Cytoreductive surgery plus intraperitoneal hyperthermic chemotherapy (HIPEC) has recently been established as the treatment of choice for selected patients with peritoneal carcinomatosis of colonic origin. Until recently, the simultaneous presence of peritoneal and hepatic dissemination has been considered a contraindication for surgery. The aim of this paper is to analyze the morbidity, mortality and survival of patients with simultaneous peritoneal and hepatic resection with HIPEC for peritoneal carcinomatosis secondary to colon cancer.
MethodsBetween January 2010 and January 2015, 61 patients were operated on, 16 had simultaneous peritoneal and hepatic dissemination (group RH+), and 45 presented only peritoneal dissemination (group RH−).
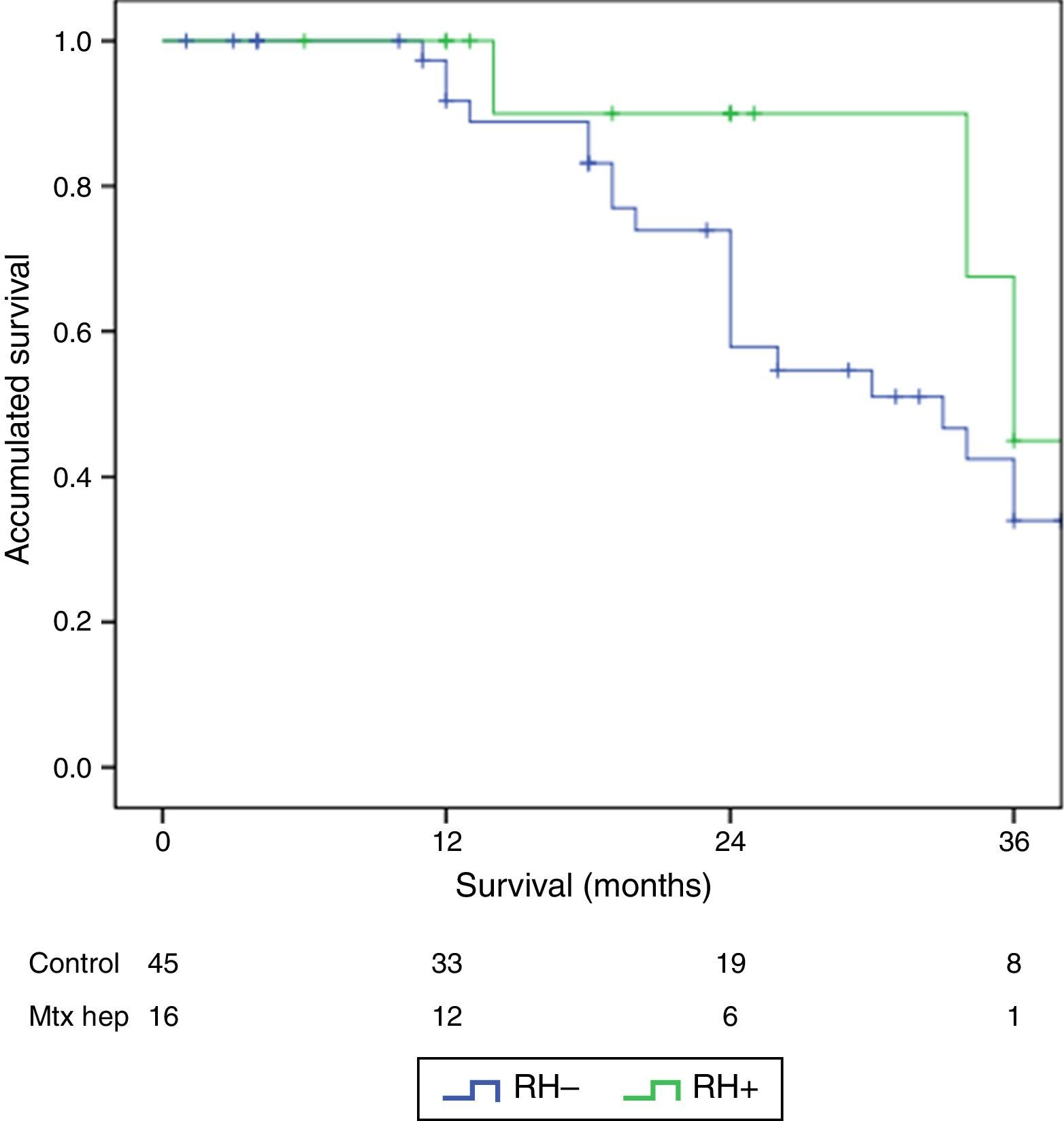
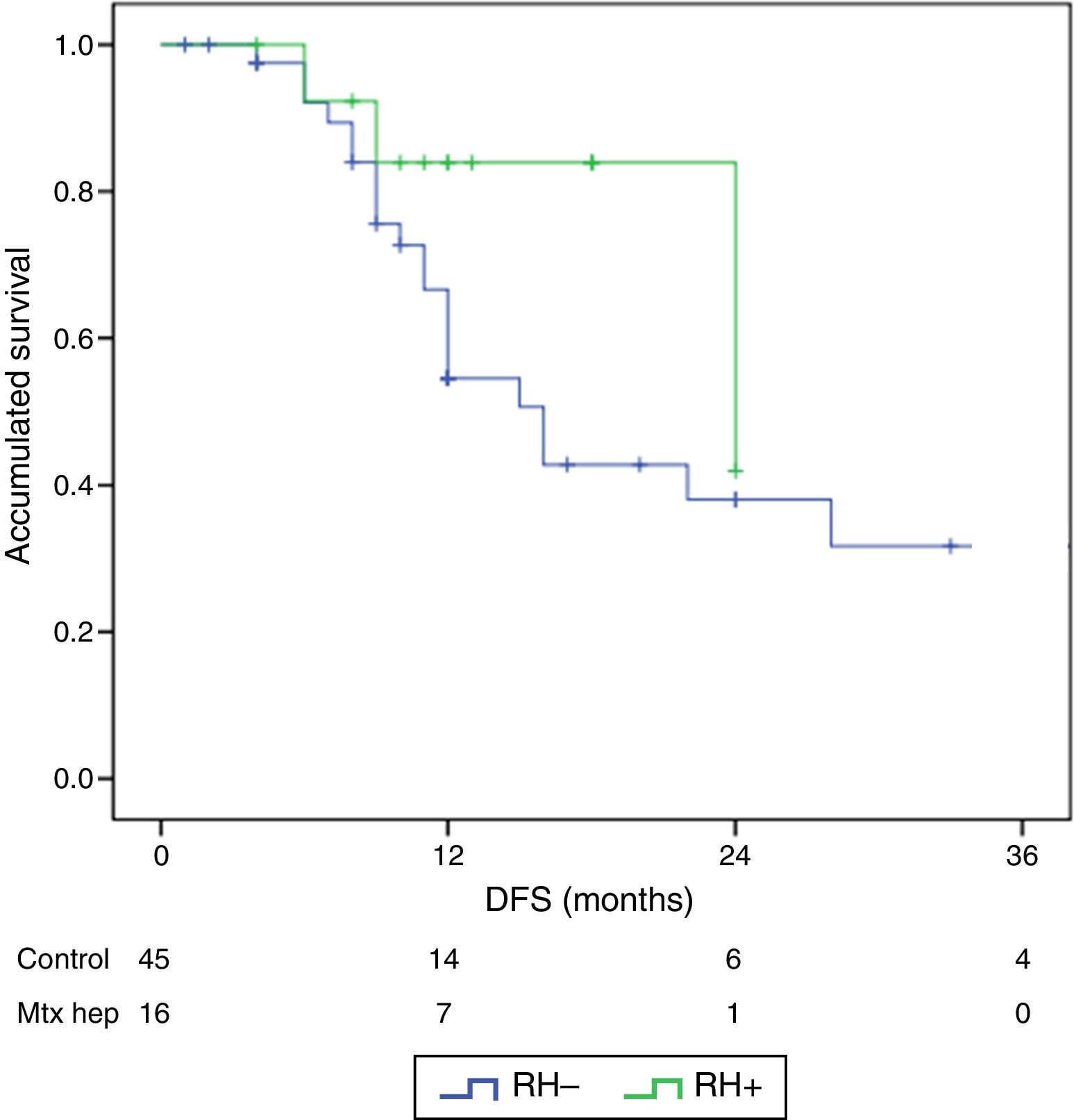
ResultsThere were no differences between the groups in terms of demographic data, length of surgery and extension of peritoneal disease. Postoperative grade III–V complications were significantly higher in the RH+ group (56.3 vs 26.6%; P=0.032). For the whole group, mortality rate was 3.2% (two patients in group RH−, and none in group RH+). Patients with liver resection had a longer postoperative stay (14.4 vs 23.1 days) (P=.027). Median overall survival was 33 months for RH−, and 36 for RH+ group. Median disease-free survival was 16 months for RH−, and 24 months for RH+ group.
ConclusionsSimultaneous peritoneal cytoreduction and hepatic resection resulted in a significantly higher Clavien grade III–V morbidity and a longer hospital stay, although the results are similar to other major abdominal interventions. The application of multimodal oncological and surgical treatment may obtain similar long-term survival results in both groups.
La citorreducción con quimioterapia intraperitoneal hipertérmica (HIPEC) se ha postulado como tratamiento de elección en pacientes seleccionados con carcinomatosis peritoneal por cáncer de colon. La presencia simultánea de diseminación peritoneal y hepática ha sido considerada una contraindicación para esta cirugía. El objetivo del presente estudio es analizar la morbimortalidad y supervivencia de los pacientes con carcinomatosis peritoneal por cáncer de colon, intervenidos mediante una citorreducción peritoneal y resección hepática simultánea con HIPEC.
MétodosEntre enero de 2010 y enero de 2015 se intervinieron 61 pacientes, 45 pacientes con carcinomatosis peritoneal (grupo RH−) y 16 con carcinomatosis peritoneal y metástasis hepáticas (grupo RH+).
ResultadosNo hubo diferencias significativas entre los 2grupos en los datos demográficos, ASA, duración de la intervención, ni extensión de la enfermedad peritoneal. Las complicaciones postoperatorias Clavien III-V fueron significativamente superiores en el grupo RH+ (56,3 vs 26,6%; p=0,03). La mortalidad global de la serie fue del 3,2% (2 pacientes en el grupo RH− y ninguno en el grupo RH+). Los pacientes con resección hepática presentaron una estancia hospitalaria significativamente más larga (14,4 vs 23,1 días; p=0,027). La mediana estimada de supervivencia global fue de 33 meses para RH− y de 36 meses para RH+, y la de supervivencia libre de enfermedad fue de 16 meses para RH− y de 24 para RH+.
ConclusionesLa citorreducción peritoneal con resección hepática simultánea presenta una morbilidad postoperatoria y una estancia hospitalaria significativamente mayores, aunque las cifras son similares a las de otras cirugías abdominales mayores. La aplicación de un tratamiento oncológico y quirúrgico multimodal permite obtener resultados de supervivencia similares en ambos grupos.
Hepatic and peritoneal progression in colon cancer have incidences of 50% and 15%, respectively, and are the 2 most frequent causes of death.1,2 Multimodal oncologic therapy has been making important advances in the prognosis of these patients.3 Surgical treatment of colorectal cancer liver metastases has demonstrated a 5-year survival rate of 25%–30%, with an operative mortality rate of approximately 3%.2 Furthermore, the application of cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) in selected patients with peritoneal dissemination of colon, appendiceal and ovarian cancer has improved prognosis in recent years and has obtained survival rates higher than those reached until now with systemic chemotherapy, as demonstrated by 2 randomized trials and several multi-center studies.3–7 In spite of these advances, until now, the unexpected intraoperative finding of peritoneal dissemination in a patient scheduled for liver resection contraindicated the resection, and vice versa: the finding of hepatic dissemination likewise limited peritoneal cytoreduction. With improvements in the radiological evaluation of tumor extension and the advances in perioperative care and surgical technique, some groups have recently proposed the simultaneous surgical treatment of hepatic and peritoneal disease during the same procedure.8–12
The objectives of this study were to analyze the morbidity and mortality of a select group of patients with simultaneous hepatic and peritoneal dissemination, treated with peritoneal and hepatic resection and HIPEC in the same surgery, and to evaluate survival rates. The results were compared with a control group of patients with peritoneal carcinomatosis (PC) without hepatic dissemination who underwent peritoneal cytoreduction with HIPEC alone.
MethodsThe study design consisted of a retrospective analysis from a prospective database. Between January 2010 and January 2015, 61 patients with PC of colorectal origin consecutively underwent CRS with HIPEC. Out of these patients, 16 presented synchronous hepatic dissemination that was resected together with the peritoneal disease plus HIPEC in the same surgery (RH+ group). The other 45 patients presented PC alone (RH− group) and were treated with CRS and HIPEC. The preoperative evaluation of the patients was agreed upon by the multidisciplinary Hepatic Oncologic Surgery and Peritoneal Surgery committees, including surgeons, oncologists, radiologists, pathologists and gastroenterologists. The study was approved by the local ethics committee and was conducted in accordance with guidelines for proper clinical practice, while preserving the confidentiality of the data at all times.
For selection, patients underwent physical exploration, colonoscopy, hepatic biochemistry, tumor marker studies and abdominopelvic CT scan. Magnetic resonance imaging of the liver and positron-emission tomography were ordered selectively in order to define the liver disease and rule out extra-abdominal disease. Resectability criteria included: acceptable comorbidity (ASA I and II), good general condition (ECOG 0 or 1), a maximum of 3 liver metastases, lack of tumor progression during treatment with systemic chemotherapy, absence of extra-abdominal disease and possibility to achieve complete peritoneal and hepatic resection.
The surgical technique performed entailed midline laparotomy with/without right transverse extension depending on the type of liver resection. The PC index (PCI) was calculated intraoperatively,13 and intraoperative liver ultrasound was carried out. The decision to perform simultaneous peritoneal and hepatic resections was based on the possibility to achieve complete resection. R0 hepatic resection was defined by macroscopically negative margins. The evaluation of the peritoneal tumor resection grade was determined by the Completeness of Cytoreduction Score (CC-Score)14 as: CC-0, absence of macroscopic disease; CC-1, visible peritoneal disease smaller than 2.5mm; and CC-2, presence of peritoneal nodules larger than 2.5mm. As no major liver resections were performed, we did not consider it necessary to estimate the percentage of residual liver volume.
HIPEC was only conducted in cases of optimal tumor resection (CC-0 and CC-1). After surgical cytoreduction, patients with colon cancer received intravenous 5FU (400mg/m2) and folinic acid (20mg/m2) one hour before hyperthermic chemotherapy. Afterwards, 460mg/m2 of oxaliplatin diluted in 3L/m2 of glucose at 1.5% were infused for 30min, to a mean temperature of 42°C, using the Coliseum open technique.15 In patients with hypersensitivity to oxaliplatin, mitomycin C was used (12.5mg/m2). In all cases, safety measures were employed for the management of cytostatic drugs and control of possible spills based on the recommendations for this type of procedures.16,17
Morbidity was recorded according to the Clavien-Dindo classification.18 Postoperative mortality was defined as mortality occurring during hospitalization.
Statistical AnalysisThe data are presented as mean±standard deviation (with a 95% confidence interval), median and interquartile range, or as percentage (%). The Kolmogorov–Smirnov test was used to determine whether the variables followed normal distribution. The continuous variables with normal distribution were compared using independent samples of the Student's t test; the non-parametric continuous variables were analyzed with the Mann–Whitney U test, and the categorical variables were compared between groups using the chi-squared or Fisher's exact tests. The survival and disease-free survival analyses were calculated by applying the Kaplan–Meier method, and the log-rank test was used to establish the existence of significant differences between the study populations. The relative risk, or hazard ratio, was estimated using the Cox proportional risk regression method for the factors of survival and disease-free survival. The statistical calculations were carried out with SPSS® software (v. 24 for Windows®). The survival results shown are actuarial.
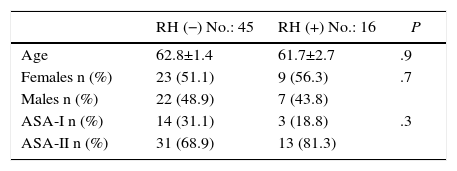
ResultsAs for the demographic data, between January 2010 and May 2015, 61 patients diagnosed with PC were consecutively treated with CCR+HIPEC, with curative intent. Two study groups were established: the RH− group, including patients with PC without liver involvement (45 patients); and the RH+ group, including patients with PC and simultaneous liver involvement (16 patients; 26.2%). The liver invasion of the patients in the RH+ group was diagnosed preoperatively in all cases. No significant differences were observed in the 2 groups in terms of age, sex or ASA (Table 1).
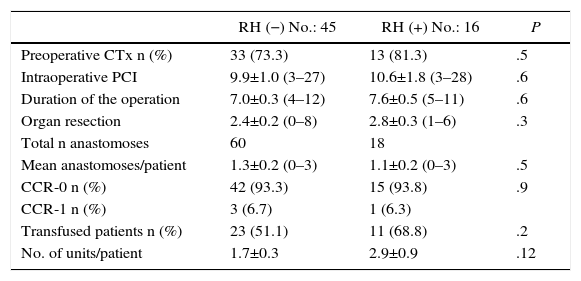
As for perioperative data, 73% of patients from the RH− group received preoperative chemotherapy, versus 81% of the RH+ group (P=.5). The most frequently used regimens were FOLFOX (52.7%), FOLFOX-bevacizumab (27%), FOLFIRI-bevacizumab (11.3%) and FOLFOX-FOLFIRI-bevacizumab (9%). All patients included in the study presented lack of tumor progression after the end of chemotherapy. The PCI, the number of organs removed and the duration of the intervention were similar between the 2 groups. Likewise, the number of anastomoses carried out showed no significant differences (1.3 vs 1.1 anastomoses/patient). Complete resection (CC-0) was achieved in 93.3% of the patients in the RH− group and in 93.8% of the RH+ group. Likewise, cytoreduction CC-1 (residual nodules smaller than 2.5mm) was also similar in the 2 groups (6.7% in the RH− group versus 6.3% in the RH+ group; P=.9). The preoperative transfusion rate was higher in the RH+ group, although the difference was not significant. The perioperative transfusion rate of the RH− group was 51.1% (23 patients) with 1.7 units of packed red blood cells transfused per patient. 68.8% of the patients from the RH+ group received transfusion (11 patients), with a mean of 2.9 units per patient (Table 2).
Perioperative Variables.
| RH (−) No.: 45 | RH (+) No.: 16 | P | |
|---|---|---|---|
| Preoperative CTx n (%) | 33 (73.3) | 13 (81.3) | .5 |
| Intraoperative PCI | 9.9±1.0 (3–27) | 10.6±1.8 (3–28) | .6 |
| Duration of the operation | 7.0±0.3 (4–12) | 7.6±0.5 (5–11) | .6 |
| Organ resection | 2.4±0.2 (0–8) | 2.8±0.3 (1–6) | .3 |
| Total n anastomoses | 60 | 18 | |
| Mean anastomoses/patient | 1.3±0.2 (0–3) | 1.1±0.2 (0–3) | .5 |
| CCR-0 n (%) | 42 (93.3) | 15 (93.8) | .9 |
| CCR-1 n (%) | 3 (6.7) | 1 (6.3) | |
| Transfused patients n (%) | 23 (51.1) | 11 (68.8) | .2 |
| No. of units/patient | 1.7±0.3 | 2.9±0.9 | .12 |
With regard to resections, the mean number of resected liver lesions was 1.2 per patient, with a maximum of 2 lesions; mean size was 2.3cm (ranges: 1–4). The disposition of the lesions was intraparenchymal in 15 patients (93.8%) and subcapsular in one patient (6.2%). The most frequent locations were segment VII (33.5%), segment IV (22.2%), segment III (22.2%), segment IV (16.6%) and segment V (5.5%). The procedures performed included 11 segmental resections (68.7%), 4 metastasectomies (25%) and a left lobectomy (6.3%). In all patients, free resection margins were achieved. Additionally, deferred hepatic radiofrequency ablation was performed 1.5 months after surgery in one patient with a central lesion that was 2cm in diameter.
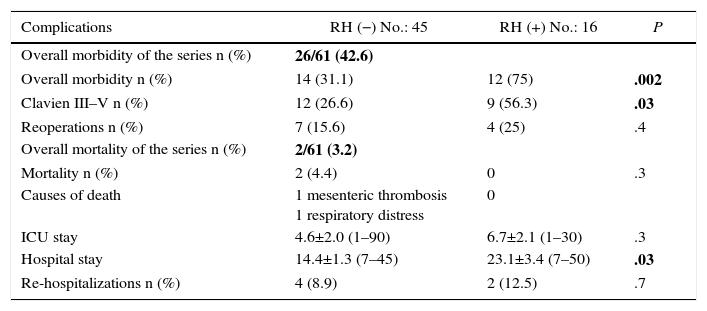
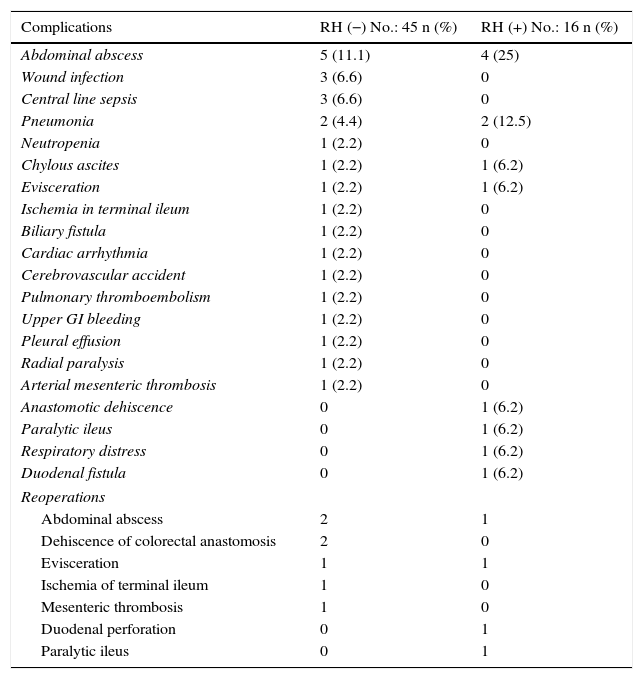
Regarding morbidity, mortality and reoperations, it should be mentioned that the overall morbidity of the series was 42.6%. Clavien-Dindo grades III–V complications were significantly lower in the group without liver resection (26.6 vs 56.3%; P=.03%). 50% of all the complications recorded were infectious; the only anastomotic dehiscence reported in the series (1.6%) occurred in the liver resection group. The postoperative mortality of the series was 3.2% (2 patients in the RH− group, 0 in the RH+ group). The causes were massive arterial mesenteric thrombosis with refractory multiple organ failure in one patient and respiratory distress associated with nosocomial bilateral bronchopneumonia in another patient. Tables 3 and 4 enumerate the complications recorded in the 2 groups.
Morbidity and Mortality.
| Complications | RH (−) No.: 45 | RH (+) No.: 16 | P |
|---|---|---|---|
| Overall morbidity of the series n (%) | 26/61 (42.6) | ||
| Overall morbidity n (%) | 14 (31.1) | 12 (75) | .002 |
| Clavien III–V n (%) | 12 (26.6) | 9 (56.3) | .03 |
| Reoperations n (%) | 7 (15.6) | 4 (25) | .4 |
| Overall mortality of the series n (%) | 2/61 (3.2) | ||
| Mortality n (%) | 2 (4.4) | 0 | .3 |
| Causes of death | 1 mesenteric thrombosis 1 respiratory distress | 0 | |
| ICU stay | 4.6±2.0 (1–90) | 6.7±2.1 (1–30) | .3 |
| Hospital stay | 14.4±1.3 (7–45) | 23.1±3.4 (7–50) | .03 |
| Re-hospitalizations n (%) | 4 (8.9) | 2 (12.5) | .7 |
Overall morbidity and mortality of the series in bold.
Postoperative Complications and Reoperations.
| Complications | RH (−) No.: 45 n (%) | RH (+) No.: 16 n (%) |
|---|---|---|
| Abdominal abscess | 5 (11.1) | 4 (25) |
| Wound infection | 3 (6.6) | 0 |
| Central line sepsis | 3 (6.6) | 0 |
| Pneumonia | 2 (4.4) | 2 (12.5) |
| Neutropenia | 1 (2.2) | 0 |
| Chylous ascites | 1 (2.2) | 1 (6.2) |
| Evisceration | 1 (2.2) | 1 (6.2) |
| Ischemia in terminal ileum | 1 (2.2) | 0 |
| Biliary fistula | 1 (2.2) | 0 |
| Cardiac arrhythmia | 1 (2.2) | 0 |
| Cerebrovascular accident | 1 (2.2) | 0 |
| Pulmonary thromboembolism | 1 (2.2) | 0 |
| Upper GI bleeding | 1 (2.2) | 0 |
| Pleural effusion | 1 (2.2) | 0 |
| Radial paralysis | 1 (2.2) | 0 |
| Arterial mesenteric thrombosis | 1 (2.2) | 0 |
| Anastomotic dehiscence | 0 | 1 (6.2) |
| Paralytic ileus | 0 | 1 (6.2) |
| Respiratory distress | 0 | 1 (6.2) |
| Duodenal fistula | 0 | 1 (6.2) |
| Reoperations | ||
| Abdominal abscess | 2 | 1 |
| Dehiscence of colorectal anastomosis | 2 | 0 |
| Evisceration | 1 | 1 |
| Ischemia of terminal ileum | 1 | 0 |
| Mesenteric thrombosis | 1 | 0 |
| Duodenal perforation | 0 | 1 |
| Paralytic ileus | 0 | 1 |
A total of 11 patients (18%) were reoperated on, 7 in the RH− group (15.6%) and 4 in the RH+ group (25%; P=.2). Hospitalization in the intensive care units was shorter in the group without liver resection, although the difference was not significant (4.6±2.0 days; range 1–90), versus 6.7±2.1 days; range 1–30; P=.3). Nonetheless, hospitalization was significantly shorter in the group without liver resection at 14.4±1.3 days (range 7–45), versus 23.1±3.4 days (range 7–50; P=.027). The liver resection group presented a higher rate of re-admissions (8.9 vs 12.5%), but with no significant differences.
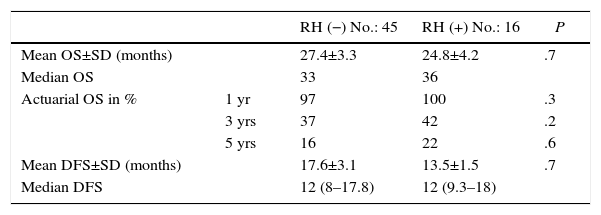
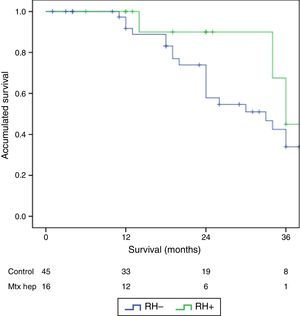
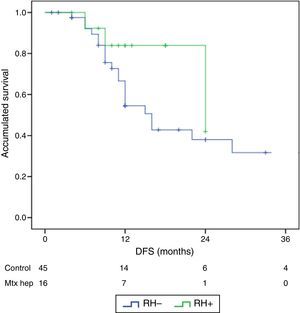
The follow-up period was between 6 and 33 months. Mean overall survival was 27.4±3.3 months in the RH− group (range 5–96) and 23.4±3.8 months in the RH+ group (range 6–32; P=.7). Mean disease-free survival was 17.6±3.1 months in the RH− group and 13.4±1.6 months in the RH+ group (P=.4). Median overall survival, estimated by the Kaplan–Meier method, was 33 months for the RH− group and 36 for the RH+ group. Median disease-free survival was 16 months for the RH− group and 24 months for the RH+ group. 31.2% of the RH+ patients presented hepatic recurrence, with a mean of 11.4 months after surgery. Actuarial overall survival after 1, 3 and 5 years was 97, 37 and 16%, respectively, in the group without liver resection and 100, 42 and 22% in the group with liver resection. There were no significant differences between the two groups (Table 5, Figs. 1 and 2).
Oncologic Results.
| RH (−) No.: 45 | RH (+) No.: 16 | P | ||
|---|---|---|---|---|
| Mean OS±SD (months) | 27.4±3.3 | 24.8±4.2 | .7 | |
| Median OS | 33 | 36 | ||
| Actuarial OS in % | 1 yr | 97 | 100 | .3 |
| 3 yrs | 37 | 42 | .2 | |
| 5 yrs | 16 | 22 | .6 | |
| Mean DFS±SD (months) | 17.6±3.1 | 13.5±1.5 | .7 | |
| Median DFS | 12 (8–17.8) | 12 (9.3–18) |
SD: standard deviation; OS: overall survival; DFS: disease-free survival.
Hepatic and peritoneal progression are the two most frequent causes of death in patients with colorectal cancer.12 At the time of diagnosis, 25% of patients present liver metastases, while 35%–55% develop metastases after resection of the primary tumor.19 In recent years, multimodal treatment with systemic chemotherapy, CRS and HIPEC have modified the prognosis of patients with PC secondary to colon cancer.1,3,5,7,13 In spite of the lack of phase III trials, surgery is today the treatment of choice for resectable liver metastases in colorectal cancer, with a 5-year survival of 30%–35%.2,20–24 Peritoneal dissemination is present in 10%–15% of patients with colon cancer at the time of diagnosis and appears in up to 40% of patients after primary tumor resection. Furthermore, it represents the form of tumor progression with the worst prognosis, showing a poor response rate to systemic chemotherapy and a survival rate that is 30% lower than other forms of distant metastasis.22,25–27 In the 1990s, studies by Sugarbaker demonstrated that, before systemic expansion, peritoneal invasions could be considered a locoregional entity during a limited time and, therefore, susceptible to specific treatment with CCR+HIPEC in select cases.13 In recent years, a randomized trial and several studies have been published demonstrating the efficacy of CCR+HIPEC in select patients with PC secondary to colon cancer.5,9,28–30 Until recently, peritoneal dissemination represented a contraindication for the resection of liver metastases, and vice versa: the finding of hepatic metastases in patients with peritoneal disease limited the indication for applying the new CRS with HIPEC criteria.8,25,31 Later studies observed that morbidity, mortality and survival rates were similar in patients with hepatic metastases or peritoneal disease in whom complete tumor resection was achieved.20,21,32 In addition, the reduction in postoperative morbidity and mortality achieved in recent years has increased the safety of these interventions and, consequently, several groups have recently suggested the possibility of conducting liver and peritoneal resection simultaneously in select patients in whom it is possible to achieve complete tumor cytorreduction.8–11,22,25
The present study analyzes the preliminary results of a group of patients with simultaneous hepatic and peritoneal resection due to PC secondary to colon cancer in our setting. Patient selection is a key factor: while it is recommended to not perform CCR+HIPEC in patients with a PC index higher than 18 points33 in colon cancer because of its elevated morbidity and limited benefits for survival, there is also an agreement to limit simultaneous hepatic and peritoneal resection to patients with 3 or less liver lesions and to avoid major liver resections.9,22 In our series, the mean number of resected hepatic lesions was 1.2 lesions/patient and mean PCI was 12.8.
Advances in perioperative care and surgical technique have reduced postoperative mortality, which is currently 3%–6%.8,10,20,21 In our study, overall mortality was 3.2%, with a higher mortality rate in the group without liver resection (4.4 vs 0%), but there were no significant differences. The extension of the disease, measured by PCI, the number of resected organs and the duration of the intervention was similar in both groups. However, grade III–V complications were significantly higher in the groups with liver resection, and infectious complications were the most frequent, which provides evidence of the greater complexity of simultaneous hepatic and peritoneal resection. These morbidity and mortality results are similar to the standards recommended in other major abdominal surgeries, such as pancreaticoduodenectomy or esophagectomy, which suggests its feasibility in well selected patients and in hospitals with elevated patient volumes.8,10,22,34–37
We realize that the size of the group with peritoneal and hepatic disease is small, and this is the main limitation of our study. However, we think that the data are relevant and that this present study can be considered a pilot study for a future multicenter study with a larger patient volume. The follow-up results reflect the evolution of this subgroup of patients which, until recently, was considered inoperable. It is believed that patients undergoing surgery for simultaneous peritoneal and hepatic dissemination have poorer prognoses than patients operated on for peritoneal dissemination alone. However, mean survival is 24.8 months, with a disease-free survival time of 13.5 months and a 5-year survival rate of 22%. These results justify and explain the recent tendency to incorporate peritoneal cytoreduction with simultaneous liver resection within a multimodal oncological therapeutic plan in well selected patients, as it can increase survival in this select group of patients with advanced disease who were considered inoperable until recently.
The differences in the survival rates of both groups were not statistically significant. This fact may be due to the limited sample size of the RH+ group, so it is necessary to extend the study with a larger number of patients.
In conclusion, these results show that peritoneal cytoreduction and HIPEC with simultaneous liver resection has greater postoperative morbidity and longer hospitalizations compared to cytoreduction and HIPEC without liver resection. Nonetheless, these results are comparable with published reports in other complex abdominal surgeries. The application of this multimodal treatment option could increase the survival of a select group of patients with advanced disease that had been considered unresectable until recently.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Morales Soriano R, Morón Canis JM, Molina Romero X, Pérez Celada J, Tejada Gavela S, Segura Sampedro JJ, et al. Influencia de la resección hepática y peritoneal simultánea, en la morbimortalidad y supervivencia de los pacientes con cáncer de colon intervenidos mediante cirugía citorreductora con quimioterapia intraperitoneal hipertérmica. Cir Esp. 2017;95:214–221.
Partial data from this study were presented at the 6th National Conference of the Spanish Group for Peritoneal Surgery (Grupo Español de Cirugía Peritoneal), held in Madrid in November 2015.