Radical surgery is the standard treatment for localized gastrointestinal stromal tumors (GIST). A series of primary GIST, their treatment and pre-established risk of recurrence after their follow-up are evaluated.
Materials and methodsA retrospective, descriptive and multicenter study was conducted on primary, non-metastatic GIST operated on between June 2007 and December 2008. The variables of greater relevance were analyzed, including, location, size, mitotic index, and NHI and AFIP recurrence prognostic criteria, and their correlation with the disease-free survival (DFS) of the patients.
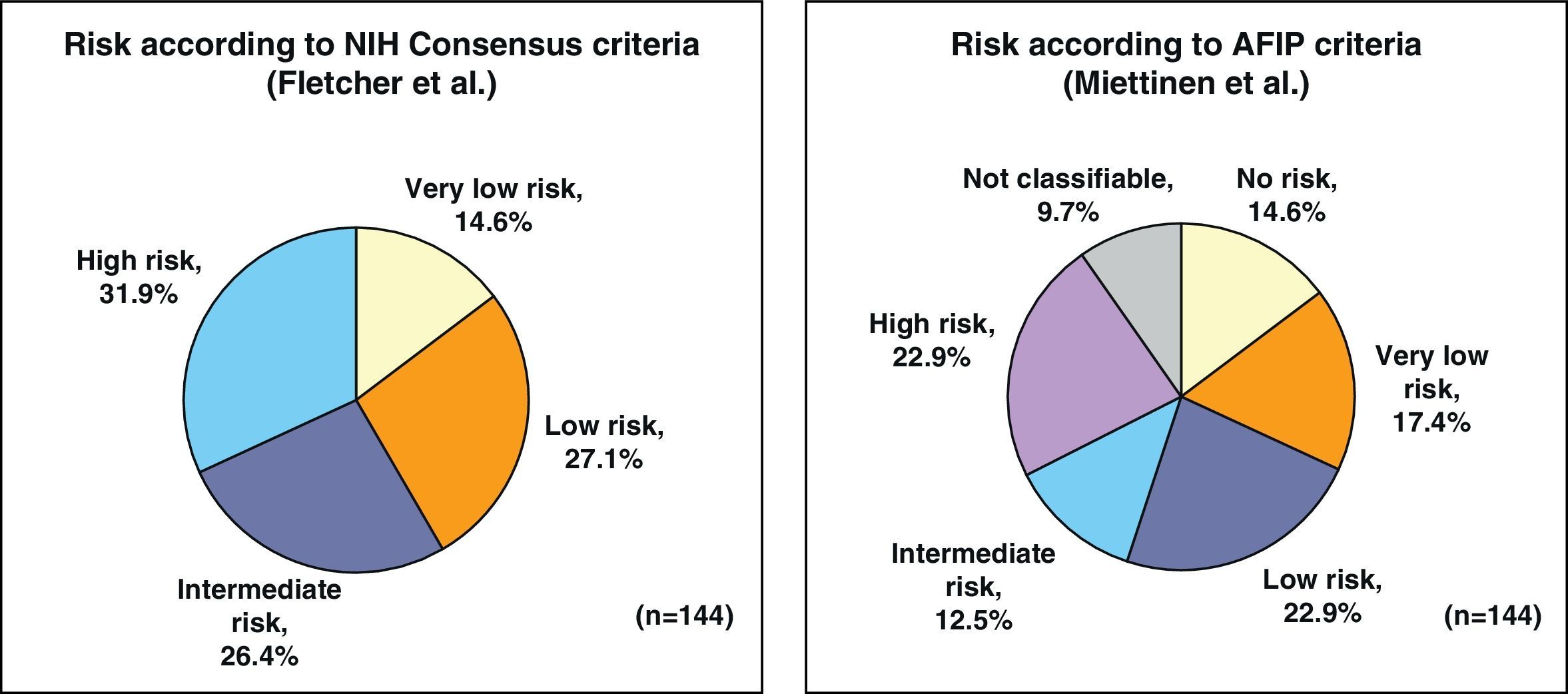
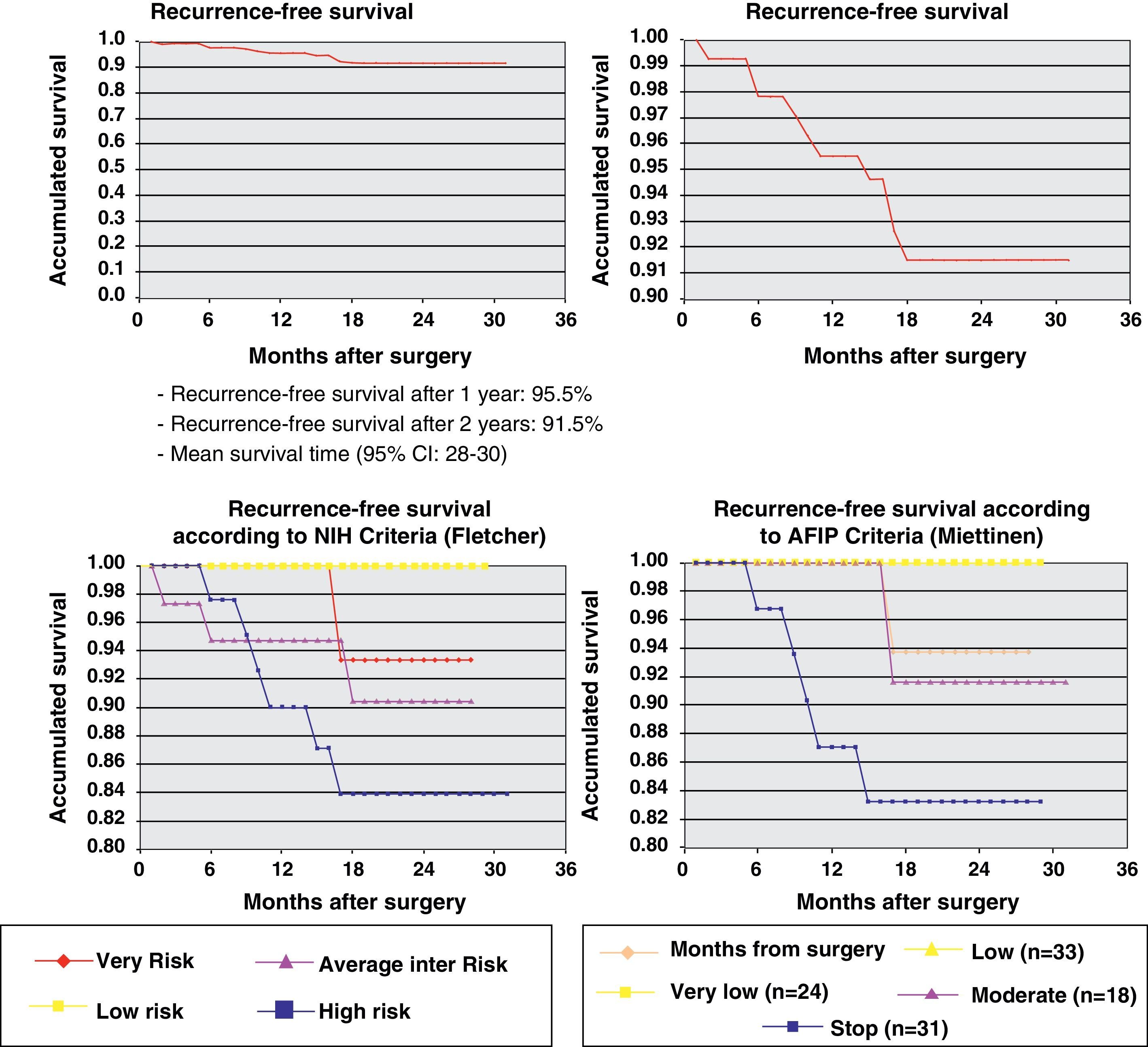
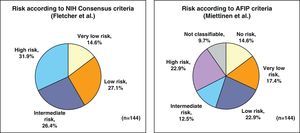
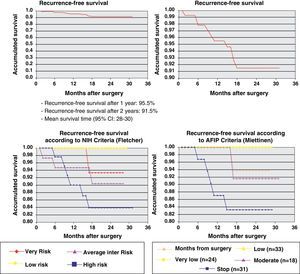
ResultsThe series included 141 patients with a mean age of 65 years. The most frequent GIST location was in the stomach (70.8%) and small intestine (22.9%), and with a mean tumor size of 6.7cm (0.5–35cm). The surgery was R0 in 97.2% of cases (laparoscopic approach, 21.5%). The distribution according to NHI/Flescher criteria was, high (31.95%), and intermediate (26.4%), and according to AFIP/Miettinen criteria it was, high (22.9%) and intermediate (12.5%). After a mean follow-up of 20.3 months, there was a 7.1% (10 cases) recurrence, with only 2 cases belonging to the group with a “low risk” using the NHI and AFIP prognostic criteria. The DFS at one year was 95.5% and 91.5% at 2 years.
ConclusionsThe series showed a high DFS and a good correlation with both the Flescher and the Miettinen criteria. However, the risk of recurrence varied according to the AFIP criteria (intermediate/high, 58.3%), or the AFIP criteria (intermediate/high, 35.4%) which included the tumor location. For this reason, we consider these latter criteria as the most adequate for assessing the prognostic risk of GIST recurrence.
La cirugía radical es el tratamiento estándar en el GIST primario localizado. Se valora una serie de GIST primarios, su tratamiento y el riesgo preestablecido de recaída tras seguimiento de los mismos.
Material y métodosEstudio retrospectivo multicéntrico y descriptivo de GIST primarios no metastásicos intervenidos entre junio de 2007 y diciembre de 2008. Se analizan las variables de mayor relevancia: localización, tamaño, índice mitótico y criterios pronóstico de recidiva NHI y AFIP y su correlación con la supervivencia libre de enfermedad (SLE) de los pacientes.
ResultadosSerie de 141 pacientes, edad media 65 años, con GIST de localización más frecuente en estómago (70,8%) e intestino delgado (22,9%) y con tamaño medio tumoral de 6,7cm (0,5-35). La cirugía fue R0 en el 97,2% de los casos (abordaje laparoscópico 21,5%). Distribución según criterios de NHI/Flescher: alto (31,95) e intermedio (26,4%), y según criterios AFIP/Miettinen: alto (22,9%) e intermedio (12,5%). Tras un seguimiento medio de 20,3 meses se detectó recaída en 7,1% (10 casos) perteneciendo tan solo 2 casos al grupo de «bajo riesgo» por criterios pronóstico NHI y AFIP. La SLE a un año fue del 95,5% y a los 2 años del 91,5%.
ConclusionesLa serie mostró una alta SLE y una buena correlación con los criterios pronóstico tanto de Flescher como de Mietinen. No obstante, el riesgo de recaída varió siguiendo los criterios de NIH (intermedio/alto 58,3%) o los criterios de AFIP (intermedio/alto 35,4%) que incluyen la localización del tumor. Por ello consideramos estos últimos criterios como los más adecuados para la valoración prónostica de riesgo de recidiva del GIST.
Gastrointestinal stromal tumors (GIST) are the most common mesenchymal neoplasms of the digestive tract. Their incidence is between 10 and 20 cases per million inhabitants.1,2 For years, GIST have been confused with other types of intestinal tumors; however, immunohistochemical and mutational studies help differentiate them from smooth muscle neoplasms (leiomyomas, leiomyosarcomas) and those derived from Schwann cells (schwannomas) because these tumors, unlike GIST, do not express the KIT protein.3,4
Radical surgical resection is the standard treatment in patients with primary localized GIST. Nevertheless, a considerable percentage of patients present recurrence of the disease after surgery. Therefore, all GIST should be considered potentially malignant tumors, with a significant risk of recurrence and metastatic progression after the complete resection of the primary tumor.5
The objective of this study was to analyze the characteristics of patients with primary non-metastatic GIST who were treated surgically in our country in order to know the treatment that they underwent and the degree of risk of progression of the disease. The recurrence-free survival (RFS) according to the clinical–pathological characteristics of the GIST tumor was also evaluated.
Materials and MethodsThis was a retrospective, multicenter, observational study of primary, non-metastatic GIST that were surgically resected between June 2007 and December 2008. The data collection was done in December 2009 and in December 2010 in 38 hospitals with a homogenous geographical distribution covering the entire Spanish territory, with an estimated case incidence higher than 3 cases/year of GIST.
The study was designed by a multidisciplinary team of 4 surgeons, an oncologist and a pathologist, supported by the Spanish Sarcoma Research Group (GEIS, Spanish acronym for Grupo Español de Investigación en Sarcomas) and approved by the Ethics Committee for Clinical Research at the Hospital Santa Creu i Sant Pau in Barcelona.
The study participants collected data for all the patients based on the information from the medical files. We were thus able to analyze demographic variables, concomitant diseases, GIST diagnostic methods, data related with surgical and pharmacological treatment and data of the tumor regarding location and pathology study. The GIST were classified in prognostic risk groups according to the criteria of the NIH consensus6 and according to AFIP criteria.7 The RFS of the patients was analyzed in each of the categories of the NIH and AFIP classifications.
The statistical evaluation (STAR version 1.20, Pulse Train Technology) contemplates the cross tabulation of all the variables collected with those that were considered most relevant (age, sex, time since GIST diagnosis, location of the primary tumor, neoadjuvant treatment, tumor size, mitotic rate, NIH criteria, AFIP criteria). Kaplan– Meier curves were applied for the assessment of RFS.
ResultsForty-two surgeons from around the country participated in our study, which made it possible to compile the characteristics of 141 patients with primary non-metastatic GIST after complete tumor resection. Mean patient age was 65 (range 22–85), with no differences in sex and with a high comorbidity rate of 72.9%.
Mean time from the first symptom until surgery was 2.0 months. There was a predominance of patients who were symptomatic at diagnosis (82.6%), mainly upper gastrointestinal bleeding (43.7%) and abdominal discomfort (38.7%). The diagnosis of GIST was established with abdominal CT in 91.2% and by endoscopy in 71.5% of the cases.
The most frequent location of GIST was the stomach in 102 cases (70.8%) and in the small intestine in 33 cases (22.9%). In 9 cases (6.3%), GIST originated in other locations, such as the esophagus in 3 cases, the duodenum in 2 cases or the rectum in 4 cases. The most frequent gastric location was the greater curvature (42.2%) and the body of the stomach (40.2%), while in the small intestine the main location was the jejunum (75.0%). In 3 cases of gastric and one case of jejunal GIST, the tumor invaded neighboring organs (esophagus, pancreas or root of the mesentery).
By applying the criteria for risk of relapse according to the NIH/Fletcher consensus, 27% of the patients were classified as being at intermediate risk and 31% as high risk. By applying the AFIP/Miettinen criteria, 12.5% of the patients were classified as intermediate risk and 22.9% as high risk. Finally, 9.7% of the patients were unclassifiable (Fig. 1).
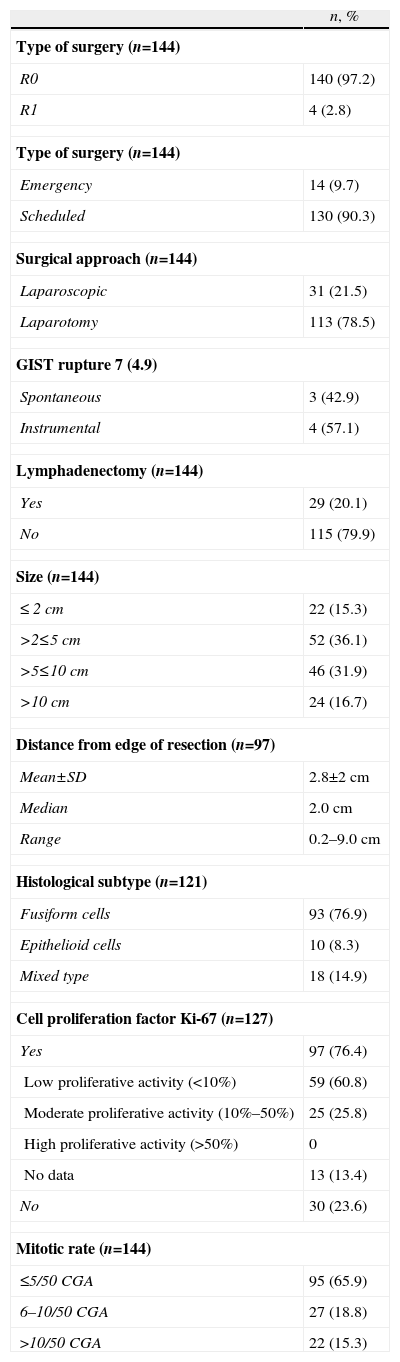
In 100% of the cases, R0 was obtained, using a laparoscopic approach in 31 (21.5%) of the GIST (28 gastric, 2 intestinal and one esophageal). After the pathology study, the surgery was R1 in 4 patients as the margins of the surgical specimen were affected (3 cases of open surgery and one of laparoscopic surgery) (Table 1). There were second primary neoplasms seen in 24 patients (17%), most of which were unexpected findings made during the diagnostic examinations of the first neoplasm. 16.0% of the patients presented post-operative complications (30 days post-op); infection and anastomotic dehiscence were the most common. There was no surgical mortality.
Details of Surgery and Pathology.
| n, % | |
| Type of surgery (n=144) | |
| R0 | 140 (97.2) |
| R1 | 4 (2.8) |
| Type of surgery (n=144) | |
| Emergency | 14 (9.7) |
| Scheduled | 130 (90.3) |
| Surgical approach (n=144) | |
| Laparoscopic | 31 (21.5) |
| Laparotomy | 113 (78.5) |
| GIST rupture 7 (4.9) | |
| Spontaneous | 3 (42.9) |
| Instrumental | 4 (57.1) |
| Lymphadenectomy (n=144) | |
| Yes | 29 (20.1) |
| No | 115 (79.9) |
| Size (n=144) | |
| ≤ 2cm | 22 (15.3) |
| >2≤5cm | 52 (36.1) |
| >5≤10cm | 46 (31.9) |
| >10cm | 24 (16.7) |
| Distance from edge of resection (n=97) | |
| Mean±SD | 2.8±2cm |
| Median | 2.0cm |
| Range | 0.2–9.0cm |
| Histological subtype (n=121) | |
| Fusiform cells | 93 (76.9) |
| Epithelioid cells | 10 (8.3) |
| Mixed type | 18 (14.9) |
| Cell proliferation factor Ki-67 (n=127) | |
| Yes | 97 (76.4) |
| Low proliferative activity (<10%) | 59 (60.8) |
| Moderate proliferative activity (10%–50%) | 25 (25.8) |
| High proliferative activity (>50%) | 0 |
| No data | 13 (13.4) |
| No | 30 (23.6) |
| Mitotic rate (n=144) | |
| ≤5/50CGA | 95 (65.9) |
| 6–10/50CGA | 27 (18.8) |
| >10/50CGA | 22 (15.3) |
The pathology results are shown in Table 1. Mean tumor diameter was 6.7±6cm, with an average of 5.0cm (0.5–35cm). Immunohistochemistry was positive for CD117 (97.2%), CD34 (89.2%) and actin (31.9%), detecting mutations in 10 out of 14 cases studied that mainly affected the KIT exon 11. In 3 cases, although initially a GIST diagnosis was established and it was treated as such, the final diagnosis after the mutational study was leiomyosarcoma; these cases were excluded from the present study.
A total of 36 patients (25.5%) received adjuvant treatment with imatinib (400mg/day): 22 (61.1%) high-risk cases, 10 (27.8%) cases with intermediate risk and 4 (1.1%) cases with low risk according to the criteria of the AFIP consensus. The average time from surgery until the start of adjuvant treatment was 1.4 months, and mean treatment duration was one year.
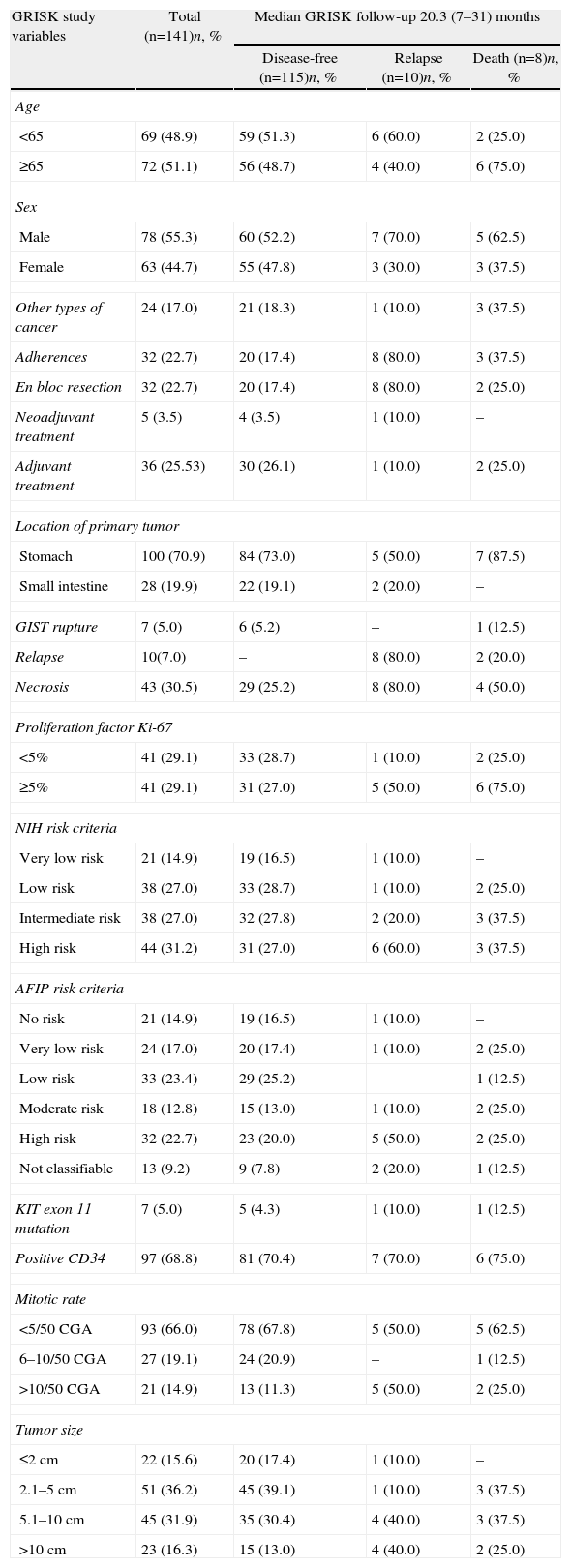
After an average follow-up of 20.3 months, 115 patients (81.6%) remained disease-free (Table 2) while 10 cases could not be evaluated due to loss to follow-up. The recurrence rate was 7.1% (10 cases); 8 patients died (5.8%). Only one patient out of the 10 with recurrence had been treated with imatinib 400mg/day after surgery. The average time to recurrence was 11 (2–18) months and in 6 cases there was early recurrence, with an average time elapsed since surgery of 6.7 months. In 9 cases, the recurrence was in a distant location and in 1 it was local. No cases of recurrence had previous evidence of GIST rupture during surgery. Only 2 of the 10 patients belonged to the low-risk group for recurrence according to NIH and AFIP criteria. The causes of death included progression of the disease (2 patients), cerebrovascular accidents (3 patients), acute myocardial infarction (1 patient), disseminated lung cancer (1) and post-operative septic problems (1).
Current Clinical Situation of the Patients (With the Main Variables of the GRISK Study).
| GRISK study variables | Total (n=141)n, % | Median GRISK follow-up 20.3 (7–31) months | ||
| Disease-free (n=115)n, % | Relapse (n=10)n, % | Death (n=8)n, % | ||
| Age | ||||
| <65 | 69 (48.9) | 59 (51.3) | 6 (60.0) | 2 (25.0) |
| ≥65 | 72 (51.1) | 56 (48.7) | 4 (40.0) | 6 (75.0) |
| Sex | ||||
| Male | 78 (55.3) | 60 (52.2) | 7 (70.0) | 5 (62.5) |
| Female | 63 (44.7) | 55 (47.8) | 3 (30.0) | 3 (37.5) |
| Other types of cancer | 24 (17.0) | 21 (18.3) | 1 (10.0) | 3 (37.5) |
| Adherences | 32 (22.7) | 20 (17.4) | 8 (80.0) | 3 (37.5) |
| En bloc resection | 32 (22.7) | 20 (17.4) | 8 (80.0) | 2 (25.0) |
| Neoadjuvant treatment | 5 (3.5) | 4 (3.5) | 1 (10.0) | – |
| Adjuvant treatment | 36 (25.53) | 30 (26.1) | 1 (10.0) | 2 (25.0) |
| Location of primary tumor | ||||
| Stomach | 100 (70.9) | 84 (73.0) | 5 (50.0) | 7 (87.5) |
| Small intestine | 28 (19.9) | 22 (19.1) | 2 (20.0) | – |
| GIST rupture | 7 (5.0) | 6 (5.2) | – | 1 (12.5) |
| Relapse | 10(7.0) | – | 8 (80.0) | 2 (20.0) |
| Necrosis | 43 (30.5) | 29 (25.2) | 8 (80.0) | 4 (50.0) |
| Proliferation factor Ki-67 | ||||
| <5% | 41 (29.1) | 33 (28.7) | 1 (10.0) | 2 (25.0) |
| ≥5% | 41 (29.1) | 31 (27.0) | 5 (50.0) | 6 (75.0) |
| NIH risk criteria | ||||
| Very low risk | 21 (14.9) | 19 (16.5) | 1 (10.0) | – |
| Low risk | 38 (27.0) | 33 (28.7) | 1 (10.0) | 2 (25.0) |
| Intermediate risk | 38 (27.0) | 32 (27.8) | 2 (20.0) | 3 (37.5) |
| High risk | 44 (31.2) | 31 (27.0) | 6 (60.0) | 3 (37.5) |
| AFIP risk criteria | ||||
| No risk | 21 (14.9) | 19 (16.5) | 1 (10.0) | – |
| Very low risk | 24 (17.0) | 20 (17.4) | 1 (10.0) | 2 (25.0) |
| Low risk | 33 (23.4) | 29 (25.2) | – | 1 (12.5) |
| Moderate risk | 18 (12.8) | 15 (13.0) | 1 (10.0) | 2 (25.0) |
| High risk | 32 (22.7) | 23 (20.0) | 5 (50.0) | 2 (25.0) |
| Not classifiable | 13 (9.2) | 9 (7.8) | 2 (20.0) | 1 (12.5) |
| KIT exon 11 mutation | 7 (5.0) | 5 (4.3) | 1 (10.0) | 1 (12.5) |
| Positive CD34 | 97 (68.8) | 81 (70.4) | 7 (70.0) | 6 (75.0) |
| Mitotic rate | ||||
| <5/50CGA | 93 (66.0) | 78 (67.8) | 5 (50.0) | 5 (62.5) |
| 6–10/50CGA | 27 (19.1) | 24 (20.9) | – | 1 (12.5) |
| >10/50CGA | 21 (14.9) | 13 (11.3) | 5 (50.0) | 2 (25.0) |
| Tumor size | ||||
| ≤2cm | 22 (15.6) | 20 (17.4) | 1 (10.0) | – |
| 2.1–5cm | 51 (36.2) | 45 (39.1) | 1 (10.0) | 3 (37.5) |
| 5.1–10cm | 45 (31.9) | 35 (30.4) | 4 (40.0) | 3 (37.5) |
| >10cm | 23 (16.3) | 15 (13.0) | 4 (40.0) | 2 (25.0) |
One year later, recurrence-free survival (Kaplan–Meier) was 95.5% and two years later it was 91.5%. Fig. 2 expresses the correlation of RFS and its relationship according to the NIH and AFIP risk criteria showing a greater rate of progression in high-risk patients in the 2 classifications.
DiscussionGIST are the most common mesenchymal neoplasms of the digestive tract. Diagnosis is established based on the secondary symptoms of the disease; 21% of cases are incidental findings and 10% are detected during autopsy ordered for other causes. In our observational and retrospective study, a diagnosis of presumed primary non-metastatic GIST was initially established in 144 patients who underwent R0 resection. Later mutation studies confirmed that in 4 patients the initial diagnosis was erroneous as they were actually leiomyosarcomas. Therefore, when given a presumed diagnosis of GIST and negative CD117 results, a complete mutational study8 is always recommended.
The clinical manifestations of GIST depend on the tumor diameter and location. GIST in the small intestine are usually diagnosed later and have a poorer prognosis. The most common location was gastric, followed by the small intestine, and the mitotic rate was low (<5/50HPF) in 65.9% of cases, intermediate (6–10/50HPF) in 18.8% and high (>10/50HPF) in 15.3%. These characteristics of the series are similar to data from other published series.7
The presence of a GIST as a second neoplasm should not be considered uncommon, especially because the presence of silent gastric GIST is more frequent after a certain age, when the development of other neoplasms is likewise more frequent.9 It is interesting to comment that, in the series studied, 26 (16.6%) patients were diagnosed with second primary neoplasms in different locations. In most cases, a completely asymptomatic GIST was discovered that was not related with the primary tumor. These incidental findings of GIST were, in most cases, small-sized GIST found in the stomach with low risk for recurrence. No patients from this group presented recurrence during follow-up.
Radical surgery is the standard treatment of patients with primary localized GIST. In our series, the surgery was mostly R0 (97.2%), meaning radical surgery with free margins. The use of laparoscopic surgical techniques is currently widely accepted, as long as radical (R0) surgery criteria are met.10–14 This type of approach was used in 21.5% of the patients in our series, most of which were located in the stomach. It is striking that, although regional lymphadenectomy is not indicated, a group of 28 patients underwent lymphadenectomy that was unnecessary in the context of surgical treatment of GIST.15
GIST are tumors that are potentially malignant, and patients may therefore present a significant risk for local recurrence and/or metastatic progression of the disease, even after complete resection of the tumor.5 In 2002, Fletcher et al.6 published the first stratification for risk of recurrence according to the National Institute of Health (NIH) consensus, based on GIST mitotic rate and size. Later, Miettinen et al. evaluated other key factors such as tumor location.7 Although both classifications are recommendable and easily used in clinical practice, it was confirmed that the risk of recurrence expressed with the AFIP better weighs the risk for tumor recurrence.16,17 Last of all, the introduction of mutational studies in these tumors can offer additional beneficial information for both diagnosis (especially in c-KIT-negative GIST) as well as prognosis. In this manner, some studies suggest a greater risk of recurrence in patients with mutations in exon 11 (deletion 557–558) compared with mutations in exon 9 and PDGFRa.18
In the results of our study, we have observed how by following NIH criteria more than 50% of patients had an intermediate-high risk of recurrence, while this occurs in little more than one-third of the patients when the assessment was done with AFIP criteria. The relapse rate detected in our study after a 2-year follow-up was 7%, specifically in patients with greatest risk following either the NIH or AFIP criteria.
The discovery of imatinib mesylate as a target substance in the treatment of GIST has generated important studies about its use as adjuvant treatment in situations of high risk of recurrence in recent years.19,20 The results published after the ACOSOG Z9001 study showed benefits in RFS after its administration at a dosage of 400mg/day for one year, especially in patients with moderate-high risk of recurrence.21 Furthermore, the recently published results of the SSGXVIII/AIO study, which evaluated adjuvant imatinib at 400mg/day for one year versus its administration for 3 years in tumors at high risk for relapse, confirm the aforementioned data as they show a statistically significant improvement of both RFS and SG with the 3-year branch.22 Thus, it seems logical to recommend adjuvant treatment with imatinib in GIST with intermediate-high risk of recurrence. In our study, 25.5% of the patients with resected GIST received adjuvant treatment with imatinib at 400mg/day for an average of 12 (5–19) months both in the context of a clinical trial or as regular care.
Last of all, we should comment that only 68% of the patients of this observational study were referred to oncology after surgery. We believe that, given the special characteristics of these tumors, the risk of recurrence and current advances in medical therapies all justify it being treated by multidisciplinary oncological committees, which can clearly improve both diagnosis as well as adequate therapeutic focus for these patients.8
Conflict of InterestsThis study was financed by Novartis Oncology.
Logistical support, monitoring and statistical analyses were provided by Sociología y Comunicación, S.L.
Participants in the GRISK study: Abradelo de Usera M, Alcántara Gijón F, Aranda Danso H, Bernal Jaulín J, Bibiloni Truyols M, Bustamante Montalvo M, Cáceres Alvarado N, Calle Baraja M, Castro Boix S, Cuberes Montserrat AR, Delgado Rivilla S, Farran Teixidor L, Franco Osorio JD, Galindo Galindo A, García de Polavieja Carrasco M, García Fidalgo G, García Navarro A, García Plaza G, González de Francisco T, Grande Posa L, Gutstein D, Hachem Ibrahim A, Lacueva Gómez FJ, López Pardo R, Martí Obiol R, Martín Pérez E, Martínez Alarcón M, Martínez Molina E, Miró Martín M, Morlán López MA, Osorio Aguilar J, Pérez-Ricarte Pérez P, Rufián Peña S, Ruiz del Castillo J, Sánchez Hidalgo JM, Suárez Muñoz MA, Talavera Eguizabal P and Zapata Salamé C.
Please cite this article as: Artigas Raventós V, et al. Cirugía de los tumores del estroma gastrointestinal primarios no metastásicos. Resultados del estudio GRISK. Cir Esp. 2013;91:96–102.