To analyze the cases of pancreatic metastases due to renal carcinoma operated on in our hospital between the years 2000 and 2011.
Material and methodsA retrospective study using the variables of 8 patients who were subjected to surgery of pancreatic metastases due to renal carcinoma, and a comparison of our data with those from the literature.
ResultsThe incidence of metastatic disease of the pancreas due to renal carcinoma in our series was 1.2%. All the metastases were metachronous, with both sexes being affected equally. The mean time between resection of the renal tumor and the diagnosis of the metastasis was 12.42 years (range: 1.62–30.13 years). The therapeutic approach to the pancreatic lesions was surgical in all cases. Seven patients are currently still alive.
ConclusionMetastatic disease of the pancreas due to renal carcinoma is uncommon (1%–2.8%). The interval between the primary resection and the metastasis can be quite long. Pancreatic metastasis must always be suspected in patients who present with a pancreatic mass and a history of renal carcinoma. Aggressive surgical treatment is recommended in selected cases. The surgery in these cases improves survival and the quality of life.
Analizar los casos de metástasis pancreáticas por carcinoma renal intervenidos en nuestro Hospital entre los años 2000 y 2011.
Material y métodosEstudio retrospectivo donde se recogen diferentes variables de 8 pacientes con metástasis pancreáticas por carcinoma renal intervenidos. Comparación de nuestros datos con los de la literatura.
ResultadosLa enfermedad metastásica del páncreas por carcinoma renal en nuestra serie ha sido de 1.2%. Todas las metástasis han sido metacrónicas. La afectación por sexo ha sido igual. El tiempo medio entre la resección del tumor renal y el diagnóstico de las metástasis ha sido de 12.42 años (rango: 1.62-30.13 años). La actitud terapéutica ante las lesiones pancreáticas ha sido quirúrgica en todos los casos. Hasta la fecha, 7 pacientes continúan vivos.
ConclusiónLa enfermedad metastásica del páncreas por carcinoma renal es poco frecuente (1-2.8%). El intervalo entre la resección primaria y las metástasis puede ser bastante largo. Siempre debe sospecharse metástasis pancreática en los pacientes que presenten masa pancreática e historia de carcinoma renal. Se recomienda un tratamiento quirúrgico agresivo en casos seleccionados. La cirugía en estos casos mejora la supervivencia y la calidad de vida.
Pancreatic metastases of any primary tumor are rare and represent between 2% and 5% of pancreatic tumors. Renal cell carcinoma as a primary tumor is uncommon, representing between 1% and 2.8% of pancreatic metastases.1–10
Due to the rarity and peculiarity of these pancreatic lesions, we decided to analyze the cases of pancreatic metastasis from renal carcinoma treated in our hospital between 2000 and 2011, and to analyze the frequency and characteristics compared with the literature.
Materials and MethodsWe present a retrospective study of patients with a history of renal neoplasm that underwent pancreatic surgery from January 2000 to March 2011. To identify these patients, the diagnoses in the Hospital database were reviewed. We also reviewed patients with previous renal tumors treated by nephrectomy. A total of 11 patients were identified, 3 of whom were excluded because the pancreatic tumor was the primary tumor (adenocarcinoma or endocrine tumor) and not metastasis of the renal cell carcinoma.
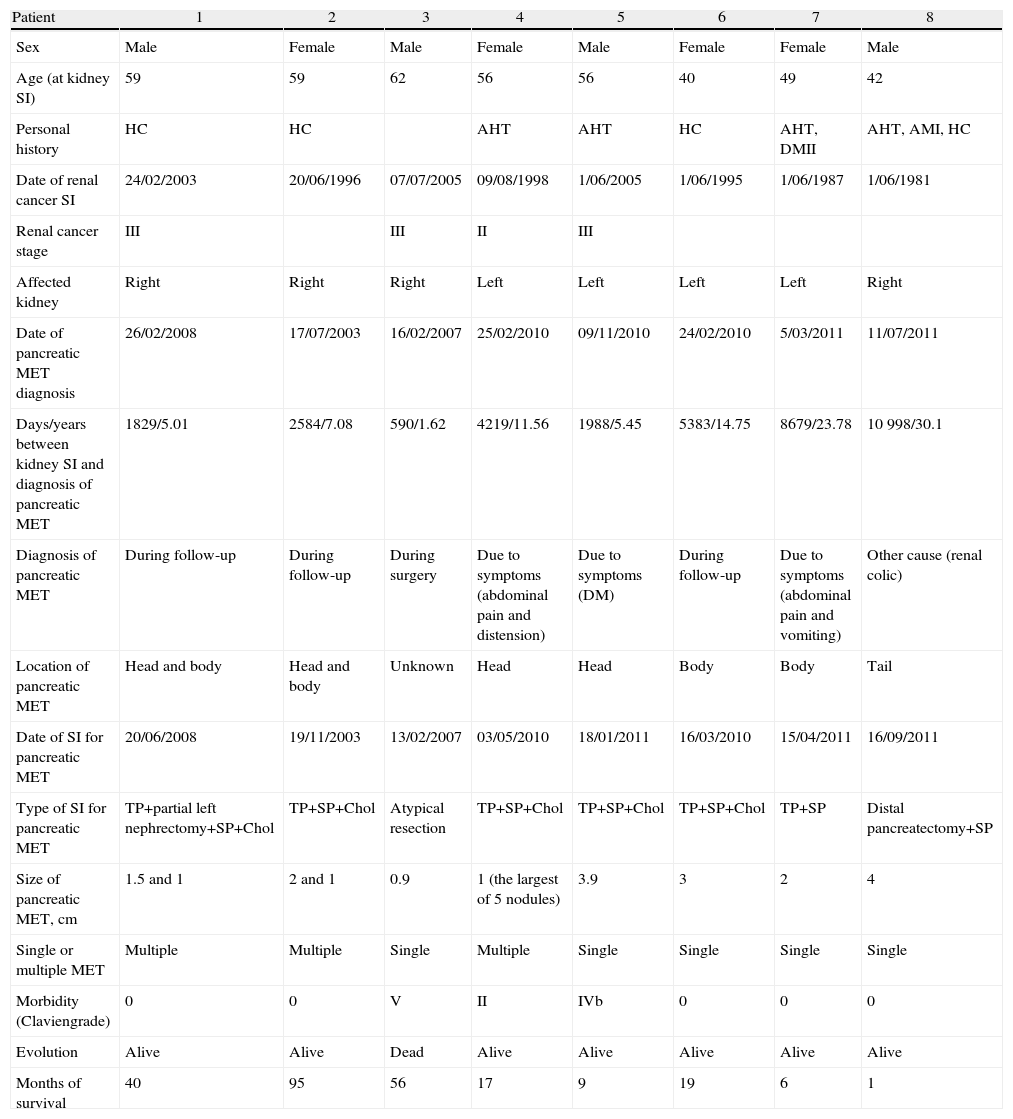
Different variables were collected, including patient variables as well as those referring to the renal and pancreatic tumors they presented (Table 1). Due to the fact that some of the patients had undergone surgery many years beforehand and/or in other centers, we could not obtain all the renal tumor data.
Characteristics of the Patients Diagnosed With Pancreatic Metastases of Renal Cell Carcinoma.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Sex | Male | Female | Male | Female | Male | Female | Female | Male |
| Age (at kidney SI) | 59 | 59 | 62 | 56 | 56 | 40 | 49 | 42 |
| Personal history | HC | HC | AHT | AHT | HC | AHT, DMII | AHT, AMI, HC | |
| Date of renal cancer SI | 24/02/2003 | 20/06/1996 | 07/07/2005 | 09/08/1998 | 1/06/2005 | 1/06/1995 | 1/06/1987 | 1/06/1981 |
| Renal cancer stage | III | III | II | III | ||||
| Affected kidney | Right | Right | Right | Left | Left | Left | Left | Right |
| Date of pancreatic MET diagnosis | 26/02/2008 | 17/07/2003 | 16/02/2007 | 25/02/2010 | 09/11/2010 | 24/02/2010 | 5/03/2011 | 11/07/2011 |
| Days/years between kidney SI and diagnosis of pancreatic MET | 1829/5.01 | 2584/7.08 | 590/1.62 | 4219/11.56 | 1988/5.45 | 5383/14.75 | 8679/23.78 | 10998/30.1 |
| Diagnosis of pancreatic MET | During follow-up | During follow-up | During surgery | Due to symptoms (abdominal pain and distension) | Due to symptoms (DM) | During follow-up | Due to symptoms (abdominal pain and vomiting) | Other cause (renal colic) |
| Location of pancreatic MET | Head and body | Head and body | Unknown | Head | Head | Body | Body | Tail |
| Date of SI for pancreatic MET | 20/06/2008 | 19/11/2003 | 13/02/2007 | 03/05/2010 | 18/01/2011 | 16/03/2010 | 15/04/2011 | 16/09/2011 |
| Type of SI for pancreatic MET | TP+partial left nephrectomy+SP+Chol | TP+SP+Chol | Atypical resection | TP+SP+Chol | TP+SP+Chol | TP+SP+Chol | TP+SP | Distal pancreatectomy+SP |
| Size of pancreatic MET, cm | 1.5 and 1 | 2 and 1 | 0.9 | 1 (the largest of 5 nodules) | 3.9 | 3 | 2 | 4 |
| Single or multiple MET | Multiple | Multiple | Single | Multiple | Single | Single | Single | Single |
| Morbidity (Claviengrade) | 0 | 0 | V | II | IVb | 0 | 0 | 0 |
| Evolution | Alive | Alive | Dead | Alive | Alive | Alive | Alive | Alive |
| Months of survival | 40 | 95 | 56 | 17 | 9 | 19 | 6 | 1 |
Chol: cholecystectomy; DM: diabetes mellitus; SP: splenectomy; HC: hypercholesterolemia; AHT: arterial hypertension; AMI: acute myocardial infarction; SI: surgical intervention; MET: metastasis; TP: total pancreatectomy.
In our center during the period selected, 502 malignant renal neoplasms were treated surgically; the most frequent histologic type was clear cell (402 patients [80.1%]). Out of the 502 renal neoplasms, 6 (1.2%) had metastasized in the pancreas. Another 2 patients in the series were operated on for renal neoplasia in other centers, so we cannot add them to this percentage, although their data are included since their surgeries for pancreatic metastasis were done at our center. All the pancreatic metastases were metachronous (Table 1). Distribution was equal in both sexes. Mean age at the time of the renal neoplasia was 52.87 years (range 40–62).
As a primary tumor, the 8 patients had presented a clear cell renal carcinoma. Half of the patients presented left kidney affectation, while the other half presented right renal tumors.
The average time between renal tumor resection and the diagnosis of pancreatic metastases was 4533.75 days, or 12.42 years (range: 1.62–30.13 years), and the latest presentation of the series was more than 30 years after the nephrectomy.
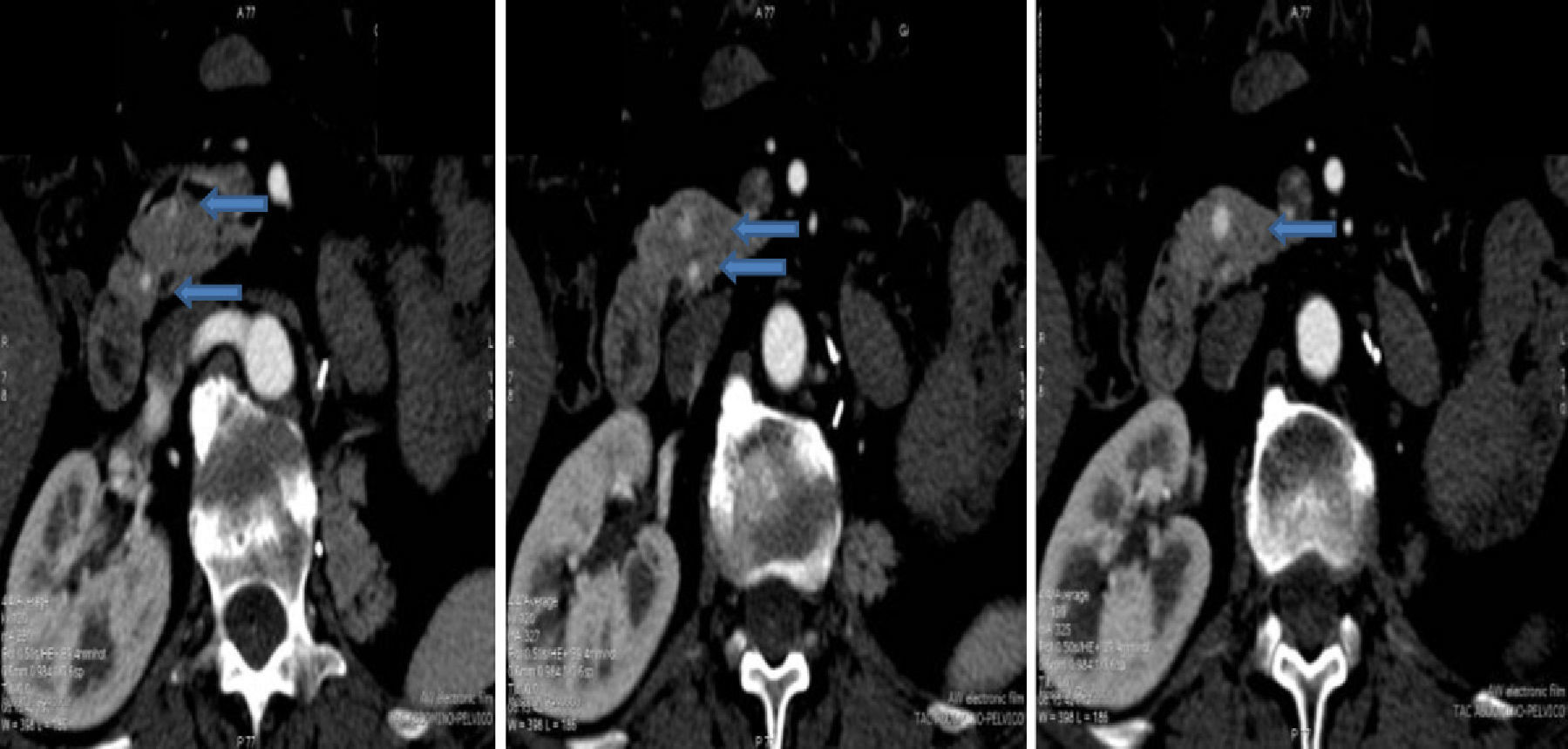
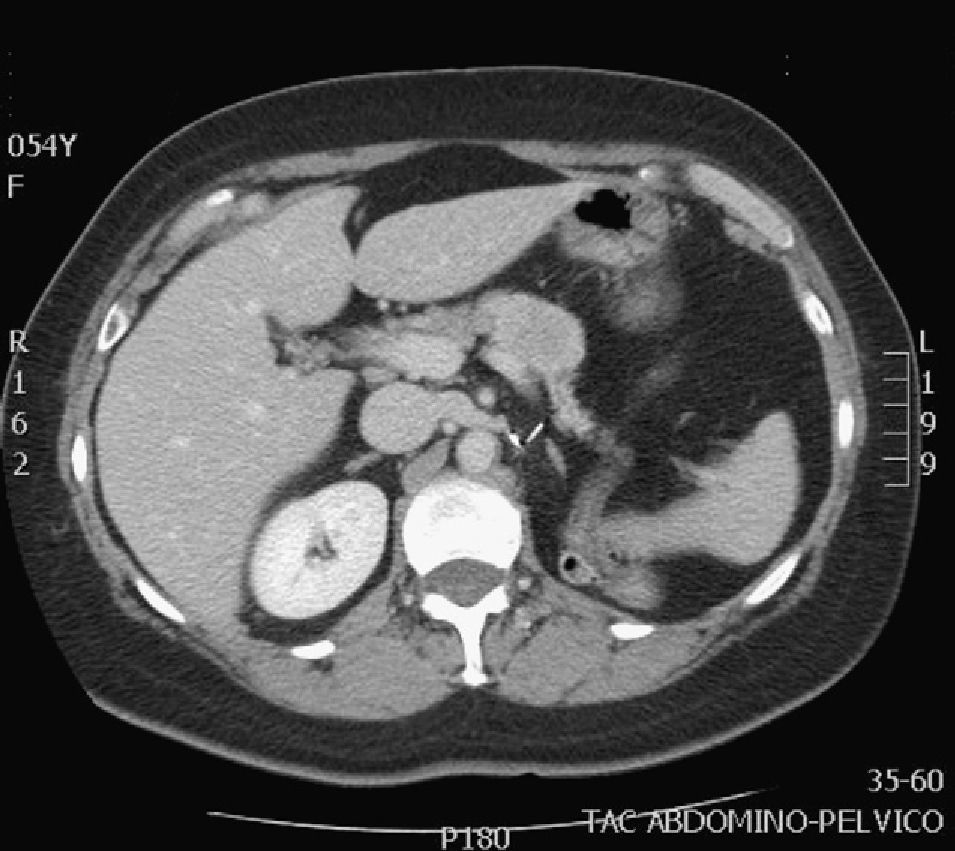
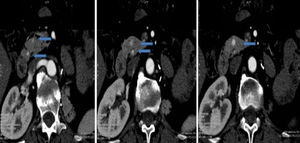
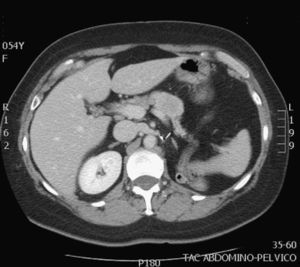
The diagnosis of the pancreatic lesions was made during follow-up after nephrectomy in 3 patients (37.5%), due to the appearance of symptoms in 3 (37.5%) (abdominal pain in 2 and debut of diabetes mellitus with diabetic decompensation in another), in a study for another cause (renal colic) in one patient (12.5%) and as an intraoperative finding in another (12.5%) (Suspicion of left suprarenal metastasis; the pathology study reported pancreatic metastasis). The most widely used imaging methods in the diagnosis of the pancreatic lesions were ultrasound and CT. The diagnosis was not confirmed histologically by FNA prior to surgery in any case. The lesions were located in the head, body and tail of the pancreas (Figs. 1 and 2).
The therapeutic approach for the pancreatic lesions was surgery in all cases because all the lesions were resectable (Table 1). The intervention performed most frequently was total pancreatectomy with splenectomy and cholecystectomy (n=4, 50%) (in one, partial left nephrectomy was also performed due to tumor invasion). In one of the patients, an atypical resection was performed, thinking that there was a left suprarenal metastasis. The same surgical team performed all the interventions. No new pancreatic recurrences were detected.
All the histologic tests demonstrated that the pancreatic lesions corresponded with metastases of clear cell renal carcinoma. Five patients (62.5%) presented single pancreatic metastases, while the other 3 (37.5%) presented multiple lesions, as seen on pre-operative imaging tests.
None of the patients received adjuvant or neoadjuvant therapy for the pancreatic metastases.
As for postoperative morbidity, one patient presented hemorrhage of the hepaticojejunal anastomoses on the tenth post-op day, requiring transfusion of platelet concentrates and plasma (grade ii of the Clavien classification). Another patient presented pseudomembranous colitis with acute renal failure and compartment syndrome requiring surgery to reduce intra-abdominal pressure (placement of vacuum system), hemofiltration and tracheotomy (Clavien grade ivb). One patient died after an atypical resection due to a torpid post-operative course (Clavien grade v). The remaining patients presented no complications. To date, 7 patients are still alive (87.5%) and only one has died (12.5%).
During the follow-up after pancreatic lesion resection, 3 patients presented metastasis in other organs: one in the contralateral kidney requiring tumorectomy (nearly 7 years after pancreatic resection); one in the thyroid, treated with thyroidectomy (4 months after pancreatic intervention); and one in the bronchus of the left lower lobe (2 months after pancreatic intervention), who is currently being treated with chemotherapy (sunitinib) and bronchoscopic ablation. The remaining 4 patients (since one had died during post-op) have not presented evidence of disease (disease-free survival: 50%). Mean follow-up was 2.56 years (range: 0.11–7.94 years).
Patient follow-up after the pancreatic intervention included periodic patient visits to our outpatient clinic.
DiscussionPancreatic metastases of any primary tumor are rare and represent between 2% and 5% of pancreatic tumors. The most frequent primary tumors are lung, colon, breast, skin and brain. Metastatic disease of the pancreas due to renal cell carcinoma is rare (1%–2.8%), since the most frequent places for renal carcinoma metastasis are: the lungs (50%–60%), bones (30%–40%), liver (30%–40%), suprarenals and brain (5%).1,2,10 The percentage of pancreatic metastases due to renal tumors reported in our series is 1.2%, similar to what has been described in the literature.
Renal clear cell carcinoma is the most common histological type within the renal cell carcinomas, representing 70%–80%; in our series, the percentage of clear cell carcinomas was 80.1%.
The presentation of pancreatic metastases of renal carcinoma may be synchronous or metachronous, with cases being reported some 27 years after nefrectomy.1–10 In our series, all the metastases were metachronous, and the latest presentation of the series was more than 30 years after nephrectomy (range: 1.62–30.1 years). 85% of the recurrences occur within the first 3 years after nephrectomy.1,2 It is, therefore, extremely important to take into account the medical history as these patients can present metastasis at any time, in any location and with different symptoms. Given the suspicion for metastatic renal disease, imaging studies are essential and the confirmation diagnosis should be done in accordance with the location.1
The mean age of our patients was 52.87, which is similar to the results of other authors, although somewhat younger (e.g. 56, 63.5, 62.2).9
The means of extension of renal cell carcinoma to the pancreas are controversial; it may be hematogenous or lymphatic, and direct extension to the pancreas is thought to be unusual.2,3,7 Renal carcinoma cells have an affinity for the pancreatic parenchyma and they can metastasize in the pancreas without there being metastasis in other organs.5,7,8 Due to this affinity, there may be recurrences after previous pancreatic resection.5
No relationship has been found between the location of the pancreatic metastases and the location of the primary renal carcinoma. The tumors on the left side do not metastasize more frequently in the tail of the pancreas, nor do the tumors on the right side metastasize more frequently in the head of the pancreas.3,7
The presentation is usually as single, asymptomatic metastases. If there are symptoms, these may be very diverse and indistinguishable from other types of pancreatic cancer.1,2,4,7,9 In our cases, 5 patients (62.5%) presented single metastasis and 3 presented multiple metastases (37.5%); only 3 patients (37.5%) presented symptoms.
The multifocal nature of pancreatic metastases has been reported to range from 20% to 45%.7 Our percentage is 37.5%.
As for their diagnosis, metastases are usually diagnosed during follow-up studies. Pancreatic metastasis should always be suspected in patients who present a pancreatic mass and history of renal carcinoma.4,5,7,10 Diagnostic methods aid in making the differential diagnosis, which should include primary adenocarcinoma of the pancreas, neuroendocrine tumor, etc.2,6
Ultrasound is more sensitive for detecting small pancreatic metastastatic foci than CT.2 The lesions appear hypoechogenic when compared with the pancreatic parenchyma. Abdominal CT can be useful for the differential diagnosis of pancreatic lesions: in premature phases, after the administration of intravenous contrast, these lesions are seen as hypervascular in the pancreatic parenchyma, unlike primary adenocarcinoma of the pancreas, which is typically hypovascular. These findings are not pathognomonic since endocrine pancreatic tumors also appear as hypervascular lesions.1,2,4–7,10 The use of MRI for the diagnosis of these lesions is increasing.7 Standard imaging methods (CT, MRI) cannot provide an adequate differential diagnosis.7 The role of PET-CT has yet to be defined, but it can be useful for ruling out unsuspected metastasis.4,5 Some authors, given the diagnostic difficulties in certain cases, use FNA. This has its risks and disadvantages, which is why other authors make the diagnosis after surgical resection of the surgical resection.4,7 In none of the cases presented FNA was used for the diagnosis.
In selected patients, when the primary tumor is controlled, the pancreas is the only organ affected by metastasis and the lesion or lesions are resectable, aggressive surgical treatment is recommended as long as there are no comorbidities that contraindicate it. Surgery in these cases improves survival and quality of life, especially in patients with asymptomatic metastases that occur after a disease-free period of more than 2 years.1,3–9 Aggressive surgical management for single pancreatic metastasis of renal cell carcinoma is justified. In contrast, in the case of multiple metastases, this is more controversial. Some authors consider that if there are multiple metastases it is because the disease is disseminated; nevertheless, as there is no other treatment, surgery is used. In conclusion, surgery is done as long as the disease is not locally unresectable, there is no comorbidity that contraindicates it and there is no unresectable extra-pancreatic disease.1–3,5,7
All our patients presented resectable lesions, and the pancreas was the only organ affected by metastasis, so they were therefore treated surgically. The type of pancreatic resection is conditioned by the location of the lesions and the need to obtain free margins with preservation of the exocrine and endocrine functions of the pancreas.2,4,5,7 All the metastases seen on radiography and during surgical exploration should be resected. There is no need for radical lymph node dissection as the literature indicates that lymph node affectation in pancreatic metastatic disease is very rare.1–3,8 In our case, only one patient with multifocal disease presented lymph node affectation.
There are many articles that suggest that previous resection of renal carcinoma metastasis in other organs, such as the thyroid, suprarenal glands or lungs, should not rule out aggressive treatment of the secondary pancreatic metastases after having confirmed that it is the only organ with disease relapse.7,8
Chemotherapy, hormone therapy and radiotherapy have not been demonstrated to be effective for primary renal tumors or their metastases. In recent years, promising results have been seen in phase iiiassays with sunitinib, sorafenib, temsirolimus and bevacizumab.2,3,7
The resection of pancreatic metastases by other, non renal cancers has a poor prognosis as it is a sign of disseminated metastatic disease. Contrarily, survival after the resection of pancreatic metastases of renal cell carcinoma is clearly superior7,8: the 5-year survival rate in patients with pancreatic metastases after resection is very variable, from 29% to 88% depending on the series.2,3,5–7 Survival is better than that of primary pancreatic tumors.1,2,7 In our series, there was only one death, 3 months after the procedure, due to surgical morbidity; the remaining patients are still alive (87.5%). Disease-free survival is 50%. Survival after the pancreatic resection is 30.37 months, although the patients present different follow-up times (0.11–7.94 years).
Factors associated with a favorable prognosis include a prolonged disease-free period, a single metastasis with central necrosis and complete resection with histologically negative margins. Others have reported that the tumor grade of pancreatic metastases correlates with the tumor grade of the primary renal tumor and that tumor grade is a predictor for survival, with a mean survival of 42 months for grade 2 cancer and 10 months for grade 3.7
ConclusionsPancreatic metastases of any primary tumor are rare. Pancreatic metastasis should always be suspected in patients who present a pancreatic mass and history of renal cell carcinoma. The interval between the primary resection and metastasis can be quite long. Patients may or may not present symptoms, which vary widely. Thus, we should consider the length of follow-up in patients affected by renal tumors and determine whether these follow-ups are cost-effective, for which more studies should be done with a larger number of cases.
In selected patients, when the primary tumor is controlled, the pancreas is the only organ affected by metastasis and the lesion or lesions are resectable, aggressive surgical treatment is recommended. Surgery in these cases improves survival and quality of life.1–9
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Markinez I, et al. Metástasis pancreáticas por carcinoma renal. Nuestra casuística y revisión de la literatura. Cir Esp. 2013;91:90–5.