Hiliar cholangiocarcinoma is the most common type of cholangiocarcinoma, an represent around 10% of all hepatobiliary tumors. It is an aggressive malignancy, resectable in around 47% of the patients at diagnosis. Complete resection is the most effective and only potentially curative therapy, with a survival rate of less than 12 months in unresectable cases. Axial computerised tomography and magnetic resonance are the most useful image techniques to determine the surgical resectability. Clinically, jaundice and pruritus are the most common symptoms at diagnosis; preoperative biliary drainage is recommended using endoscopic retrograde cholangiography or percutaneous transhepatic cholangiography. Surgery using extended liver resections with an en bloc resection of the liver with vascular reconstruction is the technique with the highest survival. Complete resection with histologically negative resection margins (R0), nodal involvement and metastases are the most important prognostic factors.
El colangiocarcinoma hiliar es el colangiocarcinoma más frecuente, representando hasta un 10% de todos los tumores hepatobiliares. Es un tumor agresivo con una resecabilidad al diagnóstico del 47% y una supervivencia sin cirugía inferior a 12 meses. Las pruebas de imagen más utilizadas para valorar estadificación y resecabilidad son la tomografía computarizada y la colangiorresonancia magnética. La mayoría de los pacientes presentan prurito e ictericia al diagnóstico, por lo que el drenaje biliar preoperatorio está indicado, pudiendo realizarse por colangiopancreatografía retrógrada endoscópica o colangiografía transparietohepática. En la actualidad, el único tratamiento curativo consiste en la resección quirúrgica, siendo la resección amplia con resección en bloque y reconstrucción vascular la técnica que ha conseguido una supervivencia mayor a largo plazo. La resección R0, la afectación ganglionar y las metástasis a distancia siguen siendo los factores pronóstico más importantes.
This neoplasia, which originates in the epithelium of the biliary ducts, or cholangiocarcinoma (CC), represents 10% of hepatobiliary tumours and 2% of malignant tumours.1,2 CC can be divided into 3 subtypes, depending on their anatomical origin within the biliary duct: intrahepatic or peripheral CC (ICC), perihilar CC or Klatskin's tumour (PHC) and distal CC.
PHC, which is the object of this revision, is the most frequent, and it represents around 40%–60% of all CC.2,3 This tumour is aggressive and silent, with non-specific symptoms until advanced stages, leading to late diagnosis and short survival without surgery of from 6 to 12 months.4 Surgery, which is the only available curative option, is only possible in approximately 47% of patients at the moment of diagnosis.5–8 The most important prognostic factors for this tumour are usually associated with surgical options, and tumour stage, size, ganglia and vascular involvement, intrahepatic metastasis and histological type are the most important factors.9–11
Anatomical SpaceThe anatomical space occupied by PHC would be delimited by the entry to the cystic duct at distal level, and the bifurcation of the right and left hepatic ducts at the proximal level.1,12 The most widely used classifications include all of the CC that originate in the biliary confluence or its surroundings. Some groups have suggested that the CC originating in the hepatic parenchyma sometimes can invade the biliary confluence, with an origin in the anatomical space delimited by the source of the rear right portal vein branch and the falciform ligament.13–15 These ICC involving the biliary confluence would be treated in the same way as tumours with an extrahepatic origin, and survival is similar to PHC in the same stage. They are usually highly developed tumours with locoregional vascular and lymph node invasion. Doubts about the biological behaviour of these tumours have led many groups to exclude them from perihilar tumours. In a study published by Ebata et al.13,15 of 250 patients resected for CC with involvement of the confluence, stage and survival were analysed according to whether the tumour was intrahepatic with involvement of the confluence (ICC), or if the tumour origin was in the extrahepatic biliary duct (PHC). A total of 83 patients presented ICC and 167 PHC. When stages were compared, patients with ICC displayed a higher frequency of vascular and lymph node involvement, with a TNM higher than that of the PHC group, presenting stages iii and iv in 59% of cases, in comparison with 38% in the PHC group. Nevertheless, if survival is divided according to stages in both groups, there are no statistically significant differences at 5 years, with slightly higher overall survival in the PHC group (20% vs 29%, respectively; P=.057), so that it was concluded that they are comparable in terms of treatment and survival. With the improved diagnostic tests and histopathological knowledge, in the future we may be better able to define whether they are 2 distinct entities or if they are clinically and biologically the same.
Histopatological Characteristics90% of PHC are adenocarcinomas. 10% are adenosquamous or squamous carcinomas, which in some cases have been associated with a history of lithiasis, cysts or anomalies of the biliary ducts. According to the appearance of the tumour, growth type and the biological and clinical behaviour of PHC, they are classified as16,17:
- -
Tumour or “mass-forming” CC: this is the most common form of presentation in ICC, although it can also be found in a large number of PHC. It is characterised by the formation of a tumour mass with clearly defined margins. It has a major fibrotic reaction and central necrosis is also frequent. This tumour originates in the opening of the biliary duct, invading the wall and disseminating by growing three-dimensionally, forming a nodular mass that gives rise to obstructive symptoms.16–19
- -
Infiltrating periductal CC: tumours of this type grow along a biliary duct in the form of a concentric longitudinal thickening through the connective tissue around the duct, causing stenosis or complete obstruction of the affected biliary duct.19 The majority of PHC are of this type, and they are difficult to identify using imaging techniques.17
- -
Intraductal or intraductal papillary CC: this variety is characterised by the presence of superficial and intraluminal tumours in the biliary duct. They produce mucin and cause the partial obstruction and dilatation of the ducts.16 This tumour has a low degree of malignancy and is usually small in size, although it may spread through the biliary mucus, giving rise to multiple tumours (papilomatosis or papillary carcinomatosis).20
The importance of this differentiation lies in the variations in survival depending on the subtype in question. Several studies have shown that patients with the intraductal papillary type have a better prognosis than those with the scleral-nodular varieties,21 with an average survival of 55 and 33 months, respectively,22 while vascular and ganglion involvement are less frequent in the intraductal papillary subtype.
To summarise, the majority of PHC is adenocarcinomas with a periductal growth pattern that gives them a poorer prognosis, while the variant with intraductal growth is the least frequent, although it has a better prognosis.
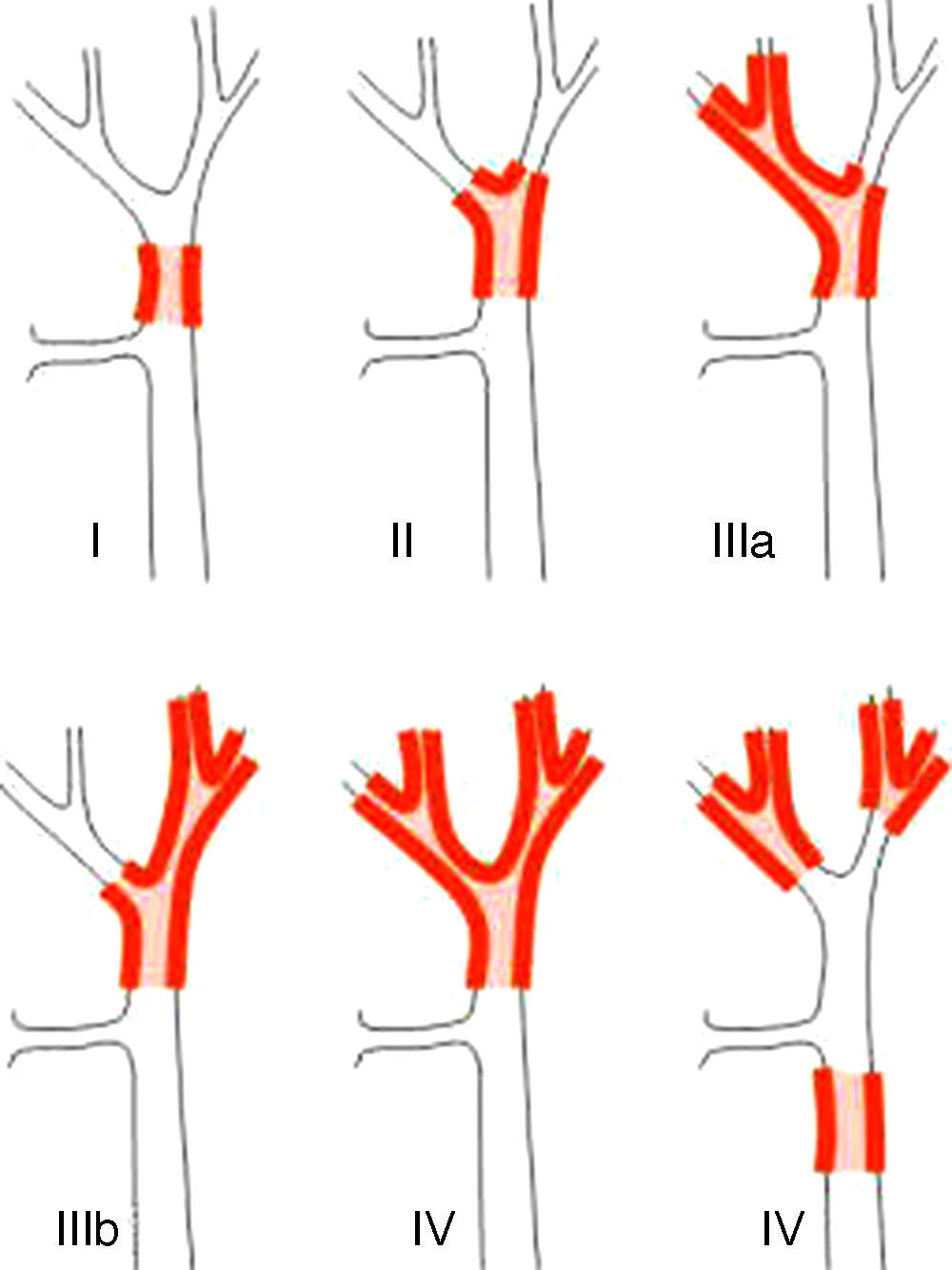
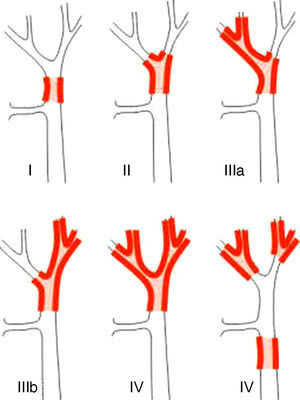
Staging SystemsStaging systems for PHC may be differentiated into pre- and postoperative classifications. Of the first, which are used when planning surgery, the most important system is the Bismuth-Corlette classification. This indicates which lobe is preferentially affected, and it therefore shows the type of hepatectomy which should be used (Fig. 1).23 This classification was invented in the 1970s and takes neither lymph node involvement nor metastasis into account, so that it now has less prognostic value.
The Bismuth-Corlette23 classification.
Another preoperative classification used in the USA is the one published by Jarnagin et al.10 of the Memorial Sloan Kettering Cancer Centre, New York. This classification aims to predict the resectability of tumours, taking 3 local extension factors into account. These are biliary extension, vascular involvement and lobe atrophy. This classification takes neither lymph node involvement nor metastasis into account, so that it has less prognostic value. As resectability differs from group to group, while the tendency is towards increasingly aggressive treatments, its value will depend on the criteria for non-resectability of each centre. Local involvement should now not be a criterion for non-resectability, on condition that we are able to achieve an R0 with surgical resection.
Of the postoperative classifications, the most widely used is the TNM classification of the Union for International Cancer Control (UICC), seventh edition.12 This classification takes pathological data into account, such as local extension, vascular involvement, lymph node involvement and metastasis, to establish a classification that includes the extension of the tumour and is prognosis in nature.
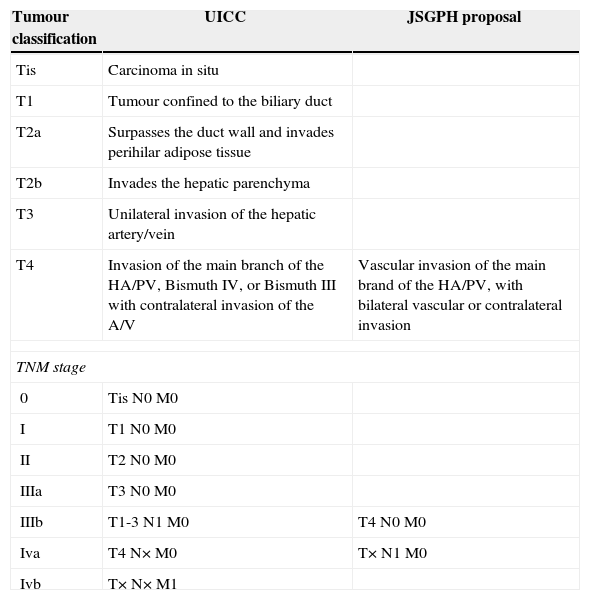
Recently the “Japanese Study Group on Perihilar Cholangiocarcinoma” (JSGPH) published a study which proposed modifying the classification of the “Union for International Cancer Control”. The basic differences are shown in Table 1, and they chiefly consist of13:
- 1.
Not considering Bismuth IV to be T4. The classification of the JSGPH therefore does not take bilateral biliary extension into account as a poor prognosis if an R0 resection is achieved.
- 2.
With respect to the stages, it prioritises lymph node involvement as the worst prognosis. They therefore consider lymph node involvement to be stage iva and not iiib (TNM7).
Comparison of the Basic Differences Between the UICC TNM 7 Classification and the Proposal by the JSGPH.
| Tumour classification | UICC | JSGPH proposal |
|---|---|---|
| Tis | Carcinoma in situ | |
| T1 | Tumour confined to the biliary duct | |
| T2a | Surpasses the duct wall and invades perihilar adipose tissue | |
| T2b | Invades the hepatic parenchyma | |
| T3 | Unilateral invasion of the hepatic artery/vein | |
| T4 | Invasion of the main branch of the HA/PV, Bismuth IV, or Bismuth III with contralateral invasion of the A/V | Vascular invasion of the main brand of the HA/PV, with bilateral vascular or contralateral invasion |
| TNM stage | ||
| 0 | Tis N0 M0 | |
| I | T1 N0 M0 | |
| II | T2 N0 M0 | |
| IIIa | T3 N0 M0 | |
| IIIb | T1-3 N1 M0 | T4 N0 M0 |
| Iva | T4 N× M0 | T× N1 M0 |
| Ivb | T× N× M1 | |
HA, hepatic artery; JSGPH, Japanese Study Group on Perihilar Cholangiocarcinoma; TNM, tumour lymph node metastasis; UICC, Union for International Cancer Control; PV, portal vein.
At a European level an international registry of perihilar tumours treated surgically has been created, led by the “International Cholangiocarcinoma Group for the Staging of PHC”. This group has published a new classification1 which takes tumour size into account, together with biliary, venous, arterial, ganglion and metastatic involvement, preoperatively as well as postoperatively. Nevertheless, the restrictions of this classification are that it merely describes tumour characteristics and the surgery to be performed, without going on to divide patients into groups or stages according to these variables. As a result is it not possible to extract prognostic conclusions and it is also impossible to compare the results of different groups.
Previous publications show that the preoperative classification used the most widely now to decide on the type of resection is Bismuth-Corlette, while the TNM 7 classification is used to define the long-term prognosis.
Diagnostic StrategiesThe symptoms associated with PHC more frequently are: jaundice (90%), weight loss and abdominal pain (35%), pruritus (26%) and acute cholangitis (10%).17 Due to these symptoms the initial diagnosis is usually made using by abdominal ultrasound, and this is a good screening test. Other diagnostic tests for this type of tumour would be:
- •
Abdominal Doppler Ultrasound: this makes it possible to evaluate arterial and portal permeability (thrombosis), which may condition the resectability of the tumour and therapeutic strategy, although it is not the test of choice for the evaluation of vascular involvement.
- •
Abdominal computerised axial tomography: this is useful for diagnosis of the primary tumour and disease extension, with 80% sensitivity in the evaluation of biliary extension. It is the technique of choice for preoperative evaluation of vascular anatomy, with a sensitivity of 93% and 87% for the evaluation of arterial and portal involvement, respectively. It has low sensitivity for the preoperative evaluation of lymph node involvement (50%).14,24 It is also useful in performing volumetric tests and calculating the hepatic volume remaining after surgical resection. It is also the most economical test for preoperative staging.
- •
Magnetic resonance and magnetic resonance cholangiography (RM-cholangiography): the best test for the diagnosis of the primary tumour and to evaluate biliary extension. It has a sensitivity of 86%–100%, and it is better than direct cholangiography, while it is also a non-invasive test.25 On the other hand, it has low sensitivity for the evaluation of vascular involvement (73%) and a sensitivity of 80% for invasion of the hepatic parenchyma.
- •
Direct cholangiography: endoscopic retrograde cholangiopancreatography (ERCP) and transparietal hepatic cholangiography (TPHC): these inform us about the level of biliary obstruction and make it possible to take samples from the lesion for cytology, with a sensitivity of 20%. They have now been replaced in diagnosis by MR-cholangiography. They are very useful for preoperative biliary drainage and in the palliative treatment of PHC, with the insertion of preferentially metal-coated stents.26–28
- •
Endoscopic ultrasound: this is useful for the evaluation of ganglion involvement in the area of the celiac trunk and peripancreatic region, establishing preoperative staging and making it possible to take fine needle aspiration biopsies.
- •
Positron emission tomography: this is useful in the study of patients with suspicion of metastatic involvement as well as involvement of adenopathies in the celiac trunk. Its sensitivity is controversial, and it may vary from 38% to 90%, depending on the series published.14,24,29
- •
Tumour markers: these are of limited usefulness, and CA 19.9 is the most commonly used. The majority of studies have evaluated these in pancreatic neoplasias and in CC to a lesser extent, without specifying their location or characteristics. The levels in serum of these markers are strongly influenced by biliary obstruction and jaundice due to their biliary elimination. Different normal values have been proposed, depending on the presence of hepatopathy (300U/ml) or jaundice (1000U/ml), while in pancreatic cancer sensitivities higher than 70% are obtained, with levels of specificity higher than 95%. Its concentration in these patients varies widely and does not correlate with tumour size, although it does correlate with metastatic involvement. The sensitivity and specificity of this marker can be increased by combining it with CEA, above all in Lewis A negative cases (non-producers of CA 19.9).30
It may be deduced from the above data that we usually commence study using an ultrasound scan for the diagnosis of PHC. This takes place in the context of a patient with jaundice, while computerised tomography and MR-cholangiography are the best staging tests and the most recommendable prior to surgery. MR or computerised tomography may be used for volumetric testing, depending on the type of apparatus or computer programmes available in each hospital. If there is doubt about spread into the lymph nodes which may contraindicate surgery positron emission tomography or fine needle aspiration endoscopy is recommended.
Non-Resectability CriteriaSurgery is the only curative treatment for PHC, and it offers the best long-term survival. The criteria adopted for surgical resection have expanded over recent years, from those described initially by the team of the Memorial Sloan Kettering Cancer Center by Burke et al.31 in 1998, until the recent introduction of approaches using vascular resection and extended hepatectomies.3,5,32–34 The criteria for non-resectability vary from hospital to hospital, and the most widespread are: vascular involvement on one side with contralateral biliary involvement up to the division of second-level radicals, distant hepatic metastases, vascular involvement of both hepatic lobes, extrahepatic or peritoneal involvement and adenopathic involvement of the celiac trunk, the upper mesenteric artery or the paraaortic region.3,5,8,31
In a multicentre study published by De Jong et al.34 which analysed 305 patients operated for PHC in 7 different centres in the USA and Europe, and which included patients with portal involvement, in multivariable analysis the only 2 statistically significant prognostic factors were involvement of the resection margin and lymph node involvement (P=.02). In the study published by Ebata et al.,15 analysing 1352 patients operated in 8 Japanese hospitals for PHC with curative intent, multivariable analysis of the statistically significant prognostic factors showed them to be: vascular invasion, invasion of the pancreas, lymph node involvement, the presence of metastasis and involvement of the resection margin. Lymph node involvement and metastasis were the factors which led to poorer survival at 5 years in comparison with the others (10%, 20% and 63%, respectively). These results support the use of surgery for these tumours, if in spite of local extension it is possible to perform a R0 resection, given that it is possible to increase survival to 5 years regardless of local extension.
Due to all of the above considerations it is recommended that each case be evaluated individually, and that surgery be used if an R0 resection can be achieved in the absence of distant metastasis or peritoneal involvement. Bilateral biliary and local vascular involvement should therefore not be non-resectability criteria if it is possible to operate while preserving more than 30% of liver volume and achieving an oncological resection.
Therapeutic StrategiesBiliary DrainageGiven that the majority of PHC patients debut with jaundice, one of the most important dilemmas regards the utility of preoperative biliary drainage. However, this is not free of complications, and those associated with ERCP with the insertion of a stent are: pancreatitis, duodenal perforation, duodenal migration, catheter obstruction and, most importantly, cholangitis. Drainage by CTPH presents a lower frequency of preoperative cholangitis, but it is associated with haemorrhage, catheter migration, up to 5% tumour dissemination within the trajectory of the catheter and discomfort and pain in the entry zone. Overall, according to the published studies, ERCP has an associated morbidity of 60%, and the corresponding figure for CTPH is 31%.14,35
Due to the above reasons, the utility of preoperative biliary drainage in hepatobiliopancreatic surgery has been called into question.36,37 These studies, which include all types of hepatobiliary surgery, have shown that preoperative biliary drainage in patients with jaundice increases associated morbidity without improving survival, mainly increasing complications involving infections. The European multicentre study published in 2013 by Farges et al.38 retrospectively analysed 366 patients who had been subjected to hepatectomy or extended hepatectomy and biliary resection due to PHC. They were classified according to whether or not preoperative biliary drainage had been performed. The group without preoperative drainage (non-PBD) was composed of 186 patients, and the group with biliary drainage (PBD) contained 180 patients. The groups were homogeneous in terms of age, tumour stage and portal resection. The PBD group presented more right hepatectomies (56% vs 44%). When both groups were compared according to the type of surgery performed, those patients subjected to right hepatectomy showed a higher number of postoperative liver failure if they belonged to the non-PBD group, with an incidence of 16% vs 4% in the PBD group (P=.009). In the multivariable analysis of the factors associated with higher mortality in the right hepatectomy group, having bilirubin levels under 3mg/dL before surgery was a statistically significant factor. However, if both groups of left hepatectomy patients were compared, the PBD group presented a higher number of postoperative sepsis, with an incidence of 6%, compared to 0% in the non-PBD group (P=.014). This study shows that although it is true that biliary drainage increases the incidence of postoperative sepsis, biliary drainage should be performed in those patients who are going to be subjected to right hepatectomy to reduce the morbimortality associated with postoperative liver failure, and other studies support this theory.39 Given that in the treatment of PHC the only studies which have shown greater survival are those which support extensive resections to achieve R0 resection, biliary drainage is recommended when surgery is indicated, and it may eventually require extensive hepatectomy of more than 50% of hepatic volume or trisegmentectomy, or if there is cholangitis. There is controversy about the cut-off point in bilirubin levels to indicate drainage, and >10mg/dL is one of the most widely used.5,37 It is recommended that biliary drainage by CTPH be performed, with emplacement of external drainage, which avoids manipulation of the tumour. This has a lower incidence of infections than CPRE and makes it possible, in those patients with unilateral drainage who do not normalise their bilirubin levels, to use bilateral biliary drainage.35,40
Tumour dissemination at the puncture site has been described in up to 5%–10% of cases in which CTPH was used,14,24 although these studies do not specify when external or internal–external drainage was used, and they do not take into account the time passed until surgery. Some authors recommend the use of endoscopically positioned nasobiliary drainage to prevent dissemination at the point of puncture, with a lower incidence of cholangitis and obstruction of the stent than is the case with CPRE.41 However, the same studies admit the difficulty of preoperative bilateral biliary drainage using this system, and this hinders normalisation of bilirubin levels prior to surgery in patients in which unilateral drainage has failed.42
To reduce the morbidity associated with infections following preoperative biliary drainage, it is suggested that the bile be systematically cultured following drainage and during surgery. Several studies have shown that 78%–94% of these cultures are positive for drained patients, as opposed to 20%–30% for undrained patients,43–45 and enterococcus is the most commonly isolated organism. These groups defend the use of prophylactic antibiotics, which although they increase the antibiotic resistance of the species isolated, in published studies this is shown to achieve a postoperative infection morbidity similar to that of undrained groups. The antibiotic selected will depend on the cultures and the antibiogram, although the majority of the groups used at least a third generation cephalosporin or fluoroquinolones+metronidazole, when no culture was available or when it was negative.43,44,46
Palliative biliary drainage is used in patients who cannot undergo resection. Drainage can be by ERCP, leaving a coated metal stent, or by CTPH in those cases where it is impossible to achieve correct drainage of both biliary ducts using ERCP.
Thus definitively, preoperative biliary drainage should be used in all patients with bilirubin above or equal to 10mg/dL and in those where hepatic resections will be greater than 50% of hepatic volume. The type of approach depends on the hospital, although CTPH with external drainage is recommendable to avoid manipulation of the tumour and the lower incidence of cholangitis. Some authors recommend that drained patients receive prophylactic antibiotics suitable for their biliary cultures.
Preoperative Portal EmbolisationPreoperative portal embolisation was described in the 1980s, initially by Makuuchi et al.47 and then by Kinoshita et al.48 The aim of portal embolisation is to increase the remaining hepatic volume in those cases in which it is considered insufficient in the preoperative volumetry, reducing the probability of postoperative liver failure. In a metaanalysis published recently by Higuchi and Yamamoto49 that included 836 patients with PHC with preoperative portal embolisation, morbidity was 1% and mortality 0.09%. Tumour progression during the procedure that prevented surgery amounted to 19.4%. These results support the idea that its use in PHC is justified in those patients with a remaining preoperative hepatic volume of less than 30%.
Staging LaparoscopyWith the advances in the sensitivity and specificity of non-invasive imaging tests over recent years, staging laparoscopy is falling into disuse. The most important criteria for non-resectability are lymph node involvement, biliary extension and vascular invasion. These are difficult to evaluate during laparoscopy, the precision and efficacy of which have been falling for years. This is shown by the recent revision by Rotellar and Pardo,50 in which precision and efficacy stood at 41% and 72%, respectively, in 2002, and at 14% and 32% in studies published in 2011. This fall is chiefly due to improvement in non-invasive tests such as MR-cholangiography. Its efficacy increases if patients are selected who are at high risk of presenting peritoneal dissemination or hepatic metastasis, thereby preventing unnecessary laparotomies.50–52 Non-invasive imaging techniques are recommended in advanced stages (T2/3/4) that present possible advanced peritoneal or lymph node involvement, and which cannot be punctured using echo-endoscopy before creating the preoperative biliary drainage. We always perform staging laparoscopy using intraoperative ultrasound scan to improve sensitivity to locorregional and lymph node involvement.53
Surgical TreatmentSurgery is still the only curative treatment for PHC,3,14 and it achieves a 20%–40% survival rate at 5 years (Table 2).10,11 Surgical resection must always be performed if it is possible to achieve a R0 resection. The main factors which affect survival following surgery are involvement of the resection margins, either microscopically (R1) or macroscopically (R2) together with lymph node involvement (N1 and N2).14,17,44,54 Several studies have shown that survival increases if the resection is broadened to create negative margins.3,5,33,45 The proposed techniques include extended hepatectomies with resection of the caudate segment, biliary resection, hilar lymphadectomy up to the celiac trunk and reconstruction with hepatojejunostomy.
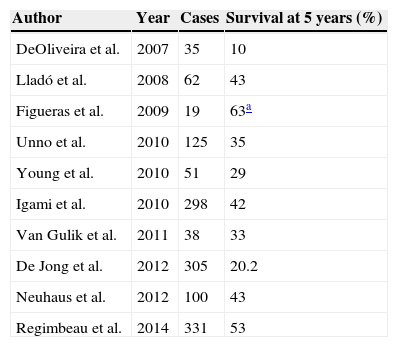
Survival Following Resection of the PHC According to the Series Published.
| Author | Year | Cases | Survival at 5 years (%) |
|---|---|---|---|
| DeOliveira et al. | 2007 | 35 | 10 |
| Lladó et al. | 2008 | 62 | 43 |
| Figueras et al. | 2009 | 19 | 63a |
| Unno et al. | 2010 | 125 | 35 |
| Young et al. | 2010 | 51 | 29 |
| Igami et al. | 2010 | 298 | 42 |
| Van Gulik et al. | 2011 | 38 | 33 |
| De Jong et al. | 2012 | 305 | 20.2 |
| Neuhaus et al. | 2012 | 100 | 43 |
| Regimbeau et al. | 2014 | 331 | 53 |
Survival following hepatic resection due to perihilar cholangiocarcinoma.
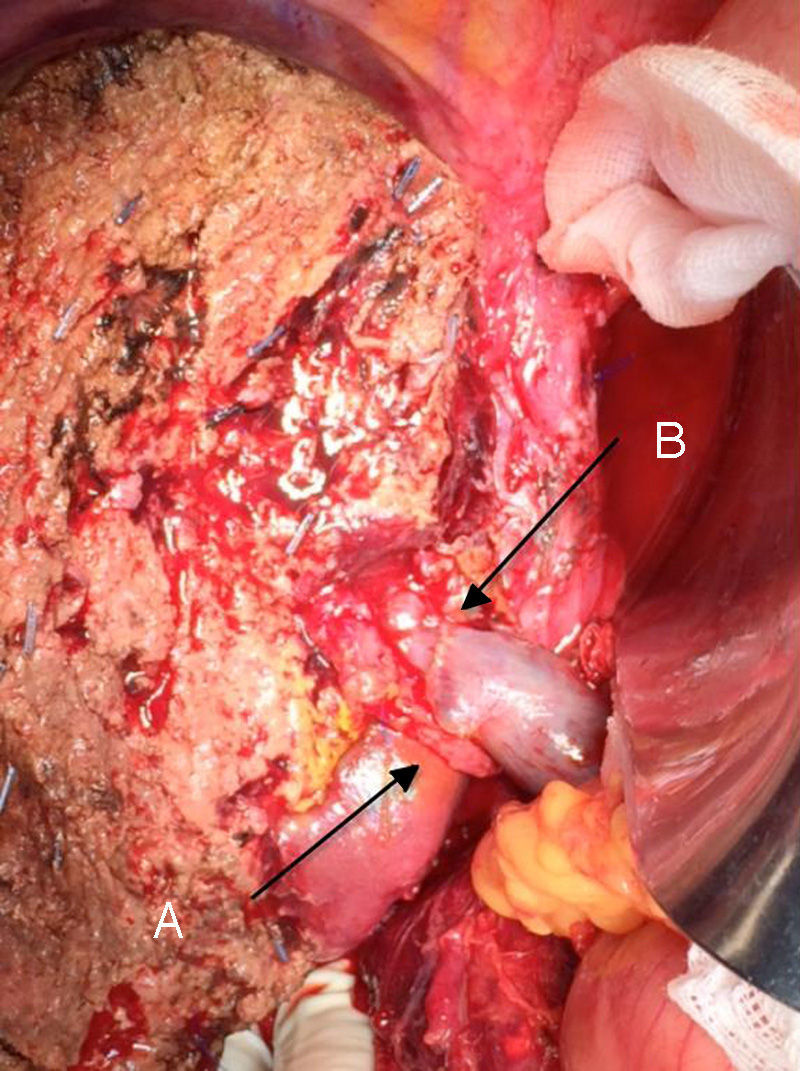
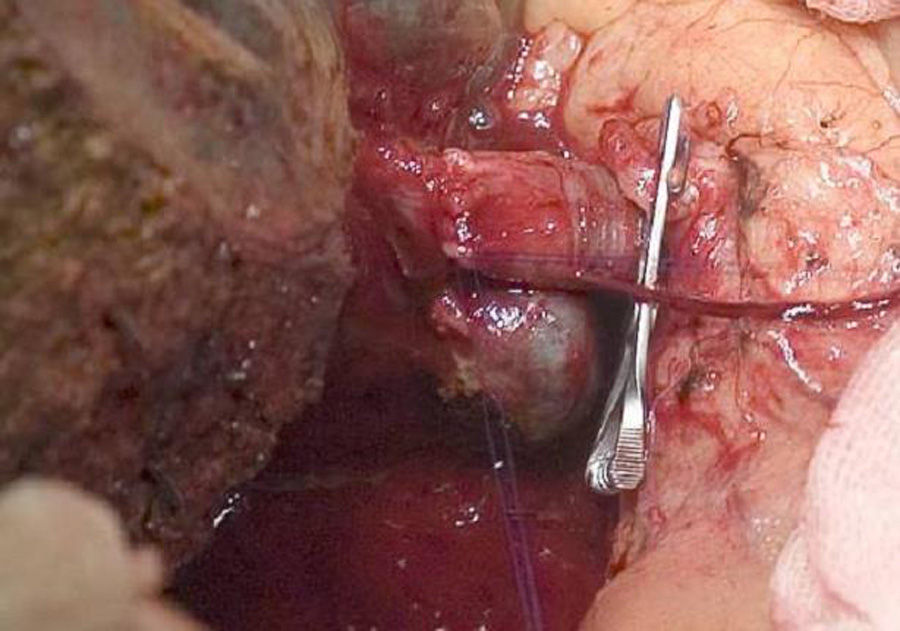
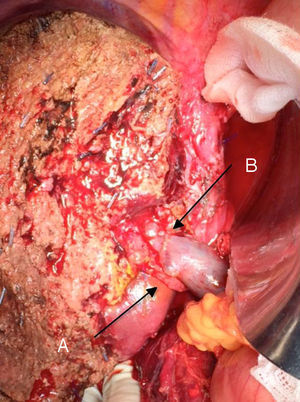
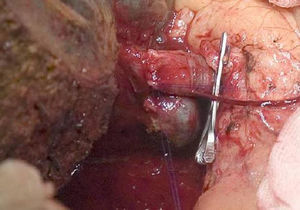
The “no-touch technique” first described by Neuhaus et al.32 involves the block resection of the hepatic hilum without manipulation of the tumour, increasing patient survival to 5 years in more than 15% of cases.3,33,34,55 This technique basically consists of the resection of the portal vein and right hepatic artery when they are close to the tumour, thereby preventing its dissection if this involves manipulation of the tumour, together with complete biliary resection with broad lymphadenectomy up to the root of the celiac trunk and reconstruction with hepaticojejunostomy. It will be preferable to select the right hepatic lobe (the essential “non-touch technique”, according to Neuhaus),33 on the condition that tumour extension makes it possible to include the right hepatic artery, which is the one closest to the tumour. This type of radical surgery leads to an acceptable rate of postoperative morbidity, of 50%-70% depending on the series in question, with a mortality of 10%–20%.3,5 PHC patient survival at 5 years stands at 20% to 40%, while studies published that include portal resection and series using the “no-touch technique” achieve 58% at 5 years (Figs. 2 and 3).3,5,33
It is also important to underline recent studies which show an increase in the survival of patients with preoperative vascular involvement following portal resection. They even achieve rates of survival that are equal to or higher than those for patients without preoperative vascular involvement and who were therefore not subjected to portal resection.33,56,57 In these studies, the incidence of hepatic and vascular complications are similar to those in the group without portal resection, except for those patients subjected to arterial resection, as these present a higher rate of morbimortality than the others. Due to all of these considerations, surgery is recommended for those patients with unilateral portal involvement or involvement of the confluence in preoperative tests, performing an en-bloc resection and vascular reconstruction. Arterial resection and reconstruction are not recommended unless the artery is clearly affected, as this is associated with poorer postoperative outcomes, so that in such cases right hepatectomy is preferable.
To summarise, the surgical principles to be followed will be: radical surgery with biliary and caudate resection and lymphadenectomy, with R0 margins and without manipulation of the tumour, even though this involves the resection and reconstruction of the portal vein and hepatic artery.
ConclusionsCurrently, the only curative treatment consists of surgical resection. Radical en-bloc resection and vascular reconstruction is the technique which has achieved the highest rate of survival in the long term. To reduce postoperative morbimortality, it is recommendable to perform biliary drainage to prevent liver failure, with antibiotic prophylaxis after drainage and portal embolisation when the future remaining hepatic volume will be less than 30%. R0 resection, lymph node involvement and distant metastasis are still the most important prognostic factors.
Conflict of InterestsThis revision has not been presented or published partially or wholly in any journal or congress.
Please cite this article as: Molina V, Sampson J, Ferrer J, Sanchez-Cabus S, Calatayud D, Pavel MC, et al. Tumor de Klatskin: diagnóstico, evaluación preoperatoria y consideraciones quirúrgicas. Cir Esp. 2015;93:552–560.