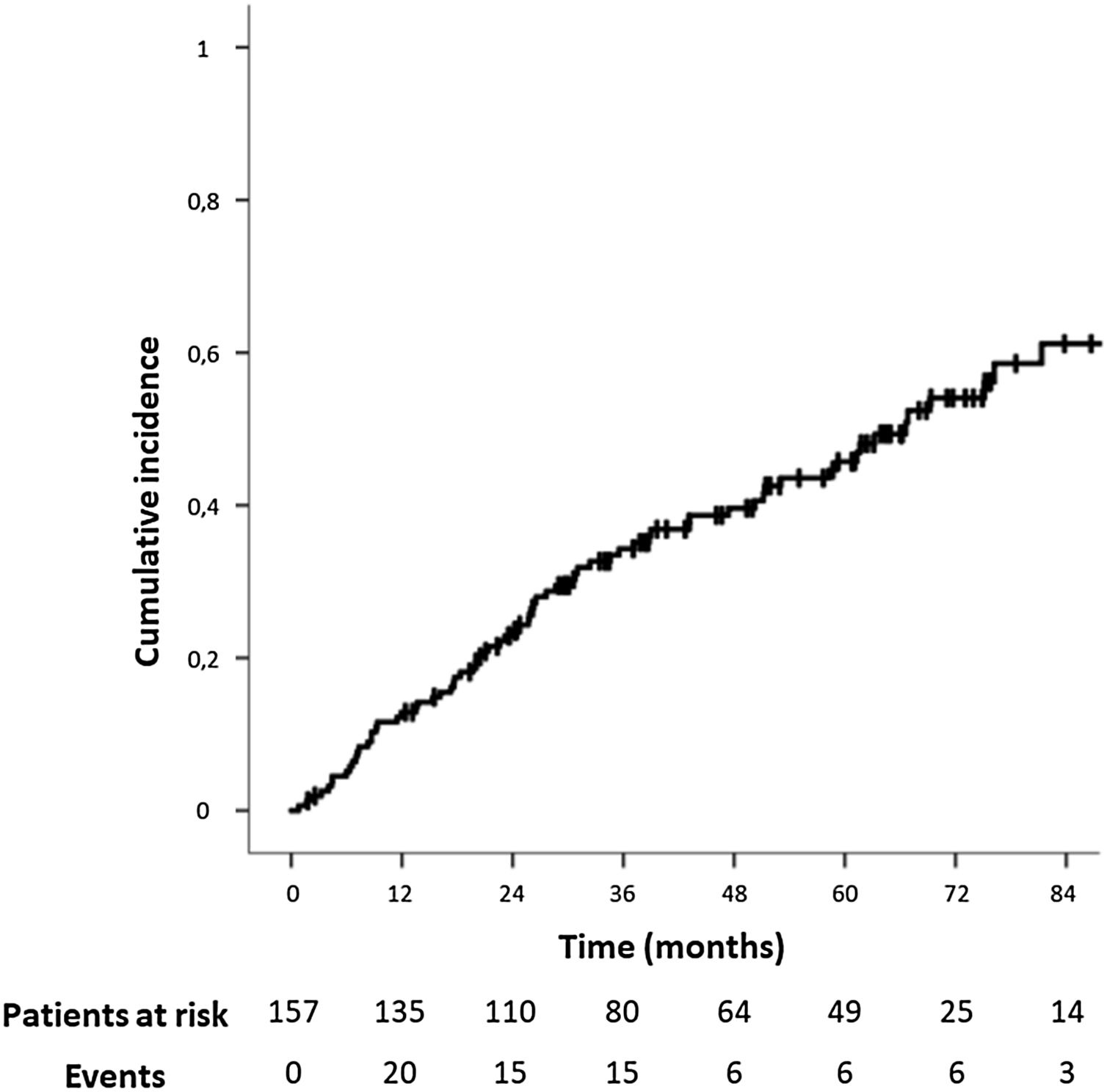
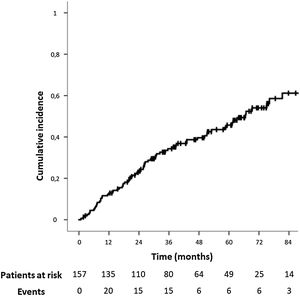
During follow-up, 73 patients (46.5%) developed an IH after open AAA repair. In this period, 98 patients (62.4%) underwent a CT scan. The IH rate was even higher in this population, and 59 of them (60.2%) presented an IH.
The median follow-up time for the study population was 37.20 months (IQR: 20.53–64.12). The median time for IH development was 24.43 months (IQR: 10.40–45.27,). The IH cumulative incidence is depicted in Fig. 2. Fourteen patients (8.9%) underwent surgical IH repair.
Risk factors for incisional herniaAll variables were explored in univariate analysis as risk factors for incisional hernia, and those found to be risk factors in univariate analysis are depicted in Table 4. The baseline characteristics were: the smoking habit, both active (HR: 4.578; 95% CI: 1.386–15.127 [p: 0.013]) and past (HR: 4.868; 95% CI: 1.501–15.787 [p: 0.008]), and chronic kidney disease (HR: 2.057; 95% CI: 1.193–3.549 [p: 0.015]). Previous history of abdominal surgery was almost statistically significant (HR: 1.621; 95% CI: 0.997–2.635; p: 0.051).
Risk factors for incisional hernia.
| Univariate analysis | HR | 95%CI | p-Value |
|---|---|---|---|
| Smoking habit | |||
| Non-smoker | 1.000 | ||
| Active | 4.578 | 1.386–15.127 | 0.013 |
| Quitted | 4.868 | 1.501–15.787 | 0.008 |
| Chronic kidney disease | 2.057 | 1.193–3.549 | 0.015 |
| Previous abdominal surgery | 1.621 | 0.997–2.635 | 0.051 |
| Aortic bypass | |||
| Aorto-aortic | 1.000 | ||
| Aorto bi-iliac | 1.780 | 1.088–2.912 | 0.022 |
| Aorto bi-femoral | 1.396 | 0.497–3.916 | 0.527 |
| Aorto-iliac and femoral | 0.293 | 0.040–2.141 | 0.226 |
| Aorto-uni-iliac | 2.601 | 0.625–10.816 | 0.189 |
| Complications according to Clavien-Dindo classification | |||
| 0 | 1.000 | ||
| I | 0.000 | 0.000–1.9×10175 | 0.955 |
| II | 1.186 | 0.708–1.989 | 0.517 |
| III | 2.771 | 1.080–7.111 | 0.034 |
| IV | 1.024 | 0.365–2.868 | 0.964 |
| V | 0.000 | 0.000–1.989 | 0.998 |
| Multivariate analysis | HR | 95%CI | p-Value |
|---|---|---|---|
| Smoking habit | |||
| Non-smoker | 1.000 | ||
| Active | 4.535 | 1.369–15.022 | 0.013 |
| Quitted | 4.652 | 1.430–15.131 | 0.011 |
| Chronic kidney disease | 2.007 | 1.162–3.467 | 0.012 |
| Previous abdominal surgery | 1.653 | 1.014–2.695 | 0.044 |
During surgery, patients with an aorto bi-iliac bypass were in a greater risk of IH compared to those with an aorto-aortic bypass (HR: 1.780; 95% CI: 1.088–2.912 [p: 0.22]). The midline incision had a reduced IH rate (61/139; 43.9%) compared to the pararectal incision (12/17; 70.6%), although it was not statistically significant in univariate survival analysis (p: 0.151). There were no statistically significant differences in IH rate according to the suture material used (p: 0.955): 47.3% (61/129) for polydioxanone monofilament (PDS®), 44.4% (4/9) for polypropylene monofilament (Prolene®), and 43.8% (7/16) for polyglactin multifilament (Vicryl®).
For the postoperative period, patients with complications requiring surgical, endoscopic, or radiological intervention (Clavien-Dindo III) had a greater risk of IH (HR: 2.771; 95% CI: 1.080–7.111 [p: 0.034]). These differences were not detected analyzing only surgical reintervention, with 44.4% (4/9) of reintervened patients having an IH (p: 1.000). Patients who had a surgical site infection had a 40% (2/5) rate of IH. The hemoglobin levels or the WBC count 48h after surgery were not predictors of further IH development.
Factors with a p-value>0.2 were not considered for multivariate analysis. Once adjusted in multivariate analysis (Table 4), the risk factors linked to IH development were all preoperative factors. The smoking habit, both active (HR: 4.535; 95% CI: 1.369–15.022 [p: 0.013]) and past (HR: 4.652; 95% CI: 1.430–15.131 [p: 0.011]), chronic kidney disease (HR: 2.007; 95% CI: 1.162–3.467 [p: 0.012]) and previous abdominal surgery (HR: 1.653; 95% CI: 1.014–2.695 [p: 0.044]) were independent risk factors.
DiscussionIn our study, the IH incidence after open repair of AAA was 46.5%. The first studies provided an IH incidence between 5.4% and 11.3%.4,5,19 However, they had several limitations, such as a high rate of loss in follow-up patients or short follow-up periods. More recent articles showed that the IH incidence ranged from 28% to 37.2%.6,20 Our IH incidence of 46.5% is only overcome by Fassiadis et al.,7 who reported a 54.05% rate.
This higher rate could be explained by the definition of IH through an appropriate measure instrument. One of the strengths of our study was the use of an abdominal CT scan for the evaluation of the abdominal wall in the majority of the subjects, which allowed detecting small defects of the abdominal wall closure that constitute the basis on which larger symptomatic hernias develop. Moreover, the study followed the recommendations published by the EHS with a follow-up period of at least two years.15 A long and appropriate follow-up time as reported in randomized control trials (RCT)6,7,20 allows the development of IH, as it is a late postoperative event. A limitation was that not all patients underwent a CT scan, or the IH diagnosis might have not been listed in the electronic medical records. Therefore, the IH rate could have been even higher.
The analysis of risk factors demonstrated the relevance of two classic predictors for IH9: the presence of previous abdominal surgery and tobacco consumption. Tobacco triggers the metalloproteinase activity that leads to increased degradation of collagen type I activity and impaired synthesis of collagen type III, which favors the IH formation.13 For our cohort, the risk of patients with an active smoking habit was similar to the risk of patients with previous history of tobacco use. An abstinence period of at least four weeks prior to surgery could reduce the wound complications21 that might lead to IH. However, patients may not recover their baseline condition.22
Significant chronic kidney disease, defined in our study as an estimated Glomerular Filtration Rate (eGFR), <45ml/min, was another risk factor for IH. Uremia toxins might result in fibroblast dysfunction, with alteration of the granulation and epithelialization during the wound healing process.23 Heller et al. found an increased risk of IH after surgery in patients with an estimated glomerular filtration rate (eGFR)<60ml/min, compared to patients with eGFR>60ml/min (Odds ratio (OR): 2.8 [95% CI: 1.2–6.1]).23 The increased risk of IH was also described by Loewe et al.,24 who reported an IH rate of 16% in patients with advanced diabetic nephropathy; compared to 5.7% without nephropathy.
Results from the Danish national database showed a 10.4% hernia repair rate after open elective aortic reconstructive surgery, in six years of follow-up.14 A similar analysis in New York State presented a 7.93% (739 of 9314) ventral hernia repair rate after open AAA surgery.25 Our results are consistent with those rates, with 14 of 157 patients (8.92%) undergoing IH repair. Given that surgical repair of complex IH is linked to a relevant risk of postoperative complications,26 and considering patients’ characteristics, some were rejected for elective surgery due to high operative risk. Since not all patients will see their IH solved, prevention of the IH through an optimal closure technique remains the key.
Current recommendations to reduce the IH rate include the use of non-midline incisions if possible, avoiding the use of rapidly absorbable suture material, and the use of the small bites technique for the closure of the abdominal wall.15 Strategies proven to reduce the IH rate in patients with an open repair of AAA are a suture to wound length ratio of 4:1, and the placement of a prophylactic mesh.8 Abdominal closure with a suture to wound length ratio of more than 4:1 resulted in a reduction of the risk of IH (Relative Risk (RR): 0.42 [95% CI: 0.27–0.65]).8 In our study, the suture to wound length ratio was not listed, and could not be analyzed due to the retrospective design, perhaps being inferior to a 4:1 ratio. Besides, some patients were closed with non-recommended suture material15 such as polyglactin multifilament. These technical factors might have increased the IH rate.
The reinforcement of the abdominal wall closure with a prophylactic mesh after AAA surgery reduces the IH rate compared to the standard sutured closure (RR 0.27 [95% CI: 0.11–0.66]).27 Besides, it is a technique linked to a low rate of postoperative complications that do not increase the surgical site infection rate or the rate of reoperations.27 Except for Bevis et al.,6 who used a biological onlay mesh, all other RCT used a polypropylene mesh, either in retromuscular/sublay20,28 or onlay position.28,29 Jairam et al.20,28 compared the onlay vs. the sublay position, not finding differences in the IH incidence (10/61 (16%) vs. 10/52 (19%); p: 0.95). Since the onlay reinforcement is easier than the retromuscular placement of the mesh, it is recommended as the standard mesh localization for abdominal wall closure in high-risk patients.27
Given the epidemic proportions of IH after open repair of AAA, we attempted to implement a homogenous surgical closure technique in our institution, with conferences to widespread the EHS guidelines recommendations.15 Besides, collaborating with the Vascular Surgery Department, the systematic placement of an onlay prophylactic mesh began. In the following years, the results of an optimal abdominal wall closure and more extensive use of the prophylactic mesh after an open repair of AAA are undoubted to be reported.
ConclusionThe incisional hernia incidence after open repair of abdominal aortic aneurysm is a relevant issue that affected a high proportion of patients. Previous abdominal surgery, chronic kidney disease, and smoking habit were independent risk factors for incisional hernia. The effect of smoking is maintained even after having abandoned the smoking habit.
Authors’ contributionsAll authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by A.G. Barranquero, J.M. Molina and Gonzalez-Hidalgo. The first draft of the manuscript was written by A.G. Barranquero and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
FundingAuthors received no financial support for the research, authorship, and/or publication of this article.
Conflicts of interestNone.