There is scant experience with robot-assisted esophagectomy in cases of esophageal and gastro-esophageal junction cancer. Our aim is to report our current experience.
Patients and methodsObservational cohort study of the first 32 patients who underwent minimally invasive esophagectomy for esophageal cancer from September 2011 to June 2014. The gastric tube was created laparoscopically. In the thoracic field, a robot-assisted thoracoscopic approach was performed in the prone position with intrathoracic robotic hand-sewn anastomosis. Patient and tumour characteristics, surgical technique, short-term outcomes (morbidity and mortality) and oncological results (radicality and number of removed nodes) were evaluated.
ResultsThirty-two patients, with a mean age of 58 years (34–74), were treated by a totally minimally invasive esophagectomy: robotic laparoscopy and thoracoscopy (11 McKeown and 21 Ivor-Lewis). Twenty-nine received neoadjuvant chemoradiotherapy. There were no conversions to open surgery. Console time was 218min (190–285). Blood loss was 170ml (40–255). One patient died from cardiac disease. Nine patients had a major complication (Dindo-Clavien grade II or higher). There was no case of respiratory complication or recurrent laryngeal nerve palsy. Five patients had intrathoracic fistula, 4 radiological and one clinical. Three had chylothorax, 2 cervical fistula and one gastric tube necrosis. The median hospital stay was 12 days (8–50). All the resections were R0 and the median of removed lymph nodes was 16 (2–23).
ConclusionsOur results suggest that minimally invasive esophagectomy with robot-assisted thoracoscopy is safe and achieves oncological standards.
La experiencia con la esofaguectomía robótica en el cáncer de esófago y de la unión esofagogástrica es limitada. El objetivo de este estudio es presentar nuestra experiencia actual.
Pacientes y métodosEstudio prospectivo, de vigilancia observacional, de las primeras 32 esofaguectomías mínimamente invasivas por cáncer con toracoscopia robótica entre septiembre de 2011 y junio de 2014. La plastia gástrica se realizó por vía laparoscópica. La toracoscopia robótica se llevó a cabo con el paciente en decúbito prono y la anastomosis intratorácica, siempre de forma manual. Se evaluaron las siguientes variables: características clínicas y patológicas, técnica quirúrgica, resultados a corto plazo (morbimortalidad) y resultados oncológicos (radicalidad y ganglios extirpados).
ResultadosA 32 pacientes con una edad media de 58 años (rango 34-74) se les realizó una esofaguectomía mínimamente invasiva en su totalidad: laparoscopia y toracoscopia robótica (11 McKeown y 21 Ivor Lewis). En 29 casos se administró quimiorradioterapia neoadyuvante. No hubo conversiones a cirugía abierta. El tiempo medio de consola fue 218min (rango 190-285) y la pérdida de sangre fue de 170ml (rango 40-255). Un paciente falleció por causa cardiológica y 9 presentaron complicaciones mayores (grado II o más de Dindo-Clavien). No hubo complicaciones respiratorias ni parálisis recurrencial. Hubo 5 fístulas intratorácicas, 4 radiológicas y una clínica, 3 quilotórax, 2 fístulas cervicales y una necrosis de la plastia. La mediana de la estancia hospitalaria fue 12 días (rango 8-50). Todas las resecciones fueron R0 y se extirparon una mediana de 16 (rango 2-23) ganglios linfáticos.
ConclusionesNuestros resultados indican inicialmente que la esofaguectomía mínimamente invasiva con toracoscopia robótica es segura y respeta los principios oncológicos.
The standard treatment of oesophageal cancer is surgery, whether it is associated or not to neoadjuvant chemotherapy or chemoradiotherapy. Oesophagectomy continues to have a high mortality rate within elective gastrointestinal surgery, with rates that range from 5% to 18%, depending on the volume of the centre.1
The use of minimally invasive surgery (MIS) with the purpose of reducing the surgical trauma, and with that the associated morbidity and mortality, should be especially beneficial in such a complex and aggressive surgical technique such as oesophagectomy,2,3 especially when intrathoracic oesophagogastric anastomosis is performed.
The robotic system “Da Vinci”, with a tridimentional and amplified vision and more freedom of movements, resolves some limitations of conventional MIS for a more precise surgical dissection and the performance of manual anastomosis. In a lot of surgical fields, such as the thorax, and without the need of frequent changes of equipment,4,5 these advantages are clearer.
So far, a few groups have adopted this kind of surgery.6–14 One of them is our group,15 which adopted such surgery with the description of a first series of robotic Ivor-Lewis in prone position and with manual anastomosis. The initial results of this series, without respiratory complications or mortality, anastomotic leak rate (7.1%) and 3 radiological fistulas (21.4%), and oncological results comparable to the ones of other approaches, point out that the technique is feasible and safe despite the scarce number of tested cases.
For that reason, we think it is interesting to show our current experience with a new series that increases in over double the number of cases of the previous publication.15
The incorporation of new cases will allow us to prove, in a still not commonly used technique, if the morbidity, mortality and oncological results are stable, which should be the first objective at the time of incorporating new procedures. On the other hand, due to the lack of new publications in the last year, we think every new contribution in that sense is appropriate.
The aim of this study is to describe the technical aspects of robotic oesophagectomy in oesophageal cancer and the short-term results of our extended series.
Patients and MethodsBetween April 2008 and June 2014, 66 patients were operated on for oesophagus and oesophagogastric junction cancer, and in 51 (77%) patients, a minimally invasive oesophagectomy was entirely performed. In all cases, the gastric tube was performed through laparoscopy. In the first 19 patients, the thoracic time was performed through a conventional thoracoscopy and, in the last 32 cases (study group), through a robotic thoracoscopy (11 McKeown and 21 Ivor-Lewis).
The clinical data were collected prospectively in a database. All the patients were diagnosed through endoscopy and biopsy. The extension study included upper GI series, thoracoabdominal computed tomography, ecoendoscopy and positron emission tomography. The clinical and pathological cancer staging was performed following the TNM classification updated from 2010.16
In 29 (90.5%) patients with locally advanced tumours (cT2-4, cN1, cM0), neoadjuvant chemotherapy was administered. All the patients were reassessed a month after they finished treatment to exclude progression of the disease, and they underwent surgery 8 weeks after finishing it. All the patients had preoperative respiratory function tests, and later they received respiratory physical therapy.
We indicated an Ivor-Lewis transthoracic oesophagectomy in localised tumours of 30cm from the dental arch and a transthoracic oesophagectomy with cervical anastomosis (McKeown technique) in those located above, except in the cases of contraindication for the thoracic approach, the only circumstance in which a transhiatal approach is indicated.
The analysed variables were the following: hospitalisation time, in-hospital postoperative mortality (regardless of the elapsed time from surgery) and morbidity. The morbidity was classified according to the Dindo-Clavien17 criteria. The anastomotic leak has been defined as symptomatic or radiological. The first one is associated with mediastinitis, abscesses or gastrointestinal content in the drain.
Radiologic leak was defined as the image in the routine upper GI series performed on the 6th postoperative day, without symptoms.18 For the postoperative respiratory complications, the criteria of Briez et al.18 were used.
The oncological results were assessed from the percentage of R0 resections and the number of removed lymph nodes, following the protocol proposed by Colina et al.19 and, since 2013, the last protocol of the College of American Pathologists.20
Surgical TechniqueAll the interventions were performed by 2 surgeons (S.T. and M.F.) with the assistance of an intern in the abdominal stage. The patient was intubated with a double-lumen tube for selective pulmonary collapse during the entire thoracic stage and an epidural or paravertebral catheter was previously placed.
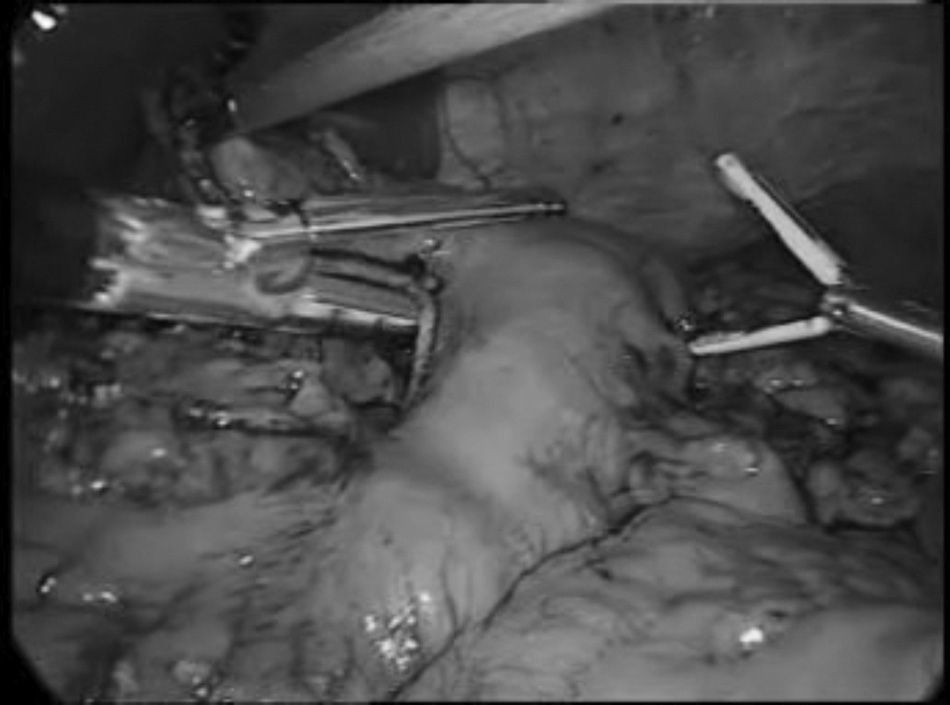
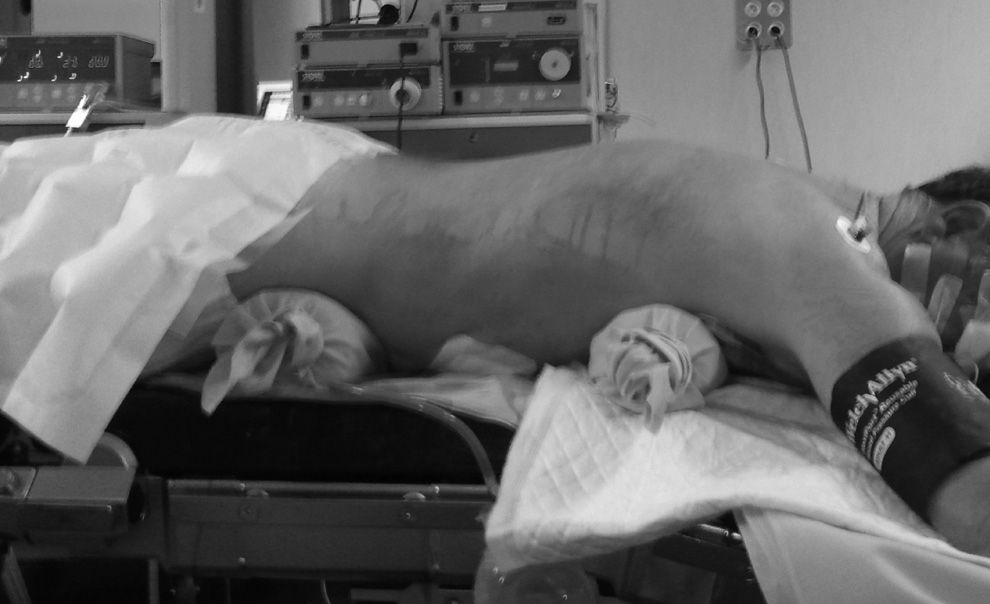
In the Ivor-Lewis technique, the oesophagectomy started by the abdominal stage for the creation of the gastric plasty according to the already described technique15 (Fig. 1). The robotic thoracoscopies were performed with the Da Vinci robot system (Intuitive, Sunnyvale, California, U.S.A.) SI model. The patients were placed in prone position, with extended arms forward and a 15–30° angulation at the scapula vertex level (Fig. 2). A conventional trocar of 12mm for the optic of 30° was placed on the 6th° intercostal, subscapularis space, and gas was insufflated at 4–6mmHg in order to widen the workspace. Under thoracoscopic vision, 2 trocars of 8mm of the robot, separated by 5cm from each other, were placed. Finally, another conventional trocar of 12mm was placed for the assistant and the robot on the left of the patient, using only 3 of its 4 arms (Fig. 3).
An oesophageal dissection was performed from the hiatus towards the thoracic vertex and a lymphadenectomy in 2 infracarinal fields widening to tracheobronchial lymph nodes and right paratracheal in the McKeown oesophagectomies with bipolar pliers in the left arm and the monopolar hook in the right one.
The thoracic duct was identified and divided between ligatures, near the hiatus. The arch of the azygos vein was cut with a lineal endostapler introduced by the trocar of the assistant and, at that height, the oesophagus was cut with monopolar scissors. After ascending the gastric plasty to the thorax, the specimen was cut with another lineal endostapler.
The anastomosis was performed manually in all patients. Interrupted sutures were used in the first cases. Then, interrupted sutures were used between the muscularis layer of the oesophagus and the gastric serous membrane in the posterior side and, after the opening of the stomach, the posterior side was completed with a continuous suture (V-Loc 2/0 Covidien, Mansfield, U.S.A.). A nasogastric tube was placed distal to the anastomosis before completing this with another continuous suture in the anterior side. The incision of one of the trocars was widened to a minithoracotomy of 4cm, protected with the Alexis® retractor (Applied Medical, Rancho Santa Margarita, California, U.S.A.), for the extraction of the specimen. Two chest tubes for drainage were placed.
Immediate extubation was always attempted, and the patients were transferred to the recovery room where early mobilisation was started and respiratory physical therapy was stimulated in the first 24h.
In the cases in which an oesophagectomy with cervical anastomosis was indicated, the surgery started with the thoracic time, and the mediastinal dissection extended towards the thoracic vertex.
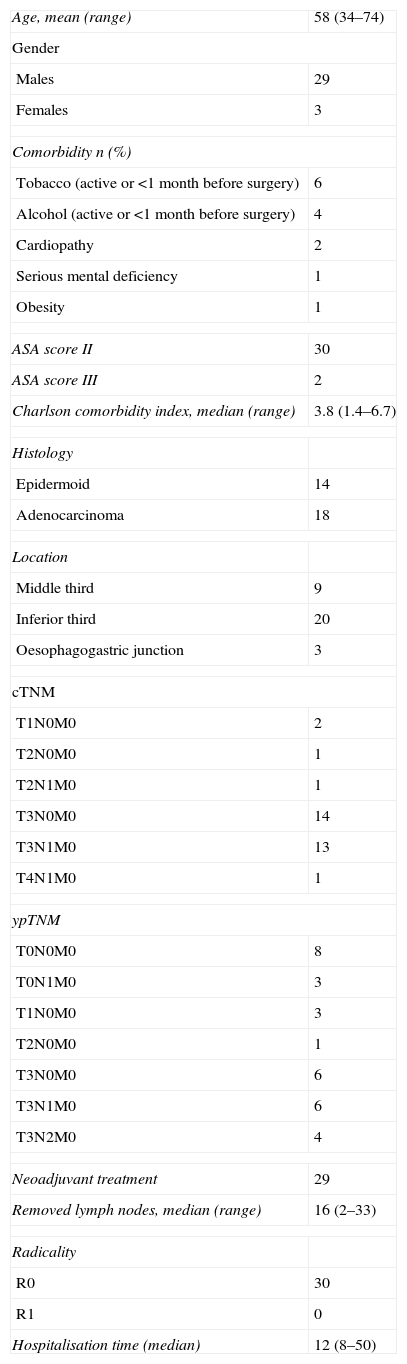
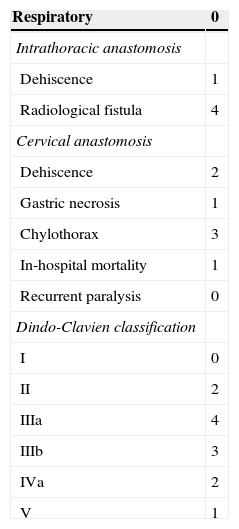
ResultsThe demographic and clinical and pathological characteristics of the patients are shown in Table 1. The postoperative complications are shown in Table 2.
Clinical Characteristics of the 32 Patients.
| Age, mean (range) | 58 (34–74) |
| Gender | |
| Males | 29 |
| Females | 3 |
| Comorbidity n (%) | |
| Tobacco (active or <1 month before surgery) | 6 |
| Alcohol (active or <1 month before surgery) | 4 |
| Cardiopathy | 2 |
| Serious mental deficiency | 1 |
| Obesity | 1 |
| ASA score II | 30 |
| ASA score III | 2 |
| Charlson comorbidity index, median (range) | 3.8 (1.4–6.7) |
| Histology | |
| Epidermoid | 14 |
| Adenocarcinoma | 18 |
| Location | |
| Middle third | 9 |
| Inferior third | 20 |
| Oesophagogastric junction | 3 |
| cTNM | |
| T1N0M0 | 2 |
| T2N0M0 | 1 |
| T2N1M0 | 1 |
| T3N0M0 | 14 |
| T3N1M0 | 13 |
| T4N1M0 | 1 |
| ypTNM | |
| T0N0M0 | 8 |
| T0N1M0 | 3 |
| T1N0M0 | 3 |
| T2N0M0 | 1 |
| T3N0M0 | 6 |
| T3N1M0 | 6 |
| T3N2M0 | 4 |
| Neoadjuvant treatment | 29 |
| Removed lymph nodes, median (range) | 16 (2–33) |
| Radicality | |
| R0 | 30 |
| R1 | 0 |
| Hospitalisation time (median) | 12 (8–50) |
ASA score: American Society of Anesthesiologists classification.
There were no conversions to open surgery. The median blood loss was 170ml (range 40–255) and the control panel time was 218min (range 190–285). There were no cases of recurrent paralysis or respiratory complications. The mortality was 3% (one patient died due to cardiological causes).
The global morbidity was 28% and the median hospitalisation time was 12 days (range 8–50). There were 4 intrathoracic radiological fistulas. In spite of being asymptomatic, they were treated with a stent, without need of drainage, and the patients started oral intake after 48h. There was a symptomatic intrathoracic leak on the 6th° postoperative day suspected due to clinical symptoms, and it was confirmed by upper GI series and computed tomography. A stent was placed, with bad results. The patient was re-operated on after 24h, performing an oesophagostomy and gastrostomy with admission to the intensive care unit (ICU) (Clavien IVa) with favourable progress.
There were 4 cases of chylothorax, 2 surgically treated, one by means of thoracotomy and another one by means of conventional thoracoscopy (Clavien IIIb). The third one was treated conservatively but needed to be admitted in the ICU (Clavien IVa). Among the patients with cervical anastomosis, there were 2 cases of cervical fistula solved with parenteral nutrition (Clavien II) and a necrosis of the proximal end of the gastric plasty which needed re-intervention with oesophagostomy and gastrostomy without admission in the ICU (Clavien IIIb).
The median of removed lymph nodes was 16 (range 2–23) and all the resections were R0. There were 8 cases (25%) of complete pathological response (ypT0, ypN0) and 3 cases with one lymph node involvement (ypT0, ypN1).
DiscussionThere is no standard procedure for oesophagectomy due to cancer.
The choice of a cervical or intrathoracic anastomosis and a transthoracic or transhiatal approach depends on the location of the tumour, the patient's characteristics and, largely, on the surgeon's experience.
The minimally invasive approaches are increasingly used in oncological surgery in general. Two recent meta-analysis analyse different approaches of minimally invasive oesophagectomy, with similar results to those of open surgery, and confirm the feasibility and security of this technique.2,3 With regard to the oncological results, minimally invasive oesophagectomy seems also comparable to open surgery.21
However, the complexity of the technique, and especially if it implies the performance of an intrathoracic anastomosis, makes MIS only suitable for the gastric or oesophageal mobilisation, followed by a cervical anastomosis in most of the series included in these revisions. The number of groups that entirely perform the minimally invasive Ivor-Lewis technique is very small, despite the fact that there are studies that describe a better quality of life with the high intrathoracic anastomosis.22
The robotic systems could resolve some of the limitations of MIS by providing a more amplified vision, in 3 dimensions and a greater width of mobility, especially in the thoracic time of an oesophagectomy.
The first oesophagectomy assisted by Da Vinci was published by Kernstine et al. in 2004.23 Since then, few and short series (6–47 patients) have been published and they were always with cervical anastomosis.6–11 The first robotic series of oesophagectomy of Ivor-Lewis with 22, 17 and 50 cases, respectively12–14 were published in 2013, and, in the last year, our series was published with 14 cases.15
The previous experience with oncological MIS in gastric and oesophageal cancer and the availability in our unit of the Da Vinci robotic system has let us accumulate a number of cases that, though small, is comparable to other series of worldwide bibliography, despite the low incidence of oesophageal cancer in our country.
Therefore, this study includes 11 robotic McKeown and 21 robotic Ivor-Lewis, among which there are cases recently published by us.15 Different authors stand up for the placement of the patient in prone position for MIS, because it favours a better exposition of the oesophagus, the accumulation of blood outside the surgical field and less handling of the lung.24,25
So far, no group, except ours, seems to use this position for intrathoracic anastomosis. Papanivelu et al.26 describe, in a series of 130 patients, only 2.3% of respiratory complications with the minimally invasive oesophageal mobilisation in that position in comparison with 18% published by Luketich et al.27 in their 222 patients in lateral recumbent position. In the more comprehensive retrospective analysis published by Kuwabara et al.,28 significant benefits of thoracoscopy performed in prone position were observed regarding blood loss, respiratory complications, duration of the postoperative hospitalisation time and number of removed lymph nodes.
In the case of robotic thoracoscopy, the prone position not only provides those advantages, but also lets us use only 3 arms of the robot in comparison with the 4 arms the rest of the groups use, because the separation of the lung is not necessary, which supposes an economic saving in clamps and decreases the conflict of space between the arms.
The performance of an intrathoracic anastomosis by MIS is not simple. In our experience, the prone position offers advantages in the dissection but makes the performance of mechanic anastomosis difficult, whether it is thoracic or transoral. In a recent review with 12 studies and 220 patients, Maas et al.29 compare the different techniques for an intrathoracic anastomosis by MIS and conclude that none of them are superior to the rest, with a leak percentage between 0% and 10%.
The robotic systems, thanks to their greater width of movements, can facilitate the performance of a manual anastomosis, thus allowing the use of the prone position and its advantages. In 2 of the 3 series published about robotic oesophagectomies with intrathoracic anastomosis, the incidence of anastomotic leaks was of 4.5% and 2%, respectively.12,14 However, the series of Sarkaria et al.,13 with 17 Ivor-Lewis, describe a 14% of clinically significant leaks, 14% of tracheoesophageal fistulas and 4 asymptomatic fistulas. The number of intrathoracic fistulas of our series, 4 radiologic (19%) and completely asymptomatic even though they were treated with stents, and a clinical leak (4.7%) do not differ from the publications up to date. Three of the radiologic fistulas and the leak took place in the first 13 cases of the series, probably because of a technical problem with suture, due to the loss of the sense of touch. Nowadays, we have replaced the interrupted suture by a double continuous suture, one in the anterior side and the other one in the posterior side. Besides, this technical modification shortens the surgical time. Apart from the technical difficulties, we have to add the necessary learning curve, another of the important results of this new series.
Our control panel time, despite being long, is difficult to assess, because it includes the performance of manual anastomosis in 21 of the 32 patients and there is no information in the literature to compare it. Only one of the robotic Ivor-Lewis series published includes manual anastomosis12 and its time is similar to ours. In the non-anastomosis series, times vary from 100 to 335min (being 180 the most common); therefore, our 218min do not seem excessive.
We have had neither conversions to open surgery nor respiratory complications.
We have had 3 cases of chylothorax (9.3%). Different studies describe a clear increase of its incidence with MIS, from the 2.4% of Smithers et al.30 to 11.6% of Luketich et al.31 Our results agree with the published studies, because we did not have cases of chylothorax with open surgery, and they are in the range of figures of the robotic surgery groups (3%–14%).7,14,23,32–34 This increase in the incidence is what led us to perform the prophylactic ligation of the thoracic duct, with no new cases since then.
Our percentage of R0 resections and the number of lymph nodes obtained are comparable to the other series of robotic oesophagectomy, as well as to the ones of open surgery. In some cases, the number of lymph nodes has been limited. However, the range, mean and median are similar to ones in the published studies. In a multicentre study that includes 299 patients of 6 specialised centres, the mean of lymph nodes obtained was 20, including cases of a one lymph node retrieved (range 1–77).35
The main limitations of this study are the number of cases and the short follow-up.
In conclusion, our results initially show that the minimally invasive oesophagectomy as a whole (including Ivor-Lewis) with robotic thoracoscopy is a feasible, safe and oncologically appropriate technique. Robotic thoracoscopy can provide the advantages of minimally invasive surgery, and it solves some of the limitations of the conventional thoracoscopy, even though this feature should be the objective of a future study.
Conflict of InterestThe authors declare that there are no conflicts of interest.
Please cite this article as: Trugeda Carrera MS, Fernández-Díaz MJ, Rodríguez-Sanjuán JC, Manuel-Palazuelos JC, de Diego García EM, Gómez-Fleitas M. Resultados iniciales de la esofaguectomía robótica en el cáncer de esófago. Cir Esp. 2015;93:396–402.
Part of the content of this document has been previously shown: in the III International Course of Robotic Surgery. Hospital San Carlos, Madrid, January 2013, in the XIX National Meeting of Surgery. Burgos, October 2013, in the XXIII National Meeting of the International Society for Diseases of the Oesophagus (ISDE), where it received the award for best oral communication. San Sebastián, May 2013.