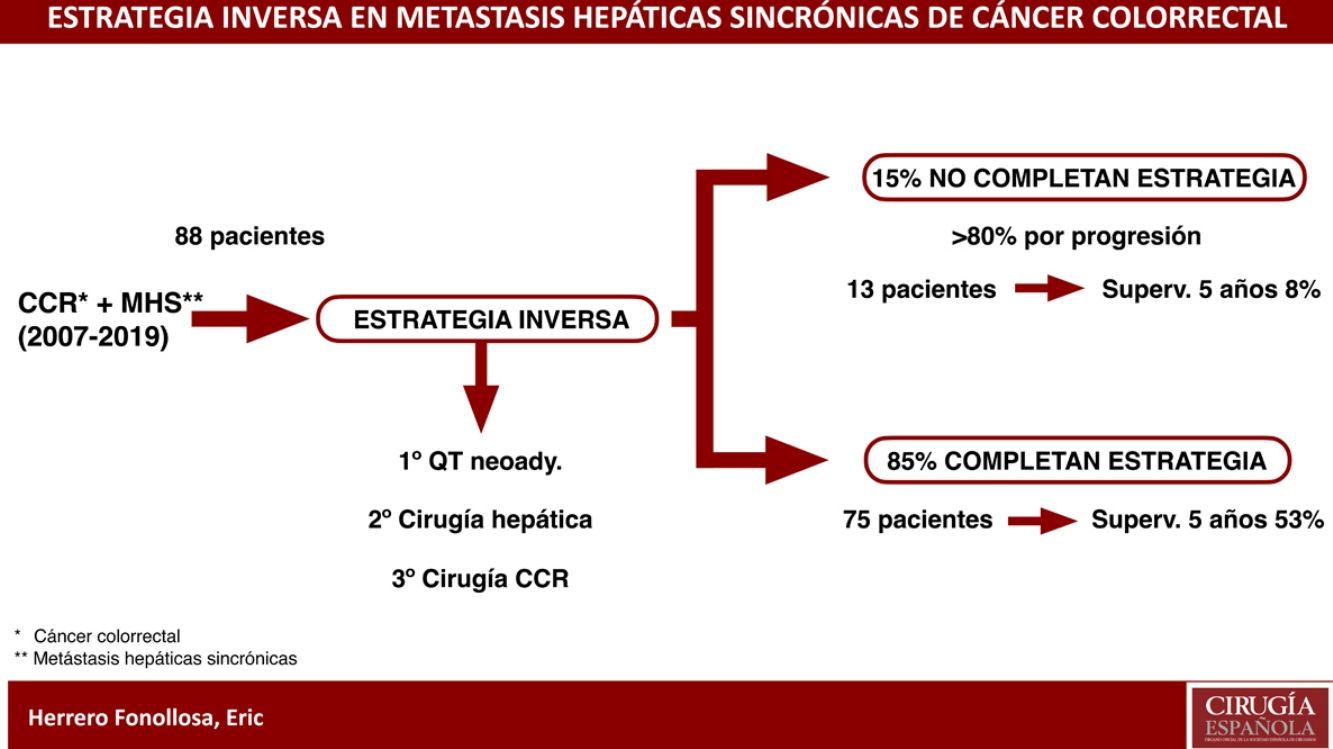
The “liver-first” approach (LFA) is a strategy indicated for advanced synchronous liver metastases (ASLM) from colorectal cancer (CRC). Includes neoadjuvant chemotherapy, resection of the ASLM followed by CRC resection.
MethodsRetrospective descriptive analysis from a prospective database of hepatectomies from liver metastases (LM) from CRC in two centers. Between 2007–2019, 88 patients with CRC-ASLM were included in a LFA scheme. Bilobar (LM) was present in 65.9%, the mean number of lesions was 5.5 and mean size 42.7 mm. Response to treatment was assessed by RECIST criteria. Progression-free survival (PFS) and overall survival (OS) were estimated using Kaplan–Meier survival curves.
ResultsSeventy-five of 88 patients (85.2%) completed the LFA. RECIST evaluation showed partial response in 75.7% and stable disease in 22.8%. Severe morbidity rate (Clavien–Dindo ≥ IIIA) after liver and colorectal surgery was present in 29.4% and 9.3%, respectively. There was no 90-day postoperative mortality in both liver and colorectal surgeries. Recurrence rate was 76%, being the liver the most frequent site, followed by the pulmonary. From the total number of recurrences (106) in 56 patients, surgical with chemotherapy rescue treatment was accomplished in 34 of them (32.1%). The mean PFS was 8.5 and 5-year OS was 53%.
ConclusionsIn patients with CRC-ASLM the LFA allows control of the liver disease beforehand and an assessment of the tumor response to neoadjuvant chemotherapy, optimising the chance of potentially curative liver resection, which influences long-term survival.
La estrategia inversa (EI) es un esquema indicado en pacientes con cáncer colorrectal (CCR) y metástasis hepáticas sincrónicas (MHS) avanzadas. Incluye quimioterapia neoadyuvante, seguido de resección hepática y, por último, resección del CCR.
MaterialEstudio descriptivo retrospectivo sobre una base de datos prospectiva de hepatectomías por metástasis hepáticas de CCR en dos centros entre 2007 y 2019. Se incluyeron 88 pacientes con CCR y MHS. La enfermedad hepática fue bilobar en un 65.9%, el número y tamaño medio de las lesiones fue 5.5 y 42.7 mm, respectivamente. La respuesta radiológica al tratamiento se evaluó mediante criterios RECIST. La supervivencia libre de progresión (SLP) y la supervivencia global (SG) media se estimaron mediante el método de Kaplan–Meier y regresión de Cox.
ResultadosResultados: De los 88 pacientes, 75 completaron la EI (85,2%). La respuesta radiológica fue parcial en el 75,7% y la estabilización en el 22,8%. La tasa de morbilidad (Clavien-Dindo ≥ IIIA) tras la cirugía hepática y colorrectal fue del 29,4 y 9,3%, respectivamente. No hubo mortalidad a los 90 días. La tasa de recurrencia fue del 76%. Se diagnosticaron 106 recurrencias en 56 pacientes. De éstos, se realizó tratamiento quirúrgico asociado a quimioterapia en 34 (32,1%). La SLP fue de 8,5 meses y la SG a 5 años fue del 53%.
ConclusionesEn pacientes con CCR y MHS la EI permite el control inicial de la enfermedad metastásica, seleccionar pacientes respondedores a la neoadyuvancia y optimizar las posibilidades de resección completa, influyendo en la supervivencia a largo plazo.
Colorectal cancer (CRC) is the third most common malignant neoplasm1 and the second leading cause of cancer death2. The liver is the organ most commonly affected by distant metastases3. Up to 25% of patients have synchronous liver metastases (SLM)4, with a worse prognosis2.
The only potentially curative treatment to prolong survival is complete resection of the CRC and metastatic disease, associated with chemotherapy5. However, the optimal sequence for the treatment of CRC with SLM remains controversial2.
The classic approach consists of CRC resection followed by chemotherapy and, lastly, surgery for the SLM. However, less than 30% of patients complete the treatment. The main disadvantage of this approach is the potential progression of the liver disease in cases of delayed adjuvant chemotherapy due to complications resulting from the colorectal surgery6.
Meanwhile, the main disadvantages of simultaneous resection of CRC and SLM are elevated postoperative morbidity and mortality, although in selected cases the results are comparable to those of other strategies1,7,8.
Since the long-term prognosis of these patients is influenced by SLM, Mentha et al described the inverse strategy (IS) or ‘liver-first’ approach in 20069. In this approach, treatment is started with neoadjuvant chemotherapy, followed by liver surgery and then CRC surgery8. It is indicated in patients with asymptomatic or symptomatic CRC treatable with stents or colostomy and potentially resectable or initially unresectable SLM. This strategy provides initial systemic control of the disease, while being able to select patients who respond to chemotherapy, thereby optimizing the possibilities of complete liver resection1.
The objective of this study was to evaluate the long-term results (overall and progression-free survival, recurrence rate, and rate of patients who completed the therapeutic regimen) in patients diagnosed with potentially resectable or initially unresectable CRC and SLM included in a liver-first treatment regimen.
MethodsWe designed a descriptive retrospective study based on a prospective database of 609 patients operated on for liver metastases from colorectal cancer at two tertiary hospitals, starting in June 2007 at Center 1 and March 2015 at Center 2 (at which time a joint hepatic surgery unit was set up with the same therapeutic protocols in both hospitals). In both study centers, patient inclusion closed December 2019 to obtain a minimum follow-up of 6 months.
Eighty-eight consecutive patients diagnosed with CRC and SLM were included in a liver-first strategy after evaluation by a multidisciplinary committee. Inclusion criteria were: (1) asymptomatic or symptomatic CRC treatable by stent or surgical bypass; (2) potentially resectable or initially unresectable SLM (according to NCCN colon cancer guidelines); (3) no unresectable extrahepatic disease; (4) patient fit for surgery (ECOG ≤ 2); (5) informed consent. Intention-to-treat failure was defined as: (1) persistence of unresectable liver metastases or their progression after neoadjuvant treatment; (2) disease progression between hepatectomy and colectomy; (3) CRC symptoms not treatable with a stent or surgical bypass.
The liver-first approach involved starting treatment with neoadjuvant chemotherapy. After the third or fourth cycle, the response to chemotherapy was evaluated with RECIST radiological criteria10, using computed tomography (CT) and magnetic resonance imaging (MRI) with a hepatospecific contrast agent (Gadoxetate disodium). In the absence of progression, hepatic surgery was considered and, finally, CRC surgery was performed. Patients with rectal neoplasm received preoperative chemoradiation therapy and re-staging of the disease by CT and pelvic MRI. Complications derived from both surgeries were recorded in accordance with the Clavien–Dindo classification11.
Neoadjuvant chemotherapyNeoadjuvant chemotherapy was based on the Folfox regimen (folinic acid, fluorouracil and oxaliplatin). In most cases, it was also associated with monoclonal antibody according to RAS/BRAF mutational status. The resectability of the SLM was reassessed in a multidisciplinary committee, and the patients underwent surgery after an interval of about 4 weeks. In patients treated with antiangiogenic agents, the interval was 6 weeks.
SurgeryThe goal of liver surgery was complete tumor resection while maintaining sufficient residual liver volume (FLR) >25% of total liver volume. Volume augmentation strategies (portal vein embolization [PVE] or liver partition [ALPPS]) were performed if the FLR was <25%. The laparoscopic approach was used when it was considered technically feasible by the surgical team.
Statistical analysisCategorical data are presented as absolute numbers and percentages. Continuous data are presented as means (with standard deviations). To study the factors that influence the R0/R1 resection rate and the recurrence rate, the variables were analyzed using the chi-squared test for the univariate study and the logistic regression method for the multivariate analysis. P-value<.05 was considered statistically significant.
For the study of the prognostic factors of survival, the Kaplan–Meier method and Cox regression (univariate) and Cox forward stepwise (multivariate) were used. Mean follow-up was considered the time elapsed from colorectal surgery to the date of the last office visit. Overall survival (OS) was defined as time elapsed from diagnosis to the date of the last visit. Progression-free survival (PFS) was considered the time from CRC surgery to the date of the first recurrence (if R0 surgery was performed and there was no metastatic disease at diagnosis) or disease progression (in R1 resections or unresected extrahepatic disease, mainly pulmonary). The analyses were performed with the SPSS program (version 26).
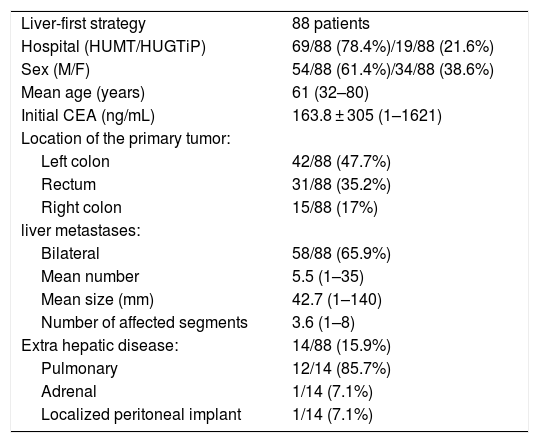
ResultsAmong the 88 patients included in the IS, 61.4% were males, with a mean age of 61 years (32–80). The most frequent location of CRC was the left colon (47.7%), followed by the rectum (35.2%) and the right colon (17%).
Liver disease was bilateral in 65.9%, with a mean of 3.6 affected segments (1–8). The mean number and size of lesions were 5.5 (1–35) and 42.7 mm (1–140), respectively.
Extrahepatic disease was initially found in 15.9% of patients, and pulmonary disease was the most common (85.7%). One patient had adrenal metastasis and another a localized peritoneal implant (Table 1).
Preoperative characteristics of patients with colorectal cancer and advanced synchronous metastatic liver disease selected for liver-first strategy.
| Liver-first strategy | 88 patients |
| Hospital (HUMT/HUGTiP) | 69/88 (78.4%)/19/88 (21.6%) |
| Sex (M/F) | 54/88 (61.4%)/34/88 (38.6%) |
| Mean age (years) | 61 (32–80) |
| Initial CEA (ng/mL) | 163.8 ± 305 (1–1621) |
| Location of the primary tumor: | |
| Left colon | 42/88 (47.7%) |
| Rectum | 31/88 (35.2%) |
| Right colon | 15/88 (17%) |
| liver metastases: | |
| Bilateral | 58/88 (65.9%) |
| Mean number | 5.5 (1–35) |
| Mean size (mm) | 42.7 (1–140) |
| Number of affected segments | 3.6 (1–8) |
| Extra hepatic disease: | 14/88 (15.9%) |
| Pulmonary | 12/14 (85.7%) |
| Adrenal | 1/14 (7.1%) |
| Localized peritoneal implant | 1/14 (7.1%) |
HUMT Hospital Universitari Mútua Terrassa. HUGTiP Hospital Universitari Germans Trias i Pujol. M: male; F: female; CEA: carcinoembrionary antigen; mm: millimeters.
Seventy-five patients (85.2%) completed the IS. Nine patients required stent placement and 3 a colostomy due to CRC symptoms, allowing treatment to be completed (Fig. 1).
Thirteen patients (14.8%) did not complete the liver-first strategy due to intention-to-treat failure. The most frequent cause was progression during neoadjuvant chemotherapy (53.8%), followed by progression between hepatectomy and colectomy (30.8%) and, finally, by CRC symptoms that were not treatable by stents or bypass (15.4%).
The 5-year OS of this group of patients was 8% (Fig. 2).
Progression-free survival curves (PFS) and 5-year overall survival (OS).
(A) OS at 5 years of the patients who completed the IE (green color) compared to those who did not complete it (blue color).
(B) OS at 5 years of patients with free liver resection margin (blue color) compared to those with affected margin (green color).
(C) PFS of the patients who completed the IS.
EI: reverse strategy; N: number of patients at risk; R0/R1: refers to the status of the resection margin.
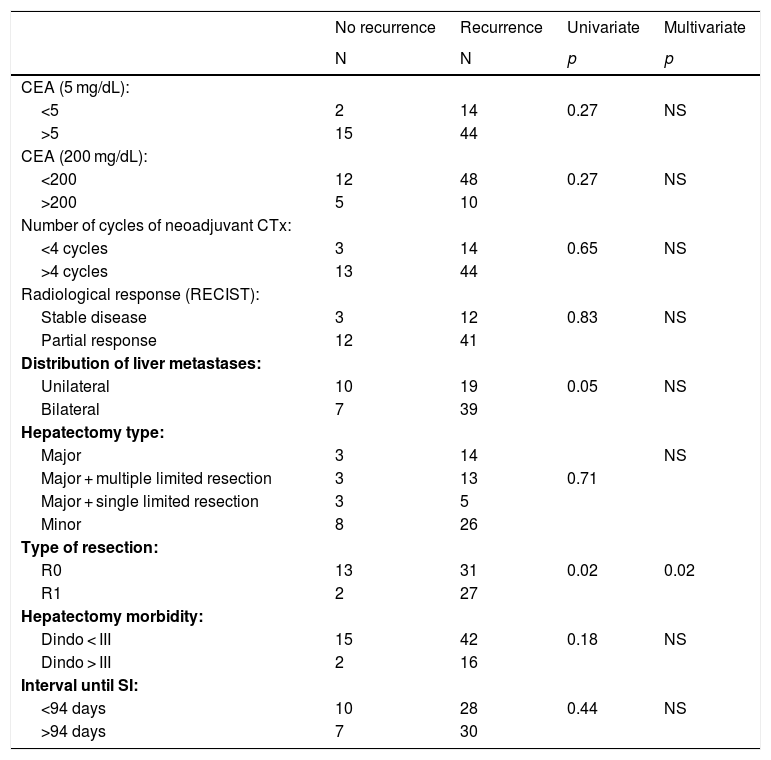
Intravenous neoadjuvant chemotherapy was administered to 93.3% of the patients. The scheme was Folfox in 84% of the cases, with a mean of 8.5 cycles ± 6.5 (2–36). An associated monoclonal antibody was administered in 54.3% (cetuximab 24.3%; bevacizumab 17.1%; panitumumab 12.9%). The radiological response was partial in 75.7% of patients and stabilization in 22.8%. The number of cycles of neoadjuvant chemotherapy (greater or less than 4 cycles) and the type of radiological response had no influence on disease recurrence (Table 2).
Analysis of factors related with recurrence in the group of pacients who completed LFS (chi-squared test [univariate]. logistic regression [multivariate]).
| No recurrence | Recurrence | Univariate | Multivariate | |
|---|---|---|---|---|
| N | N | p | p | |
| CEA (5 mg/dL): | ||||
| <5 | 2 | 14 | 0.27 | NS |
| >5 | 15 | 44 | ||
| CEA (200 mg/dL): | ||||
| <200 | 12 | 48 | 0.27 | NS |
| >200 | 5 | 10 | ||
| Number of cycles of neoadjuvant CTx: | ||||
| <4 cycles | 3 | 14 | 0.65 | NS |
| >4 cycles | 13 | 44 | ||
| Radiological response (RECIST): | ||||
| Stable disease | 3 | 12 | 0.83 | NS |
| Partial response | 12 | 41 | ||
| Distribution of liver metastases: | ||||
| Unilateral | 10 | 19 | 0.05 | NS |
| Bilateral | 7 | 39 | ||
| Hepatectomy type: | ||||
| Major | 3 | 14 | NS | |
| Major + multiple limited resection | 3 | 13 | 0.71 | |
| Major + single limited resection | 3 | 5 | ||
| Minor | 8 | 26 | ||
| Type of resection: | ||||
| R0 | 13 | 31 | 0.02 | 0.02 |
| R1 | 2 | 27 | ||
| Hepatectomy morbidity: | ||||
| Dindo < III | 15 | 42 | 0.18 | NS |
| Dindo > III | 2 | 16 | ||
| Interval until SI: | ||||
| <94 days | 10 | 28 | 0.44 | NS |
| >94 days | 7 | 30 |
CEA: carcinoembrionary antigen; CTx: chemotherapy; RECIST: Response Evaluation Criteria in Solid Tumors; R0/R1 refers to the resection margin status; SI: surgical intervention; NS: not significant.
Table 3 summarizes the liver surgery variables, including the number of procedures performed, type of liver resection, approach, volume augmentation procedures and analysis of the resection margins. In two patients, another procedure was associated with liver resection (one adrenalectomy and one resection of a localized peritoneal implant in the diaphragm). Bilateral distribution of liver metastases was identified as a risk factor for R1 resection in both the univariate and multivariate analyses. The number of affected liver segments, number of resected segments and type of liver resection performed had no influence on the status of the resection margin (Table 4).
Results of liver surgery.
| Numer of liver surgery procedures | 124 in 75 patients |
| Right hepatectomy | 23 (30.7%) |
| Left hepatectomy | 11 (14.7%) |
| Right trisectorectomy | 4 (5.3%) |
| Left trisectorectomy | 1 (1.3%) |
| Bisegmentectomy | 31 (41.3%) |
| Segmentectomy | 10 (13.3%) |
| Limited resection | 15 (20%) |
| Multiple limited resection | 29 (38.7%) |
| Laparoscopy | 22/75 (29.3%) |
| Surgery in 2 stages: | |
| Portal embolization | 3/75 (4%) |
| ALPPS | 5/75 (6.7%) |
| Resection type: | |
| R0 > 1 mm | 46/75 (61.3%) |
| R1 < 1 mm | 26/75 (34.7%) |
| R1 vascular | 3/75 (4%) |
ALPPS Associating Liver Partition and Portal vein Ligation for Staged hepatectomy. R0/R1 refers to the resection margin status.
Influence of characteristics of liver metastases and the liver resection type for obtaining complete liver resection (chi-squared test [univariate]. logistic regression [multivariate]).
| R0 Resection | R1 Resection | Univariate | Multivariate | |
|---|---|---|---|---|
| p | p | |||
| Distribution of liver metastases: | ||||
| Unilateral | 22 | 6 | 0.01 | 0.05 |
| Bilateral | 22 | 23 | ||
| Affected segments: | ||||
| <3 | 21 | 9 | 0.19 | NS |
| >3 | 23 | 19 | ||
| Resected segments: | ||||
| <3 | 23 | 9 | 0.07 | NS |
| >3 | 21 | 20 | ||
| Type of hepatectomy: | ||||
| Major | 12 | 5 | ||
| Major + multiple limited resection | 6 | 9 | 0.24 | NS |
| Major + single limited resection | 14 | 4 | ||
| Minor | 22 | 11 |
R0/R1 refers to the state of the resection margin. NS. = not significant.
Morbidity after liver surgery was mostly grade IIIA in 18.7% (14 patients required percutaneous drainage due to intra-abdominal collection), followed by grade I in 16%. Five patients required surgical reoperation (2 for hemoperitoneum and three for drainage of collections not accessible percutaneously). No postoperative mortality was registered within 90 days (Table 5).
Morbidity and mortality after liver and colorectal surgery according to the Clavien-Dindo classification.
| Morbidity (Clavien–Dindo classification) (30 days) | Hepatectomy | Colectomy |
|---|---|---|
| Grade I | 12 (16%) | 13 (17.3%) |
| Grade II | 8 (10.7%) | 8 (10.7%) |
| Grade IIIA (percutaneous drainage) | 14 (18.7%) | 3 (4%) |
| Grade IIIB (re-intervention) | 5 (6.7%) | 3 (4%) |
| Grade IVA | 3 (4%) | 1 (1.3%) |
| Grade IVB | 0 | 0 |
| Perioperative mortality (90 days) | 0 | 0 |
Regarding CRC surgery, the most frequent interventions were left hemicolectomy (45.6%), rectal surgery (31.7%) and right hemicolectomy (21.1%). The intervention was laparoscopic in 41.3% of cases. All patients with rectal cancer were administered preoperative radiotherapy. Surgery was R0 in 89.3% of patients, R1 in 6.7%, and R2 in 2.7%.
Morbidity after colorectal surgery was grade III or higher in 7 patients (9.3%). There was no 90-day perioperative mortality (Table 5).
The mean number of days between hepatectomy and colectomy was 94 days (20–536). The presence/absence of serious morbidity after hepatectomy (Dindo > III) did not influence the interval of days elapsed between the 2 operations, which were 83 and 113 days, respectively (p 0.59). After colorectal surgery, 57.3% of patients received adjuvant chemotherapy.
Follow-upDuring follow-up, 106 recurrences were diagnosed in 57 patients (76%). The most frequent was hepatic (53%), followed by pulmonary (34%) and locoregional (13%). Treatment of tumor recurrence was surgical associated with chemotherapy in 34 patients (32.1%), including 20 re-hepatectomies, 10 atypical pulmonary resections, and 2 surgeries for locoregional recurrence.
The presence of bilateral liver disease and obtaining a positive liver resection margin were identified as risk factors for disease recurrence. In the multivariate analysis, the only variable that maintained statistical significance was resection margin involvement. Other factors, such as the number of cycles of neoadjuvant chemotherapy, radiological response, CEA level, morbidity after liver surgery, and the interval between liver and colon surgery, had no influence on the recurrence rate (Table 2).
After a mean follow-up of 27.6 months, 64% of patients were alive. PFS was 8.5 months. Five-year OS in patients with completed IS was 53%, versus 8% in patients who did not complete IT; these differences were significant in the univariate and multivariate analyses.
The state of the liver resection margin (R0/R1) had an impact on 5-year overall survival, (63.3% and 27.6%, respectively) with a significance of p 0.022 in the multivariate analysis (Fig. 2). The recurrence rate, serious complications after liver surgery, or the prolonged interval between the 2 surgeries had no impact on long-term survival.
DiscussionThe inverse strategy or “liver-first approach” was introduced by Mentha et al. in 2006 for the treatment of SLM from CRC9. This includes preoperative chemotherapy, followed by resection of the SLM and, finally, resection of the CRC. This strategy makes it possible to obtain initial control of the metastatic liver disease through systemic treatment, which could optimize the chances of curative liver resection. Unlike the classic approach, it could minimize the risk of SLM progression, which often becomes unresectable after intestinal resection due to complications derived from colorectal surgery, especially in cases of advanced liver disease7,14,15.
This descriptive study evaluates the long-term results of the liver-first approach in patients with advanced CRC and SLM in two tertiary hospitals over a 12-year period.
Advanced liver disease was defined as that with a high tumor burden, generally multiple and bilobar metastases, or lesions that, due to their location, required technically complex resections.
Through this therapeutic approach, 85.2% of the patients completed the therapeutic scheme, a result that was slightly higher than that of other previously published series (65%–84%)1,12–14. This difference could be due to the fact that patients with advanced SLM diagnosed in other medical centers were only referred for evaluation by the multidisciplinary committee in case of response to neoadjuvant treatment, losing from the analysis those patients who did not have a response. This would generate a bias in favor of patients who respond to treatment and who are more likely to complete the IS.
Among the described advantages of IS are a shorter duration of chemotherapy prior to liver resection, high response rate to chemotherapy, higher response rate prior to hepatectomy, as well as maximized performance of pelvic radiotherapy in patients with rectal cancer14. The analysis of this series confirms these advantages, since the mean duration of neoadjuvant chemotherapy was 8 cycles, optimizing its effectiveness and performing liver surgery in the response phase. Only 7 patients (8%) presented disease progression during neoadjuvant therapy, who were excluded due to intention-to-treat failure. In 92% of patients, stabilization or partial radiological response was obtained according to RECIST criteria, which was similar to reports in the literature13,16,17. In this series, the response to treatment was evaluated by abdominal CT with intravenous contrast and MRI with hepatospecific contrast (Gadoxetate disodium). The latter could enable the detection of undiagnosed liver lesions by other techniques and, therefore, modify the surgical strategy in some patients22–24.
The liver-first approach is indicated in patients with asymptomatic CRC or symptoms treatable by stent placement or surgical bypass, thus allowing for treatment to be completed. Other series report symptoms derived from CRC during treatment in 5%–7% of patients1,14. In the current series, 16% of patients required treatment for symptomatic CRC, completing the therapeutic regimen.
93.3% of patients underwent intravenous neoadjuvant chemotherapy, except for 2 cases that received oral chemotherapy due to advanced age and comorbidity. A monoclonal antibody was associated in only 54.3% of cases, probably due to the different criteria of the oncologists from the 2 participating study centers regarding its indication in the neoadjuvant regimen.
In terms of liver surgery, 54.7% of cases required major hepatectomy to achieve R0 resection. The available literature reports 36%–89% major hepatectomies13,16,17. This high percentage of major liver resections could be due to the fact that, in most published series (including this one), patients selected for IS tend to present a higher percentage of multiple and bilobar liver disease. In this series, 65.9% of the cases presented bilateral SLM, with an average of >5 lesions, >4 cm in size and >3 affected segments.
In our series, 29.3% of hepatectomies were laparoscopic. The available literature shows no differences between the open and laparoscopic approach in malignant liver disease, so the decision to perform a laparoscopic approach in these cases should be based on the experience of the surgical team17.
Liver resection was R0 in 61.3% of patients (similar to reports from other series [50%–80%])13,16 and R1 in 34.7%. The rate of R1 resections is related to liver tumor burden. In the present study, only the bilateral distribution of metastases was related to a higher rate of R1 resections. The number of affected and resected liver segments greater than 3 or the type of liver resection performed did not influence the state of the resection margin. Obtaining a positive resection margin in liver surgery was associated with a higher rate of disease recurrence and shorter 5-year survival compared to patients with R0 liver resection. However, in our opinion, the possibility to achieve R1 resection after liver surgery should not be a criteria for unresectability, since the long-term results in this patient group are superior to those who do not undergo any resection18.
Morbidity was mainly grade IIIA (18.7%) after liver surgery and grade I (17.3%) after colorectal surgery, comparable to other series described19. There was no perioperative mortality at 90 days. The presence of serious complications after hepatectomy (Dindo > IIIA) a concern when considering the liver-first approach, since it could delay primary surgery and have a negative impact on the evolution of these patients. However, in this series, post-hepatectomy morbidity was neither related to a higher recurrence rate nor to an increased interval of days between hepatic and colorectal surgery, nor did it negatively impact long-term survival.
No differences in survival have been demonstrated in patients treated with the classic approach or IS14,20,21,25, although there are no randomized studies comparing both strategies. There could be a tendency to include patients with more advanced liver disease in IS, which would have a negative impact on the survival of this group.
PFS was 8.5 months. Given that most patients included in the IS had advanced liver disease and up to 15.9% had extrahepatic disease at the time of diagnosis, it was considered more appropriate to analyze PFS rather than disease-free survival. In the case of lung metastases (the most common location), these were often controlled and even became chronic with systemic chemotherapy.
Recurrence in IS series is not uncommon, but its rate is variable14. In our series, 57 patients (76%) presented at least one recurrence. Of these, 32.1% were treated with surgery and chemotherapy. The authors consider it essential to manage these patients within the framework of a multidisciplinary committee that facilitates early detection and treatment of recurrences.
The 5-year OS of patients with completed IS was 53%, similar to global hepatectomy series (30%–72%)12,16,17.
The limitations of the present study include that it is a retrospective analysis with a limited number of patients. Only two hospitals were included, and there is no control group for comparisons. Certain data that could influence prognosis, such as mutational status (RAS/BRAF), have not been analyzed.
In conclusion, when applied in patients with CRC and advanced SLM, the liver-first approach allows us to initially control the metastatic disease. 85.2%, the therapeutic regimen is completed with acceptable morbidity and mortality rates, while overall survival is similar to the global series of hepatectomies.