The relationship between preoperative malnutrition and morbi-mortality has been documented for years. Despite the existence of tools that allow its detection, and therefore treat this entity, their introduction into clinical practice is not widespread. Both perioperative insulin resistance and hyperglycemia are associated with increased perioperative morbidity and length of hospital stay. The intake of carbohydrate-rich drinks 2–4h prior to surgery reduces insulin resistance. In the immediate postoperative period, the enteral route is safe and well tolerated and its early use reduces hospital stay and postoperative complications compared with parenteral nutritional support. Inmunonutrition has been proven effective to decrease postoperative complications and hospital stay. In view of these data we opted for the adoption of these measures replacing bowel rest and the indiscriminate use of postoperative parenteral nutrition.
La relación entre desnutrición prequirúrgica y morbimortalidad está documentada desde hace años. A pesar de la existencia de herramientas que nos permiten detectar y tratar esta entidad, su aplicación en la práctica clínica es, a día de hoy, lenta. Por otra parte, tanto la insulinorresistencia como la hiperglucemia perioperatoria se asocian a mayor morbimortalidad postoperatoria y estancia media más prolongada. La ingesta de bebidas ricas en hidratos de carbono 2-4h antes de la intervención permite disminuir dicha insulinorresistencia. Otro factor que reduce la estancia y las complicaciones es el soporte nutricional enteral postoperatorio precoz en relación con el soporte vía parenteral tradicional. También las fórmulas con inmunonutrientes han demostrado ser eficaces a la hora de disminuir complicaciones posquirúrgicas y estancia media. A la vista de la evidencia científica y de las guías de práctica clínica recomendamos la adopción de estas medidas, sustituyendo a las tradicionales.
It is a consummate fact that perioperative malnutrition is associated with greater morbidity and mortality.1 Even though we are aware of this reality, some 65% of patients who are scheduled for upper gastrointestinal tract surgery present criteria for malnutrition,2 and two-thirds of surgical patients experience weight loss during their hospitalization.3 In the Spanish multi-center study Prevalence of Malnutrition and Associated Costs (Prevalencia de Desnutrición y Costes Asociados [PREDyCES©]), the prevalence of malnutrition in surgical patients was established at 10%.4
Back in 1936, Studley documented the close relationship between malnutrition and the appearance of complications in patients who had been treated surgically for peptic ulcer disease: 33% of patients with weight loss prior to surgery of more than 20% died, compared with 3.5% of those patients with weight loss of less than 20%.5
Fifty years later, despite the advance of surgical and anesthetic techniques, malnutrition prior to surgery in patients with digestive tumors was associated with a higher rate of post-surgical complications (72 vs 29%) and mortality (23 vs 4%) when compared with well-nourished patients.6
In spite of the advances made in clinical nutrition in recent years and their contribution to standard medical practice, preoperative fasting and bowel rest are still the norm in the great majority of hospitals.7 This situation is nothing new to us: in a survey done in Spain in 2005, pre- and postoperative fasting predominated over perioperative nutritional support (NS) in an alarming manner, and the NS technique that was most widely used in patients who had been treated surgically for biliopancreatic disease was parenteral NS.8
In gastrointestinal surgery, preoperative malnutrition is caused in most cases by one of the following two factors7: cancer cachexia or reduced intake.
The objective of this review is to provide scientific evidence on the new trends in perioperative NS. To do so, we searched the PubMed database for articles published in the last 15 years and selected the most relevant, including clinical practice guidelines (CPG), meta-analyses, systematic reviews of the literature and randomized prospective studies.
Preoperative Nutritional ScreeningIn recent years, several tools have been developed for the screening and diagnosis of malnutrition (for example, the Malnutrition Universal Screening Tool [MUST], Nutritional Risk Screening-2002 [NRS-2002] or Subjective Global Assessment [SGA]) as well as CPG in order to provide efficient NS. Even so, the knowledge of medical professionals in this area continues to be deficient.9
Several studies have analyzed the relationship between pre-operative nutritional state,1,10–15 risk of malnutrition and postoperative results. They confirm that malnutrition associated with disease correlates with higher morbidity, mortality and hospital stay as well as higher rates of readmission and treatment costs when compared with well-nourished patients. From these conclusions, one can deduce the importance of having proper malnutrition screening and diagnostic tools. With the existing scientific understanding, we can say that anthropometric assessment or isolated functional methods are not of great use to establish either the diagnosis of malnutrition or its potential risk,7 and sufficient studies are not available to determine whether any of the screening tools offers greater performance than the others.
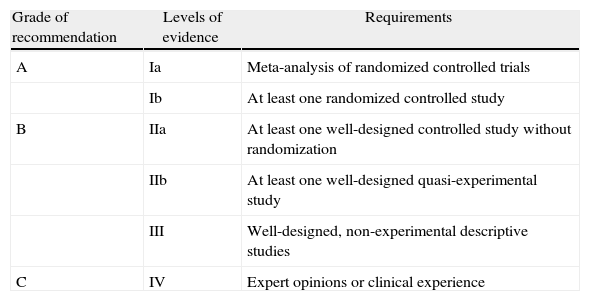
The most important scientific societies in the field of clinical nutrition are the American Society for Parenteral and Enteral Nutrition (ASPEN) and the European Society of Parenteral and Enteral Nutrition (ESPEN). Both have published their CPG for artificial nutrition based on the Healthcare Research and Quality (AHRQ) system in order to establish their recommendations16 (Table 1).
Grades of Recommendation and Levels of Evidence of the AHQR.
| Grade of recommendation | Levels of evidence | Requirements |
| A | Ia | Meta-analysis of randomized controlled trials |
| Ib | At least one randomized controlled study | |
| B | IIa | At least one well-designed controlled study without randomization |
| IIb | At least one well-designed quasi-experimental study | |
| III | Well-designed, non-experimental descriptive studies | |
| C | IV | Expert opinions or clinical experience |
AHQR: Agency for Healthcare Research and Quality.
ESPEN17 recommends determining the following parameters for the perioperative assessment of surgical patients:
- -
Weight loss of more than 10%–15% in the 6 previous months;
- -
Body mass index (BMI) below 18.5kg/m2;
- -
SGA grade C;
- -
Albumin lower than 3g/dl that cannot be justified by hepatic or renal disturbances.
Meanwhile, ASPEN, in a review of its nutritional screening guidelines,17 makes no specific recommendations for this type of patients.
Optimized Perioperative Nutritional SupportPreoperative Nutritional SupportThe objective of preoperative NS is to maintain or improve the nutritional state of patients before surgery.
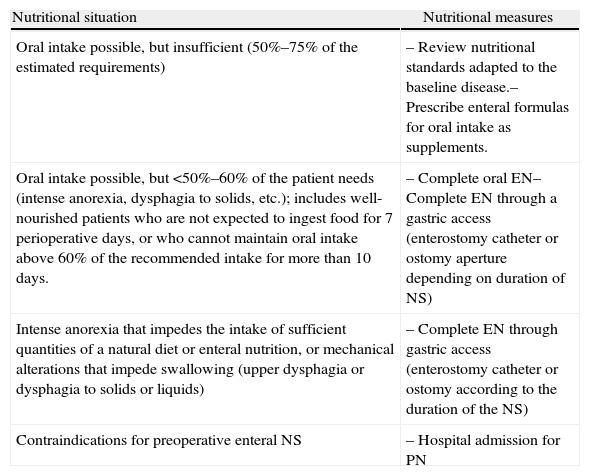
Although it is known that malnutrition is not corrected over a short period of time and that postponing surgery only provides a slight improvement in nutritional states, the scientific evidence that has been compiled about preoperative NS, especially in moderately or severely malnourished patients, has resulted in a series of recommendations in the CPG by ASPEN (2002)18 and ESPEN19 (Table 2). The following is a description of different situations in which preoperative NS would be indicated along with specific recommendations for its use20 (Table 3). Whenever possible, the NS plan should be individualized, preferably in the outpatient setting and either oral or enteral.
ASPEN and ESPEN Recommendations About Preoperative NS.
| ASPEN 200218recommendations about pre-operative NS |
| NS should be administered for 7–10 days in all patients with moderate or severe malnutrition who are scheduled for major surgery that might be postponed (A). |
| ESPEN 200619recommendations about preoperative NS |
| In patients with high nutritional risk (according to the ESPEN criteria described in the anterior section), NS is indicated for 10–14 days prior to surgery, delaying surgical intervention if necessary (A). |
| NS is indicated (preferably enteral) in well-nourished patients who are not expected to ingest food for 7 perioperative days, or who will not reach 50%–60% of their nutritional requirements orally for the 10 days prior to surgery (C). |
ASPEN: American Association of Parenteral and Enteral Nutrition; ESPEN, European Society of Parenteral and Enteral Nutrition; NS: nutritional support.
Preoperative NS Modalities.
| Nutritional situation | Nutritional measures |
| Oral intake possible, but insufficient (50%–75% of the estimated requirements) | – Review nutritional standards adapted to the baseline disease.– Prescribe enteral formulas for oral intake as supplements. |
| Oral intake possible, but <50%–60% of the patient needs (intense anorexia, dysphagia to solids, etc.); includes well-nourished patients who are not expected to ingest food for 7 perioperative days, or who cannot maintain oral intake above 60% of the recommended intake for more than 10 days. | – Complete oral EN– Complete EN through a gastric access (enterostomy catheter or ostomy aperture depending on duration of NS) |
| Intense anorexia that impedes the intake of sufficient quantities of a natural diet or enteral nutrition, or mechanical alterations that impede swallowing (upper dysphagia or dysphagia to solids or liquids) | – Complete EN through gastric access (enterostomy catheter or ostomy according to the duration of the NS) |
| Contraindications for preoperative enteral NS | – Hospital admission for PN |
EN: enteral nutrition; PN: parenteral nutrition; NS: nutritional support.
Source: Cancer et al.20
Preoperative fasting has become routine in daily clinical practice,21 even though it has been demonstrated that this practice induces a situation of insulin resistance, mitochondrial dysfunction and metabolic stress.22
Insulin resistance, characterized by a reduced tissue response to the action of insulin, has been associated with an increase in postoperative morbidity, mortality and hospital stay.23
Hyperglycemia has also been associated with an increased risk for complications.24 Improved glycemic control with intensive intravenous insulin therapy entails a reduction in morbidity and mortality of all-causes in surgical patients during their stay in intensive care.23 The main risks of this treatment, however, are hypoglycemia and the induction of glycemic variability, which has been associated with higher mortality amongst patients treated in intensive care units.25 Therefore, and despite the fact that maintaining proper glycemic control is beneficial, there has been a search for new methods to reduce insulin resistance and hyperglycemia after surgery.
The Ljungqvist group has described a simple method to achieve this effect with the ingestion of carbohydrate-rich drinks 2 or 3h before surgery.22 This strategy produces a release of insulin similar to that induced by a balanced meal and a reduction in insulin resistance associated with surgical stress, which can be 50% lower compared to patients who are not given the carbohydrate drink during the preoperative period.22
Possible mechanisms have been studied to explain how these drinks are able to reduce surgery-related insulin resistance. The following are the conclusions of these studies:
- -
Carbohydrate rich drinks replenish liver glycogen levels prior to surgery.26
- -
They favor the oxidation of carbohydrates, which is lower in situations of insulin resistance, by stimulating the transformation of pyruvate into acetyl coenzyme-A through the decrease in muscle type 4 pyruvate dehydrogenase levels.27
Currently, there is no evidence that patients who drink liquids 2 or 3h before surgery have a greater risk for aspiration or regurgitation than those who fast for more than 12h. The stomach takes less than 90min to empty after the ingestion of a carbohydrate-rich drink. Thus, in patients without risk of aspiration (excluding situations of emergency surgery or patients with known delayed gastric emptying) liquids could be given up to 2h before the surgery. In its CPG, ESPEN recommends reducing preoperative fasting to 6–8h and administering a carbohydrate-rich drink 2–3h before surgery (Table 4). In other studies, the carbohydrate drinks have been related with less hunger, thirst and perioperative anxiety in patients.28–30
ESPEN Recommendations for Preoperative Fasting.
| ESPEN 200619recommendations for preoperative fasting |
| Fasting since the night before surgery is not necessary in most patients. Patients without risk for aspiration can drink liquids up until 2h before anesthesia and ingest solid food until 6h before (A). |
| Drinking carbohydrate-rich drinks the night before (800ml) and 2h before (400ml) surgery, instead of night-long fasting, is recommended in most major surgery patients (B). |
ESPEN: European Society of Parenteral and Enteral Nutrition.
ASPEN, in contrast, makes no statement to this regard.
Postoperative NutritionOne of the most widespread dogmas in digestive surgery (in addition to preoperative fasting) is postoperative fasting. Even though its effectiveness has not been demonstrated, this practice has been perpetuated among successive generations of surgeons.7
Normally, a liquid diet is not initiated until bowel sounds have appeared, and oral intake is gradually progressed afterwards. This means that many patients are kept fasting for prolonged periods of time. Fasting favors bacterial overgrowth, and at the same time it produces a loss of trophism of the intestinal mucous membranes. In addition, bowel rest has been associated with diminished intestinal secretions and immunoglobulin A as well as atrophy of the lymphoid tissue associated with the intestines, which produces fewer defenses against the aggression of the enteric bacteria. These factors all favor bacterial translocation and the passage of toxins from the intestinal lumen to the blood circulation. This happens not only during fasting, but also in the course of total parenteral nutrition (TPN).20
For some time now, it has been known that early enteral nutrition (EN) is well tolerated. In 2001, a meta-analysis published by the British Journal of Medicine31 determined that postoperative bowel rest did not result in clear patient benefits. Furthermore, by comparing this nil per os strategy with the introduction of early EN (24h post-op), the conclusion was that the application of the second strategy led to a reduction in infectious complications and mean hospital stay.
There is also a report of a tendency toward a lower incidence of anastomotic leak and mortality, although these results were not statistically significant. In 2009, another meta-analysis32 that once again compared early EN versus bowel rest in patients who had undergone digestive tract surgery concluded that early EN was also associated with a reduction in mortality.
It is currently known that ileus after abdominal surgery is a transitory phenomenon caused by inhibited intestinal motility due to a stimulus of the sympathetic reflex secondary to surgical trauma of the abdominal cavity. The peristaltism of the small intestine is recovered 6–12h after surgery, in the stomach within 12–24h and the colon within 48–120h.20 Any procedures that are able to reduce ileus will make early oral intake easier. In this regard, new protocols are being reported of perioperative measures aimed at accelerating the recovery of patients treated with conventional or laparoscopic surgery, known as “Enhanced Recovery After Surgery (ERAS)”. This group of measures that reduce surgical stress can minimize catabolism and favor rapid anabolism in order to achieve optimal recovery and tissue healing. One of the most developed programs, used in colorectal surgery,33 declares that classic preoperative fasting is not necessary (evidence A). It advocates administering a carbohydrate drink 2 or 3h before surgery (evidence A), no mechanical preparation of the colon (evidence A), minimally-invasive surgery or horizontal incisions, the use of upper thoracic epidural anesthesia and more aggressive postoperative care with restricted fluid therapy, improved pain control, reduced nausea, liquid diet initiated in the immediate postoperative period (oral EN) and early mobilization (same day as surgery) (evidence A). These protocols have been demonstrated to reduce insulin resistance during the post-op period and mean hospital stay.
With these data, the guidelines of ESPEN about postoperative fasting19 advocate shortening postoperative bowel rest as much as possible and adapting oral intake to the tolerance of the patient and the type of surgery (Table 5). Once again, ASPEN makes no statement on this matter.18
ESPEN Recommendations for Postoperative Fasting.
| ESPEN 2006 recommendations for postoperative fasting |
| In general, the interruption of nutritional intake after surgery is not necessary in the majority of patients (A) |
| Oral intake can be introduced a few hours after surgery in most patients who undergo colon surgery (especially in an ERAS program) without differences between open surgery and laparoscopic surgery (A) |
| Oral intake after surgical intervention should be adapted to individual patient tolerance and type of surgery (C) |
ERAS: Enhance Recovery After Surgery; ESPEN: European Society of Parenteral and Enteral Nutrition.
Source: Based on Weinman et al.19
Once the patients have been treated surgically, postoperative NS should be considered. We have already indicated that oral nutrition is appropriate in patients who have undergone colon surgery, mainly through ERAS programs, although there is a group of patients in whom early oral intake after surgery is not possible. This is the case of patients who have been operated on for upper gastrointestinal tract neoplasms. For these cases, there are 2 NS modalities available: enteral and parenteral, the latter of which has been preferred for many years. In 2001, Bozzetti34 published the conclusions of a multi-center randomized prospective study that compared early EN with PN in the postoperative period of malnourished patients who had undergone gastrointestinal tract surgery. It included 317 patients and both types of NS contributed the same nutritional content in calories and protein. It concluded that EN was associated with fewer postoperative complications (34 vs 49%; P<.05) and shorter hospital stays (13.4 vs 15 days; P<.05). More recently, in 2008, a meta-analysis was published that included 2552 subjects, comparing post-op EN and PN anew.35 The results once again supported using early EN as a postoperative NS modality associated with reductions in: any type of complication (infectious and non-infectious), anastomotic leak, incidence of abdominal abscesses and hospital stay. Recently, a prospective intervention study by Jie et al.36 with 1831 patients who received enteral, parenteral or no NS demonstrated that the post-surgical complications, mainly infectious, were significantly fewer in the EN group versus the PN group and the group that received no NS, with no significant differences found between these last 2 groups.
We can therefore conclude that the enteral approach is the method of choice when applying postoperative NS if the oral approach is not feasible. To do so, digestive access is necessary. In preparation, the surgical team should place a naso-enteral feeding tube during the operation. If recovery is expected to be difficult or NS may be required for more than 6 weeks, the creation of a surgical feeding ostomy is indicated during the intervention. In cases where it has not been possible to leave in a naso-enteral feeding tube or create an ostomy, the postoperative NS can be done by PN. The PN approach will also be used in those cases in which the nutritional requirement could not be met with enteral nutrition or if there were postoperative complications with intestinal failure that would make the patient unable to meet nutritional requirements with oral intake alone for more than 7–10 days if the patient is well-nourished, or 5–7 if he/she is malnourished.
The recommendations of the main scientific associations are listed in Table 6.
ASPEN and ESPEN Recommendations for Postoperative Nutrition.
| General recommendations |
| PN should not be routinely administered in the postoperative period of patients with major gastrointestinal surgery (ASPEN, A) |
| NS should be administered after surgery in those patients with expected fasting times of at least 7–10 days (ASPEN, B) |
| EN through gastric access should be initiated in the first 24h following surgery (ESPEN, A) |
| Early EN is recommended in patients in whom early oral intake is not possible, such as: the immediate post-op of major head and neck surgery, upper gastrointestinal tract tumors, severe trauma (due to greater risk of sepsis and multi-organ failure) and in patients with severe malnutrition detected at the time of surgery (ESPEN, A) |
| Early EN is indicated in the postoperative period if there is an expected oral intake of less than 60% for more than 10 days (ESPEN, C) |
| Postoperative EN should be initiated slowly (10–20ml/h) due to the limited tolerance of the gastrointestinal tract. It can take 5–7 days to reach full rhythm, although this is not prejudicial (ESPEN, C) |
| Type of formula |
| The use of formulas with immunomodulators is recommended in the postoperative period of patients operated on by major surgery for cancer of the head, neck or upper abdomen (esophagus, stomach and pancreas-duodenum) or with severe trauma, regardless of nutritional state (ESPEN, C) |
| Formulas with immunomodulators should be initiated, if possible, in the pre-operative period (ESPEN, A) |
| In most patients with enteral NS, the enteral formula used is usually polymeric (ESPEN, C). |
| When an immunomodulatory formula is used, it should be maintained for 5–7 days after surgery (if the surgery has not been complicated) (ESPEN, C) |
| Type of enteral access |
| The placement of a nasojejunal or jejunostomy tube is recommended as a digestive access for nutritional support in patients who undergo major abdominal surgery (ESPEN, A) |
| If there is an anastomosis in the proximal gastrointestinal tract, a tube should be used that distally passes the anastomosis (ESPEN, B). |
| If EN is required for more than 4 weeks (severe traumatic brain injury), the percutaneous placement of a gastrostomy should be considered (ESPEN, C) |
The stress of surgery induces changes in the activity of the immune system, both in innate as well as adaptive immunity.37 The initial immunological response against surgical stress is local inflammation. This type of response seems to be adaptive and is seen not only in humans but also in many other species. Nonetheless, this type of response can become inappropriate and turn into a systemic response (called “systemic inflammatory response syndrome”), which can also result in multi-organ failure.37
Immunonutrition is used to try to modulate the inflammatory response and avoid systemic inflammatory response syndrome and multi-organ failure as much as possible. To do so, several nutrients can be administered with the enteral approach. Today, the most widely studied immunonutrients are: arginine, glutamine and omega 3 fatty acids.
Arginine is a conditioned essential amino acid, meaning that its synthesis by the organism does not satisfy the demand in certain situations, such as postoperative stress or sepsis.38 Arginine fulfills several metabolic activities, but it is essentially implicated in the production of polyamines through its conversion into ornithine. Polyamines are key molecules in cell growth and differentiation through the acceleration of protein synthesis. At pharmacological doses, it is able to increase the synthesis of anabolic hormones such as prolactin, growth hormone and insulin-like growth factors. In addition, it stimulates the function of the T-cells and is a precursor to nitric oxide synthesis,39 which improves microvascular circulation. In situations of stress secondary to surgery or trauma, arginine deficit alters the adaptive immune response and disrupts the proper functioning of T-cell receptors.
The clinical evidence on arginine supplements in situations of stress is still limited. Its current indications therefore depend on the underlying condition causing the stress. A meta-analysis published in 200140 concluded that its administration in septic patients lacked beneficial effects and could even increase mortality. Afterwards, another meta-analysis41 described favorable properties in surgical patients (reduced rate of postoperative infection, hospital stay and duration of mechanical ventilation during their ICU stay). Based on these accounts, the CPG by ESPEN alert professionals to the possible damaging effect of its administration in septic patients, while they state that supplementation in surgical patients may be beneficial.19
Omega 3 fatty acids derive from the elongation of the essential fatty acid α-linolenic. Its most widely studied derivatives are eicosapentaenoic acid (EPA) and docosahexaenoic acid, which have been attributed numerous anti-inflammatory properties as they are the basis for the production of series-3 prostaglandins.
Aside from its anti-inflammatory effect, there are studies that report anabolic properties in this family of fatty acids. In a double-blind, randomized, prospective study, Ryan et al.7 demonstrated that preoperative supplementation with EPA in patients who were scheduled for esophagectomy maintained their lean mass compared with those who did not receive supplements.
Glutamine is the most abundant amino acid in the human body, and its function is not just structural. In addition to its recognized utility as an energy substrate (main source of energy of enterocytes, macrophages and lymphocytes), it has also been attributed the following capabilities37:
- -
Cell signaling;
- -
Antioxidant function (precursor of glutathione synthesis);
- -
Immunomodulator: it attenuates the immune response against external aggression, making it more proportional;
- -
Increases the expression of heat shock proteins.
Glutamine is considered a conditioned-essential amino acid. In situations of stress, its deposits are rapidly used up and can cause a deficiency in this amino acid, which entails immune dysfunction and disturbance of the barrier function of the intestinal epithelium.37 Both in animal experiments as well as clinical trials, supplements with glutamine have been shown to restore the integrity of the gastrointestinal barrier and diminish bacterial translocation.37 In 2002, Novak,42 in a systematic review of the scientific literature available at that time about supplementation with glutamine, concluded that this action was able to reduce infectious complications and shorten hospital stay in surgical patients.
Today, the combination of glutamine, arginine and omega 3 fatty acids is the most frequently studied cocktail as a main immunomodulating diet therapy. Two meta-analyses published in 201043,44 determined that the use of these formulas in surgical patients reduced postoperative complications and hospital stay.
Currently, the ESPEN guidelines recommend this type of formulas during the perioperative period of patients affected by neoplasms of the upper gastrointestinal tract.
ProbioticsA new area of interest in the NS approach in surgical patients is the administration of probiotics in the preoperative period with the aim to achieve beneficial postoperative effects. The administration of Lactobacillus plantarum in some case has been able to reduce the incidence of postoperative infections (especially pneumonia),45 while in another randomized study46 no significant beneficial effects were seen. The dose of probiotics used in the second study was smaller, which could have caused these differences. However, more studies are needed with different quantities and strains of microorganisms in order to establish a definitive recommendation.
ConclusionMalnutrition increases postoperative morbidity and mortality, hospital stays and treatment costs. Assessment of the nutritional state prior to surgery enables us to detect patients with nutritional risk who will benefit from a perioperative NS program to reduce the associated postoperative complications.
Postoperative bowel rest has not been revealed to be effective, but instead it is rather counterproductive when compared with the early initiation of feeding (either oral or enteral).
Early EN has been shown to be superior to total PN in gastrointestinal surgery, but it requires the surgical team to place a feeding tube during the procedure.
In cases where enteral NS is recommended, immunomodulatory formulas are especially effective in patients who are treated surgically due to neoplasms of the upper gastrointestinal tract.
Recently, there have been new areas of interest in the approach to NS in surgical patients, for instance preoperative carbohydrate support with the administration of probiotics.
Conflict of InterestThe authors have no conflict of interests to declare.
Please cite this article as: Morán López JM, Piedra León M, García Unzueta MT, Ortiz Espejo M, Hernández González M, Morán López R, et al. Soporte nutricional perioperatorio. Cir Esp. 2014;92(6):379–386.