There is still controversy on the management of complex cryptoglandular fistulas, even after employing the newest, theoretically simple, techniques. A critical review of the literature was performed, in order to clarify the role of the surgeon, where the precarious balance between eradicating sepsis and maintaining anorectal influences the choice. Techniques, such as fistulotomy, immediate sphincter repair or ligature of the inter-sphincter trajectory, are discussed. The new sphincter preserving techniques, such as sealing, use of plugs and cell therapy are also analyzed. However, with a few exceptions, the scientific evidence is low or zero, due to the lack of clinical trials and to the large variation in the presentations and technical details that could influence the results. For this reason, experience in treating complex cryptoglandular fistulas is still essential.
Sigue habiendo controversias en el manejo de las fístulas de ano complejas de origen criptoglandular (FC) incluso tras el empleo de técnicas novedosas teóricamente más simples. Para clarificar el papel del cirujano colorrectal en su tratamiento, se efectúa una revisión crítica de la literatura basándonos en que el precario balance entre erradicar la sepsis y mantener la función anorrectal afecta la elección. Se discuten técnicas como la fistulotomía, colgajos de avance, reparación esfinteriana inmediata o ligadura del trayecto interesfintérico. También se analizan las nuevas tecnologías preservadoras del esfínter como el sellado, empleo de tapones y terapia celular. Sin embargo, con escasas excepciones, la evidencia científica es baja o nula debido a la escasez de ensayos clínicos y a que hay gran variabilidad de presentaciones y de detalles técnicos que pueden influir en el resultado. Por tanto, la experiencia en el tratamiento de las FC sigue siendo esencial.
Fistulas are one of the most common anorectal problems. They have been treated for millennia: the Corpus Hippocraticum1 discusses the use of setons or fistulotomy, as well as instruments for its treatment. In the 14th century, John Arderne2 published a monographic text with therapies similar to current treatments, and Félix de Tassy successfully operated on Luis XIV of France after a “prospective assay” amongst members of the Court.3 Nevertheless, it was Frederick Salmon, founder of the London charity institution St Mark's Hospital for Fistula and other Diseases of the Rectum, who began the trail toward a scientific approach to the problem.4
We know that most fistulas are cryptoglandular in etiology and originate from an infection of the intersphincteric space, although there is no unequivocal confirmation of this theory.5 We diagnose them better, although the evaluation of an expert surgeon is most important.6 But, how should we treat them? There is no evidence to determine how to ideally maintain the stable balance between continence control and curing the septic process,7 especially when treating complex fistulas (CF), defined as those in which the tract includes an important part of the anal sphincter or those associated with or at high risk of fecal incontinence.
Fistula surgery, feared by some and in some cases obscure, has always had a sort of artisan element. Quite possibly, the need for three-dimensional assessment, difficulties in following the trajectories, finding the internal orifice of the fistula (IOF) or not compromising the sphincters have contributed to the fact that not too many surgeons have tried to deal with difficult cases. Many aspects, however, have been clarified: we can evaluate the anatomy of the fistula and its relationship with the sphincters, there are multiple therapeutic options, and less-aggressive therapies have arisen in which the verbs dissect, cut and suture have been substituted with seal, plug or implant, encouraging even the most timid to embark on treating complex cases, driven by the industry.
Undoubtedly, we must be open to change, but what is the current state? What do new treatments provide? In short, are colorectal surgeons still required to treat a CF? The objective of the present article is to clarify this with evidence, data and a few reflections.
Risk Factors for Recurrence and Incontinence After “Classic” SurgeryExcluding Crohn's, tuberculous or rectovaginal fistulas, there is great variability in the recurrence rate, which can range between 0% and 65% although usually situated at around 5%–8%.8–10
The most widely used classification was described by Parks,11 according to which suprasphincteric and extrasphincteric fistulas are the most difficult to treat, although they are fortunately the most uncommon. In addition, the risk factors (RF) for recurrence are: horseshoe extension, number of previous interventions,8 CF,12 not finding the IOF8,9,12,13 and surgeon variables.8 In a group treating anal fistulas, the surgeon who operated on 55% of patients had a recurrence rate of 3.9% compared with 11.2% of the remainder, which attests to the influence of experience in this process.12
In addition, post-operative alterations in fecal continence (AFC) are frequent. Marks and Ritchie reported that 25% of patients had gas leaks, 17% liquid stool leaks and 3% solid stool, while 31% of patients presented mucus leaks or soiling.14 Soiling or mucus leaks are not considered in many series and there are such disparate results that some authors report less post-operative FI than other studies report before surgery.15–18 Thus, in our own case series, 14.9% of patients presented pre-operative FI,19 which is more than double that of the general population20; this percentage increased after surgery to 49.1%, as observed by other authors.13,17 If patients are not directly asked about incontinence, its frequency is obviously underestimated. Given the prevalence of soiling, it would be interesting to add this variable to classification scores such as the Cleveland Clinic incontinence score.21
In 1934, Milligan and Morgan22 observed that, “If the anorectal ring is cut, the appearance of incontinence is certain”. This still holds true today and it has been demonstrated to be a risk factor in univariate studies, along with high anal fistulas, female sex, anterior tracts, high IOF, vertical tracts, advanced age, double fistulas and previous surgery8,17,23,24; meanwhile, multivariate studies show previous incontinence and hypotonic sphincter as RF.12,23 Colorectal surgery associations recommend that if more than 30% of the external anal sphincter (EAS) is to be cut, this should be done with caution by an experienced surgeon, especially in high risk cases.25,26
Why is it Difficult to Decide on an “Ideal Treatment”?This is largely due to the variability in the concept of CF, which basically includes difficult-to-treat fistulas (with possible risk to patients’ continence), such as suprasphincteric and extrasphincteric fistulas, because fistulotomy would harm much of or all of the sphincters, and other treatments are technically difficult. High transsphincteric fistulas are also complicated, as well as those with RF, as mentioned. For their treatment, a wide variety of techniques have been described. Which is best? The levels of evidence rarely surpass that of an expert consensus. This is due to the different types of fistulas and clinical presentations, disparate results and ways of evaluation. On top of this, if we add the final opinions of each surgeon, the discussion turns into a veritable Tower of Babel.
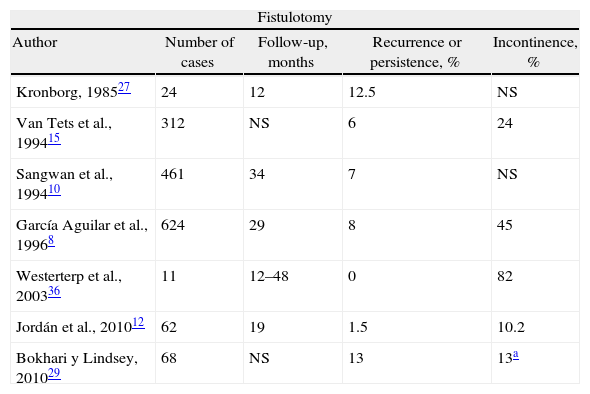
Is Fistulotomy Obsolete?Laying open the fistula tract is one of the oldest treatments. It may be used for simple fistulas with a level of evidence II and grade of recommendation B25; and with level I and recommendation B, fistulotomy can be considered better that fistulectomy, given that the latter generates a wound that takes longer to cure and causes more damage to the sphincters.25,27,28 Fistulotomy is the method that offers more possibilities for a cure, at around 90%.13 But, there have been unacceptable rates of serious FI observed in CF and even 5% in simple fistulas.29 Nonetheless, a recent article of the St. Mark's group30 shows a cure rate of 96% of CF with fistulotomy, with a low rate of FI, and they therefore recommend its use in experienced hands. The use of radiofrequency has shown no benefits.7
The use of a seton does not greatly improve the results. With cutting setons, the rate of incontinence is proportional to the speed of cutting, and recurrences and FI are similar to those of fistulotomy.26,31,32 The latter has been reported in 18%.33 But, what if the seton is snug but not tight, as in the snug seton concept by Hammond et al.34? Cutting is progressive but slow, and in 52% the seton falls out spontaneously in 6 months, while in the remainder a minimal fistulotomy is needed, with reports of minor FI. Preserving or cutting the internal anal sphincter (IAS) with the placement of the seton also changes the results, and a recent meta-analysis showed that its preservation reduced post-operative incontinence.35 In any case, fistulotomy and seton significantly deteriorate sphincter pressures and anatomy,19 and surgeons who use them navigate like Ulysses between the monsters Escila and Caribdis, since aggressive treatment would mean incontinence and timid treatment would lead to recurrence8,10,12,15,27,29,34,36–44 (Table 1). A score has been described to determine whether the patient is able to be treated with fistulotomy by evaluating RF, including type of fistula, sphincter function and defecation habits.45 Finally, leaving a loose seton in patients without Crohn's disease is associated with almost 50% of recurrences, and it is not useful in practice.46
Results of Treating Cryptoglandular CF With Fistulotomy or Staged Seton.
| Fistulotomy | ||||
| Author | Number of cases | Follow-up, months | Recurrence or persistence, % | Incontinence, % |
| Kronborg, 198527 | 24 | 12 | 12.5 | NS |
| Van Tets et al., 199415 | 312 | NS | 6 | 24 |
| Sangwan et al., 199410 | 461 | 34 | 7 | NS |
| García Aguilar et al., 19968 | 624 | 29 | 8 | 45 |
| Westerterp et al., 200336 | 11 | 12–48 | 0 | 82 |
| Jordán et al., 201012 | 62 | 19 | 1.5 | 10.2 |
| Bokhari y Lindsey, 201029 | 68 | NS | 13 | 13a |
| Seton | ||||
| Author | Number of cases | Follow-up, months | Persistence or recurrence, % | Incontinence, % |
| Ramanujam et al., 198337 | 45 | NS | 2 | 2 |
| Williams et al., 199138 | 24 | NS | 8 | 54a |
| Pearl et al., 199339 | 65 | 23 | 3 | 5b |
| Graf et al., 199540 | 25 | 46 | 8 | 44 |
| García-Aguilar et al., 199841 | 47 | 27 | 9.2 | 65.9 |
| Hasegawa et al., 200042 | 32 | 12 | 29 | 46 |
| Hammond et al., 200634 | 29 | 42 | 0 | 25a |
| Chuang-Wei et al., 200843 | 112 | 39 | 0.9 | 24a |
| Lykke et al., 201044 | 41 | NS | 12 | 61 |
NS: not specified.
What is an acceptable treatment of CF? The use of fistulotomy in recurrent cases, with predominantly posterior fibrosis, in males with good muscle mass and a normal rhythm of defecation. But, of course, all this was before the appearance of sealing techniques…
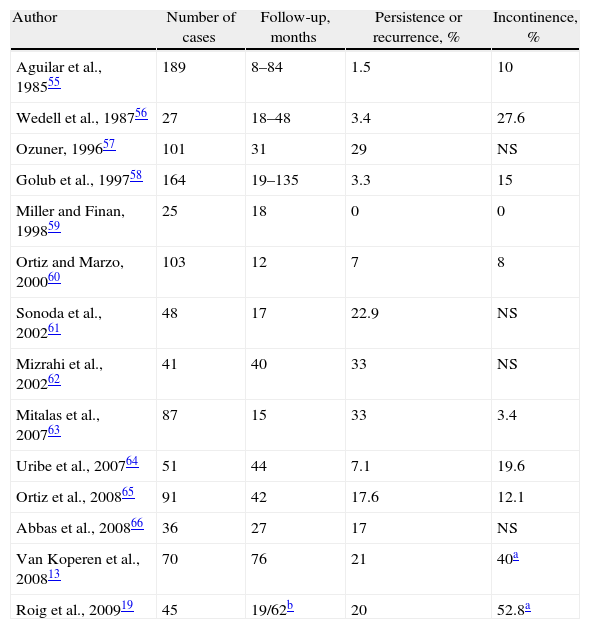
A Good Option: Advancement FlapsPossibly the gold standard for treating CF is endorectal advancement flap (EAF) surgery, as described by Noble47 at the beginning of the 20th century, with later modifications.48,49 Technically, after surgically removing the extrasphincteric part of the tract, the mucosa is resected distal to the IOF and a flap is created with a wide base using rectal submucosa or mucosa, partially including the IAS or even including its entire thickness. Afterwards, the IOF is closed and the repair is covered with the flap.
Perhaps what is most important is correct closure of the IOF, which can also be reinforced with lateral or transversal plasty of the IAS or the rectal circular muscle layer.50,51 The EAF is a good technique to treat suprasphincteric, high or even low transsphincteric fistulas in patients at risk for incontinence. However, it may be technically demanding in recurrent fistulas with scar tissue that may make it difficult to even insert a retractor. In cases with technical difficulties, an anal skin flap can be created by advancing the perianal skin proximally toward the anal canal; this has obtained slightly poorer results.52,53
The series are not homogenous: many are retrospective, some have selection biases and others have personal contributions that limit the power of the data obtained. Recurrences range from 0% to 33%, with an average of 19%54 and the lack of interest that some authors show for assessing post-op continence is striking19,55–66; alterations occur in about 13% of patients54 (Table 2).
Results of Treatment of Cryptoglandular CF With Endorectal Advancement Flap.
| Author | Number of cases | Follow-up, months | Persistence or recurrence, % | Incontinence, % |
| Aguilar et al., 198555 | 189 | 8–84 | 1.5 | 10 |
| Wedell et al., 198756 | 27 | 18–48 | 3.4 | 27.6 |
| Ozuner, 199657 | 101 | 31 | 29 | NS |
| Golub et al., 199758 | 164 | 19–135 | 3.3 | 15 |
| Miller and Finan, 199859 | 25 | 18 | 0 | 0 |
| Ortiz and Marzo, 200060 | 103 | 12 | 7 | 8 |
| Sonoda et al., 200261 | 48 | 17 | 22.9 | NS |
| Mizrahi et al., 200262 | 41 | 40 | 33 | NS |
| Mitalas et al., 200763 | 87 | 15 | 33 | 3.4 |
| Uribe et al., 200764 | 51 | 44 | 7.1 | 19.6 |
| Ortiz et al., 200865 | 91 | 42 | 17.6 | 12.1 |
| Abbas et al., 200866 | 36 | 27 | 17 | NS |
| Van Koperen et al., 200813 | 70 | 76 | 21 | 40a |
| Roig et al., 200919 | 45 | 19/62b | 20 | 52.8a |
NS: not specified.
Overall, EAF is recommended in cases in which fistulotomy would cause FI (recommendation grade B),25,26 and it has an established role in the armamentarium of colorectal surgeons. The differences in the results suggest that technical factors are fundamental. Identified risk factors for failure include smoking67,68 and the associated use of adhesives.69,70 There is still controversy about which type of flap is better,54 either complete or partial.71
The observation that in all the cases of failure the EAF had specifically failed at the IOF level has made researchers think that this may be due to persistent disease and consider whether a loose seton should be previously used or not.63 It theoretically reduces suppuration and abscess cavities and is widely used as a first stage surgery.26 However, reported cure rates are 63% if it is not used, compared with 67% when it is.72
Although theoretically it does not harm the sphincters, FI after EAF is not unusual; it is possibly caused by the anal dilation necessary to carry out a technique that is often difficult.73 So, it is one of the interventions that most affects the function of the IAS.19 It has also been speculated that a total thickness flap would have more effect on continence,74 and a series of our own showed 51% FI compared with 33% after a mucosal–submucosal flap; P=.08.75 Even a certain degree of mucosal ectropion can lead to soiling.54
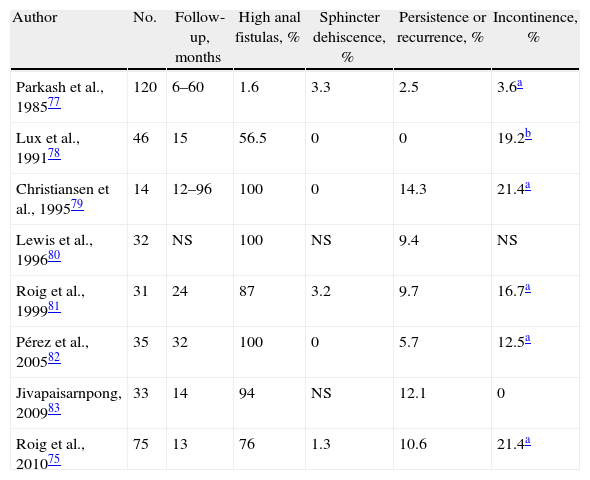
And Why Not Reconstruct the Sphincter After Cutting it?Since fistulotomy provides the best chance for a cure and its biggest problem is FI, one option would be immediate sphincter repair (ISR); it has excellent results after acute trauma if there is no loss of substance.76 This should be restricted to tracts that have no abscess cavities and when there is fibrosis that impedes creating a flap. It would be indicated in high or recurrent fistulas, preferably associated with incontinence due to previous sphincter lesions. The technique includes fistulotomy, debridement of the tract, primary sphincter reconstruction and careful closure of the mucosa of the anal canal. Despite its results75,77–83 (Table 3), the technique has been met with skepticism in surgical forums, even though it is a good option in selected cases.84 Recurrences were similar to those of EAF in a randomized study85; it does not alter continence in previously continent subjects and it improves continence in incontinent patients.81 A study of our own also compared it with flaps and observed postoperative deterioration in continence. Nonetheless, previously incontinent patients improved after ISR.75
Results of the Treatment of Cryptoglandular CF With Fistulotomy and Immediate Sphincter Repair.
| Author | No. | Follow-up, months | High anal fistulas, % | Sphincter dehiscence, % | Persistence or recurrence, % | Incontinence, % |
| Parkash et al., 198577 | 120 | 6–60 | 1.6 | 3.3 | 2.5 | 3.6a |
| Lux et al., 199178 | 46 | 15 | 56.5 | 0 | 0 | 19.2b |
| Christiansen et al., 199579 | 14 | 12–96 | 100 | 0 | 14.3 | 21.4a |
| Lewis et al., 199680 | 32 | NS | 100 | NS | 9.4 | NS |
| Roig et al., 199981 | 31 | 24 | 87 | 3.2 | 9.7 | 16.7a |
| Pérez et al., 200582 | 35 | 32 | 100 | 0 | 5.7 | 12.5a |
| Jivapaisarnpong, 200983 | 33 | 14 | 94 | NS | 12.1 | 0 |
| Roig et al., 201075 | 75 | 13 | 76 | 1.3 | 10.6 | 21.4a |
NS: not specified.
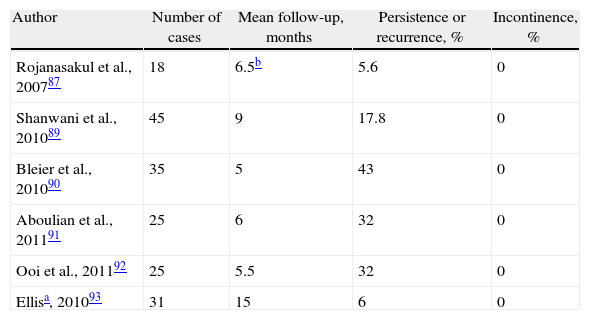
A suggestion by Matos, Lunniss and Phillips86 to preserve the sphincter has recently been modified.87 It involves making an incision at the level of the intersphincteric groove, identifying the tract, ligation close to the IOF and resection of the intersphincteric portion.88 Although it has been described as simple and reproducible, the patients must have been carefully selected or it is difficult to understand how certain fistulas were treated. The initial publication showed only one recurrence, but this has not been reproduced by other authors, although cases of FI have not been described after the procedure87,89–93 (Table 4). There are no comparative studies with other techniques.
Results of the Treatment for Cryptoglandular CF Using Ligation of Intersphincteric Fistula Tract (LIFT).
A recent modification associates the placement of a biological prosthesis to separate the ligated ends of the tract.93 Although the preliminary results have been good, in order to insert the prosthesis a more extensive dissection is required, which may harm the sphincters, and the cost of the material must also be considered.
Sealants: The End of the Risk of IncontinenceIn the era of minimally invasive surgery, the introduction of synthetic biological materials has led to the emergence of techniques for filling up the tract with a biomaterial. The sealant occludes the tract, and stimulates the migration, proliferation and activation of fibroblasts and endothelial pluripotent cells, acting as a matrix for cell growth and tissue integration.94
Fibrinogen with thrombin has been used, a mix that produces fibrin glue (Tissucol®), autologous fibrin, bovine albumin+glutaraldehyde (BioGlue®) or cyanoacrylates (Glubran®). One would think that, with all these products, we would have a standardized and reproducible technique available that would make any surgeon capable of treating fistulas with minimal morbidity. But, it is not clear if the successes are due to the biomaterial per se or to the setting in which it is placed. Is it better to previously eradicate septic cavities? Is antibiotic prophylaxis required? Debride the tract and/or use abrasives? Close the IOF or not (which is a technique in and of itself95)? Repeat sealing? For all these factors, there is a low level of evidence (level III, recommendation grade B) for their use in CF.26
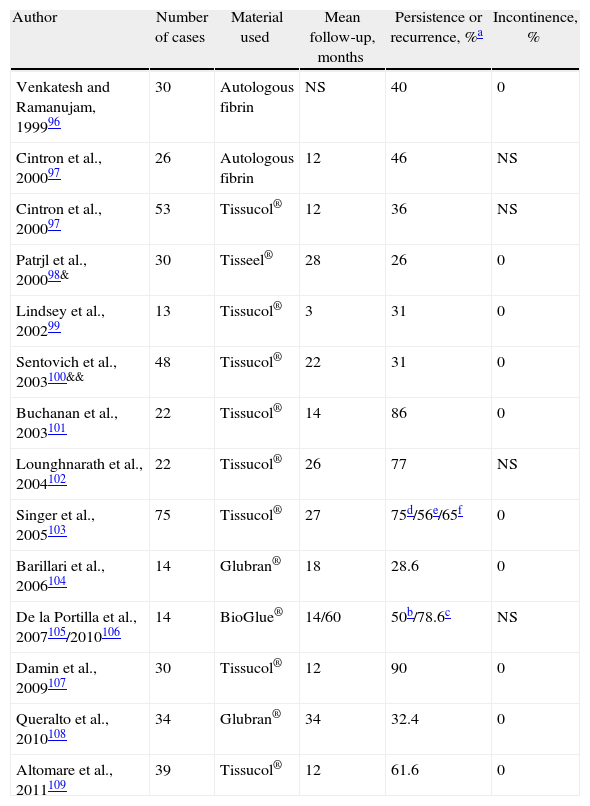
The initial success rates of 60%–70% worsened over the long term to reach a recurrence rate of 70%–100%96–109 (Table 5). However, the non-invasive nature and safety of sealants make them a reasonable first line of treatment in certain cases. A randomized study comparing setons with fibrin glue showed more favorable results for the latter, which is not surprising given the low effectiveness for a cure with a loose seton.99 Another study compared cutting setons with fibrin sealing and found 12.5% of recurrences in the former compared with 60% in the latter, but with a more uncomfortable post-operative period and significant FI using setons.109
Results of the Treatment of Cryptoglandular CF Using Biological Sealants.
| Author | Number of cases | Material used | Mean follow-up, months | Persistence or recurrence, %a | Incontinence, % |
| Venkatesh and Ramanujam, 199996 | 30 | Autologous fibrin | NS | 40 | 0 |
| Cintron et al., 200097 | 26 | Autologous fibrin | 12 | 46 | NS |
| Cintron et al., 200097 | 53 | Tissucol® | 12 | 36 | NS |
| Patrjl et al., 200098& | 30 | Tisseel® | 28 | 26 | 0 |
| Lindsey et al., 200299 | 13 | Tissucol® | 3 | 31 | 0 |
| Sentovich et al., 2003100&& | 48 | Tissucol® | 22 | 31 | 0 |
| Buchanan et al., 2003101 | 22 | Tissucol® | 14 | 86 | 0 |
| Lounghnarath et al., 2004102 | 22 | Tissucol® | 26 | 77 | NS |
| Singer et al., 2005103 | 75 | Tissucol® | 27 | 75d/56e/65f | 0 |
| Barillari et al., 2006104 | 14 | Glubran® | 18 | 28.6 | 0 |
| De la Portilla et al., 2007105/2010106 | 14 | BioGlue® | 14/60 | 50b/78.6c | NS |
| Damin et al., 2009107 | 30 | Tissucol® | 12 | 90 | 0 |
| Queralto et al., 2010108 | 34 | Glubran® | 34 | 32.4 | 0 |
| Altomare et al., 2011109 | 39 | Tissucol® | 12 | 61.6 | 0 |
NS: not specified.
The use of sealants, however, has been associated with perianal sepsis.110 It thus seems important to eliminate granulation tissue, close the IOF if it is large and avoid fragmentation of the glue to avoid trapping bacteria and detritus.105
The treatment does not alter continence and is well tolerated, but it is actually not very effective. It was suggested that a long tract favored the efficacy of sealing,96 but other authors observed that the opposite was true,100 and some found no relationship with this factor or with the location of the IOF.105 The sealant is reabsorbed in a few days, which is insufficient time to be able to serve as a matrix for tissue reparation. No one glue has been shown to be better than others as there are no comparative studies, but the material is undoubtedly another variable added to the complexity of fistulas and the technical details of their treatment. Do we see the bottle half full? With sealants we will be able to cure some patients without any risks, although, of course, we will be consuming resources. Perhaps this treatment is indicated if the sphincter function is poor or if the surgical risk is high, but the patients have to be informed of the expected results.109
It has been suggested that the association of sealants with an EAF could improve results. However, a randomized study showed 46% of recurrences in the sealant branch+EAF compared to 20% if EAF was used alone.69 Another study of this combination showed evidence of 88% persistence/recurrence.70 The biomaterial may possibly create difficulties for contact between the edges of the flap or it may block the drainage of secondary tracts.
Fistula Plugs: A Simple Technique, But Safe and Effective?Five years ago, bioabsorbable xenografts made of pig intestinal submucosa were introduced in the market. The so-called anal fistula plug (Surgisis® AFP™) is a cone-shaped device that is inserted in the fistula tract, after curetting and/or treating with abrasives, and attached to the IOF so that it occludes it. Later, it provides a framework for infiltration of connective tissue.
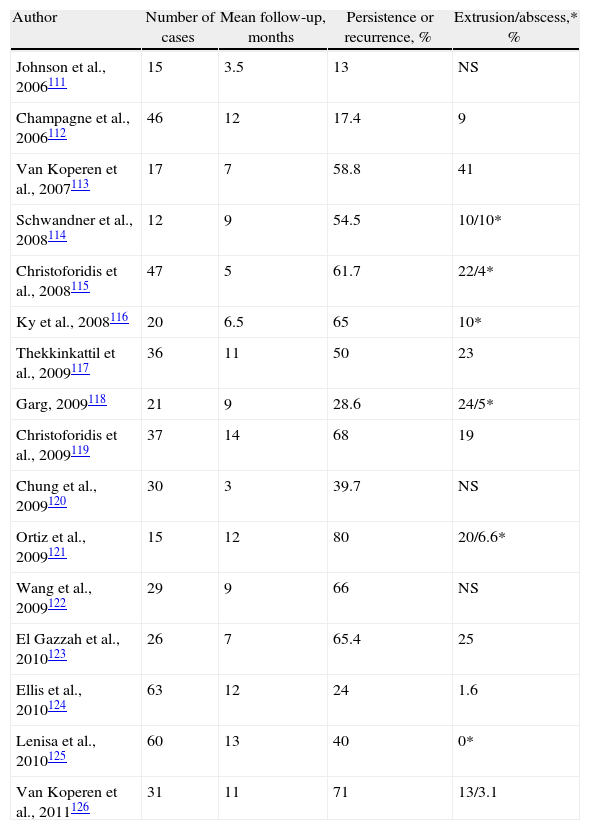
In 2006, Johnson et al.111 initially reported a success rate of 87%, much higher than using adhesives. Other publications with longer follow-ups have not reproduced these results, and the cure rates have been around 28%111–126 (Table 6). In one series, the cure rate diminished from 80% in the short term to 55% 6 months later.116 Garg et al., in a systematic review on CF, found a cure rate of 35%–87% and 13%–71% of recurrences.127 The results are worse in shorter tracts,128 and a multivariate analysis has shown that smoking, posterior fistulas and recurrences prior to a plug are risk factors for failure.124
Results of the Treatment of Cryptoglandular CF With the Use of Anal Fistula Plug.
| Author | Number of cases | Mean follow-up, months | Persistence or recurrence, % | Extrusion/abscess,* % |
| Johnson et al., 2006111 | 15 | 3.5 | 13 | NS |
| Champagne et al., 2006112 | 46 | 12 | 17.4 | 9 |
| Van Koperen et al., 2007113 | 17 | 7 | 58.8 | 41 |
| Schwandner et al., 2008114 | 12 | 9 | 54.5 | 10/10* |
| Christoforidis et al., 2008115 | 47 | 5 | 61.7 | 22/4* |
| Ky et al., 2008116 | 20 | 6.5 | 65 | 10* |
| Thekkinkattil et al., 2009117 | 36 | 11 | 50 | 23 |
| Garg, 2009118 | 21 | 9 | 28.6 | 24/5* |
| Christoforidis et al., 2009119 | 37 | 14 | 68 | 19 |
| Chung et al., 2009120 | 30 | 3 | 39.7 | NS |
| Ortiz et al., 2009121 | 15 | 12 | 80 | 20/6.6* |
| Wang et al., 2009122 | 29 | 9 | 66 | NS |
| El Gazzah et al., 2010123 | 26 | 7 | 65.4 | 25 |
| Ellis et al., 2010124 | 63 | 12 | 24 | 1.6 |
| Lenisa et al., 2010125 | 60 | 13 | 40 | 0* |
| Van Koperen et al., 2011126 | 31 | 11 | 71 | 13/3.1 |
NS: not specified.
About 19% of failed cases debut when the plug falls out, technical errors due to insufficient fixation or excessive tension. Another common cause of morbidity is post-operative abscess, which is seen in 4%–29% of cases127 and can be reduced with a meticulous technique.129 Post-operative FI has not been reported.
Although some retrospective studies show less recurrences with EAF compared with plugs in high fistulas,119,122 only two randomized prospective studies compare them. Ortiz et al.121 observed 80% recurrence in patients with plugs vs 12.5% with EAF (RR=6.4), although the study was prematurely stopped. Nevertheless, van Koperen et al.126 found no differences in either recurrences (AFP=71%; EAF=52%), nor in post-operative dysfunction or quality of life.
As in the case of association with adhesives, plugs increase recurrence if associated with an EAF,130 so its use is not recommended.
Thus, collagen plugs are a therapeutic alternative, especially in patients with long-tract CF. Attention to detail is essential during their implantation, as are measures to prevent infection (previous seton drainage) in order to improve the results, and they should be used by experienced surgeons.124,127,131 There are no data that recommend plugs as a first line of treatment in high fistulas.
In order to avoid premature failure due to extrusion, newer plugs are now commercialized with a button-like end (Biodesign™ Surgisis® Fistula Plug), with a few been reports of limited efficacy in pouch-vaginal and rectovaginal fistulas.132 Another plug (GORE® BIO-A® Fistula Plug) has a 16mm disc that can be cut to fit the defect, which is coupled to two tubes that are 9cm long but can be fitted to the tract. It is a fibrous-porous structure made up of a biodegradable synthetic copolymer of trimethylene carbonate and glycolide, so it is therefore not biological material. There are still no publications about its use.
Recently, a new treatment has been reported in a pig model using acellular collagen paste resistant to enzymatic degradation (Permacol®), either alone or associated with autologous fibroblasts, compared with a control group that underwent ‘core-out’ fistulectomy. All the filled tracts were cured, with the observation that the autologous fibroblasts were better integrated in the tissue, but only 2 out of 7 patients in the control group were cured.133 Clinically, it has been used in a phase 1 study using either strips or an injection of shredded collagen (Permacol®) diluted in fibrin glue to aid in its retention in the initial stages of healing. After 29 months, no patient had developed abscesses or FI. 54% of fistulas were cured vs 80%, although without statistical significance.134
Stem Cells: The Future of Proctology?Adult stem cell therapies have promising applications in many areas of medicine. An attractive manner to obtain them is through liposuction. The first published procedure was that of a woman with Crohn's disease and refractory rectovaginal fistula. The cells were injected in the rectal mucosa close to the IOF that had been previously sutured and a vaginal advancement flap was created. The wound healed quickly and there was no relapse during the 3 months of follow-up.135 Afterwards, García Olmo et al. designed a phase I trial that concluded that the treatment was safe and effective for inducing a cure in patients with CF, including those with Crohn's disease.136 In another phase II study, 49 patients with CF were randomized to receive stem cells and fibrin glue or isolated fibrin glue as a control group. The combination was shown to be effective and safe with 4 times more possibility for a cure than the control group.137 We are now expecting the publication of a multicenter phase III trial in cryptoglandular FC with more than 200 patients. Whatever the results are, this research will surely lead to new possibilities.
And the Endoscopic Approach…Recently, a new device has come onto the market, called the Video-Assisted Anal Fistula Treatment (VAAFT) (Karl Storz Endoskope), which consists of a rigid fistuloscope with a working channel and an irrigation channel that provides fistula treatment. The IOF is closed with endoanal sutures and stapling, using the semicircular Contour® Transtar™ device or a linear cutter-stapler, which is unlikely to be innocuous in the anal canal. The staples are also sealed with cyanoacrylate. The author affirms that “the technique is sphincter-saving…and the patients have no problems with incontinence” due to “complete destruction of the fistula from the inside”.138 There has been no series published, but it does not seem to be an advantageous option despite the cost.
Final CommentsMartin Van Butchell, a disciple of Hunter, ran an advertisement in the London Morning Herald in 1789 that read: “Fistula-in-ano: No cure, no pay. This teazing, local disease is radically cured (sometimes in one week, always in two) without cutting, dressing, medicine, cautery, injection, risk, confinement, loss of blood, or an atom found parts.139” He was an eccentric charlatan who did not live up to what he promised. More than 200 years later, we surgeons still cannot make his claim a reality, and fistulas continue to pose problems in their approach, after-effects of their treatments or the alteration in quality of life that the disease or its therapies may condition.
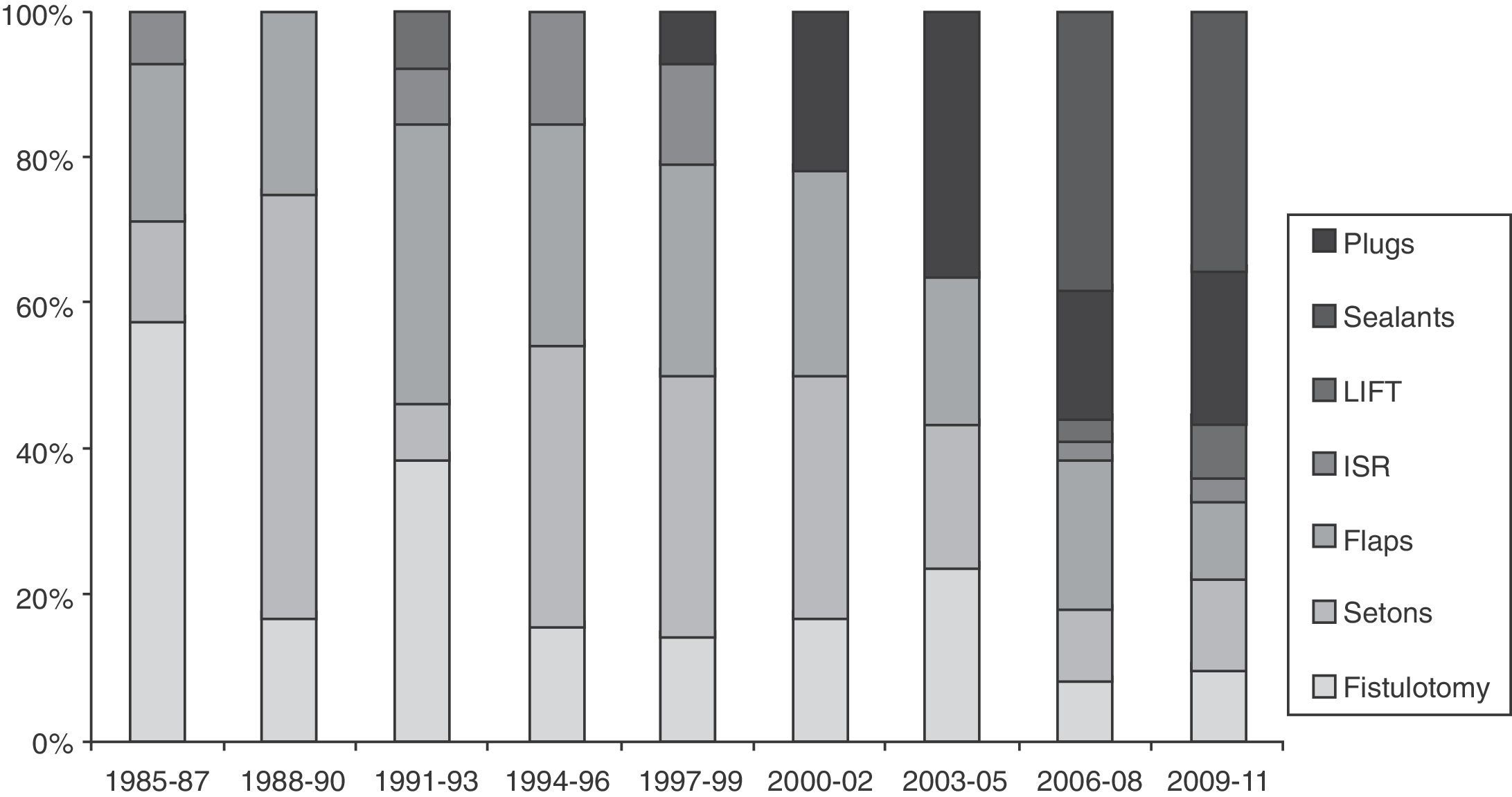
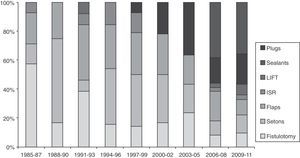
There are very few prospective and randomized studies related to fistula treatment. It seems that there are no important differences between the techniques with regards to recurrences, although the use of biomaterials and advancement flaps are associated with less FI.140,141 Many therapies have not been developed on an experimental basis; instead, the enthusiasm for adopting new techniques guided by the industry is evident (Fig. 1). Thus, there are no therapeutic standards for such a common and prevalent problem.142
Triennial evolution of the publications (PubMed) about different techniques for the treatment of anal fistulas (1985–2011). Results expressed in percentage of the total. Observe the increase in biological therapies in recent years until reaching almost 60% of the total. LIFT: ligation of intersphincteric fistula tract; ISR: immediate sphincter repair.
The community of colorectal specialists should be urged to develop greater scientific evidence to decide which option is the best for a particular patient. Furthermore, functional alterations and quality of life do not necessarily correlate, so it would be important to analyze these aspects.143
And, what is the opinion of the patients? In a survey, 74% would prefer not to risk their continence for a cure, versus 26% who would choose a fistulotomy,144 although the information that the patients were given is arguable.
What to do with a complex case? Given the low degree of evidence available, surgery based on personal experience and refection of the different options is still valid. In short, the treatment of anal CF still poses many gray areas in the era of evidence-based medicine, and the phrase by Lockhart-Mummery in 1929, “Probably more surgical reputations have been damaged by the unsuccessful treatment of a fistula than by excision of the rectum or gastroenterostomy”, still rings true.145
Fistula surgery has passed through different historical periods: manual skills, scientific basis and developing biological therapies. We are sure that for some years we will continue to work with the three, but under the direction of an expert colorectal surgeon. So, for the question posed in the title of the article, the answer is, for now, yes.
Conflict of InterestsThe authors declare having no conflict of interests.
European Board in Coloproctology.
This study has been presented at three conferences: IVth Annual Meeting, European Society of Coloproctology, Prague 2009; XIX Congresso Nacional Portugués de Coloproctología, Porto 2009; and Association of Coloproctology of Great Britain & Ireland Annual Meeting, Bournemouth 2010.
Please cite this article as: Roig JV, García-Armengol J. Tratamiento de las fístulas de ano complejas de causa criptoglandular. ¿Aún se requiere un cirujano con experiencia? Cir Esp. 2013;91:78–89.