To evaluate the association between serum levels of procalcitonin and C-reactive protein, on the first 3 postoperative days, and the appearance of postoperative intra-abdominal infection.
MethodProspective observational study including 67 patients operated on for colo-rectal, gastric and pancreatic cancer. Serum levels of procalcitonin and C-reactive protein were analysed before surgery and daily until the third postoperative day. Values of procalcitonin (PCT) and C-reactive protein (CRP) were recorded as well as their accuracy for detection of postoperative intra-abdominal infection (PIAI).
ResultsThe incidence of postoperative intra-abdominal infection was 13.4%. CRP serum levels at 72h, PCT serum levels at 24, 48 and 72h and the ratio between serum levels of CRP at 72h and serum levels of CRP at 48h (CRP D3/CRP D2) were significantly associated with the appearance of postoperative intra-abdominal infection. The highest sensitivity corresponded to PCT at 72h (88.9%); the highest specificity and positive predictive value corresponded to the ratio CRP D3/CRP D2 (96.49% and 71.4%, respectively); the highest negative predictive value to procalcitonin at 72h and 24h.
ConclusionsSerum levels of PCT are significantly associated with the appearance of postoperative intra-abdominal infection. Sensitivity and predictive positive values are low, but negative predictive value is high, even at 24h after surgery.
Evaluar la asociación entre niveles séricos de procalcitonina (PCT) y proteína C reactiva (PCR), en los 3 primeros días de postoperatorio, y la aparición de infección intraabdominal postoperatoria.
MétodoEstudio observacional prospectivo que incluye a 67 pacientes intervenidos quirúrgicamente de cáncer colorrectal, gástrico y pancreático. Los niveles séricos de PCT y PCR se midieron antes de la cirugía y a las 24, 48 y 72 h de la misma. Se registraron los valores de PCT y PCR, así como su fiabilidad para la detección de infección intraabdominal postoperatoria.
ResultadosLa incidencia de infección intraabdominal postoperatoria fue de 13.4%. Los valores de PCR a las 72 h, los valores de PCT a las 24, 48 y 72 h y el cociente entre el valor de PCR a las 72 h y el valor de PCR a las 48 h (PCR D3/PCR D2) se asociaron significativamente con la aparición de infección intraabdominal postoperatoria. La sensibilidad más alta correspondió al valor de PCT a las 72 h (88,9%); la especificidad más alta y el valor predictivo positivo (VPP) más alto, al cociente PCR D3/PCR D2 (96,49 y 71,4%, respectivamente); el valor predictivo negativo (VPN) más alto, al valor de PCT a las 72 h y a las 24 h (97,7 y 96%, respectivamente).
ConclusiónLos valores de PCT se asocian significativamente con la aparición de infección intraabdominal postoperatoria en los 3 primeros días de postoperatorio. Su sensibilidad y VPP son bajos, pero su VPN es alto, incluso a las 24h de la cirugía.
Occasionally post-operative intra-abdominal infection (PIAI) presents as symptoms of acute abdomen and is easy to diagnose. However, it more commonly manifests with covert and non-specific symptomatology and abdominal examination can be misleading. Furthermore, the most commonly used laboratory tests during the postoperative period, such as leucocyte count, are neither very sensitive nor specific. All this has meant that PIAI is usually diagnosed late, generally between the sixth and ninth postoperative day,1 despite the fact that most of its causes, such as anastomotic leak, can appear much earlier. If there were an appropriate marker, PIAI could be diagnosed earlier, probably with a notable improvement in the prognosis of these patients. To date, essentially 2 proteins have been examined, C-reactive protein (CRP) and procalcitonin (PCT).2–6 CRP is a non-specific marker of inflammation, but, in some recent studies, it has been noted that from the 4th postoperative day its levels are significantly associated with the appearance of postoperative infection.7,8 PCT, although levels can rise in different inflammatory processes of a non-infectious aetiology,9–12 is quite a specific marker of infection. To date it has been used to assess the prognosis of patients with sepsis,13 to select patients who need antibiotic treatment14,15 and to assess the prognosis of patients with acute pancreatitis16 and even to predict pancreatic necrosis infection.17 Reith et al. were the first authors to observe that PCT levels rise significantly in patients with postoperative infection4 and other authors have subsequently made similar findings.5,18–21 Nonetheless, its role in the diagnosis of postoperative infection has still not been well evaluated and its use remains controversial. Four articles have recently been published which examine the value of PCT levels in the diagnosis of PIAI, 2 of which conclude that they are better than CRP22,23 and the other 2 conclude that they are not.24,25 A prospective study was organised, not yet finished, to analyse the evolution of serum levels of both proteins in the first 3 postoperative days and to assess whether there is an association between them and the occurrence of post-operative intra-abdominal infection; the preliminary findings of the study are shown in this article.
Patients and MethodsA prospective, observational study was carried out which included 67 patients operated on for colorectal, pancreatic or stomach cancer, consecutively, between 1 January and 30 June 2011 in the Surgical Department of the Hospital Complex in Pontevedra (CHOP). Criteria for exclusion were; aged less than 18 years of age, emergency or palliative surgery, and existence of preoperative infections and preoperative levels of PCT above 0.5ng/ml. None of the patients were operated on using laparoscopy. Data of age, gender, ASA classification, type of tumour, type of surgery, postoperative complications and postoperative stay were recorded for each patient. The patients were assessed daily until discharge and were reviewed as outpatients 30 days after the operation. PCT and CRP levels were measured the day before surgery and at 24, 48 and 72h after surgery (D0, D1, D2 and D3, respectively). Blood was taken between 8am and 10am. CRP levels were determined using an immunoturbidometric method, using a Beckman Coulter AU 5420 analyser (Fullerton, California, USA). PCT levels were determined with a VIDAS® multi-parametric immunoanalyser (BRAHMS, Hennigsdorf, Germany) using an enzyme immunoassay test method. The quotients between the values obtained at a determined time and the values obtained in the preceding moments were calculated, with the exception of D0. Thus 3 quotients were calculated for each of the 2 variables: D3/D2, D3/D1 and D2/D1. Intra-abdominal infection was considered to be present when one of the following occurred26:
- (a)
Discharge of purulent liquid through a drain placed in the peritoneal cavity
- (b)
Positive culture of exudates obtained aseptically from the peritoneal cavity
- (c)
Evidence of infection by direct examination (reoperation), by histopathological study (identification of germs) or by radiological examination.
The software packages IBM SPSS (Statistical Package for Social Sciences Inc., Chicago, Illinois, USA.) version 20.0 for Mac and MedCalc version 12.2.1 for Windows (MedCalc Software, Marlakerke, Belgium) were used for the statistical analysis.
Fisher's exact test was used to compare qualitative variables. Kruskall–Wallis and Mann–Whitney tests were used to compare quantitative variables between groups. For intra-group comparisons the Wilcoxon signs test was used.
P values lower than .05 were considered statistically significant. The diagnostic precision of CRP and PCT in detecting PIAI was assessed through ROC curves construction for each day, obtaining an optimal cut-off point for which the sensitivity, specificity and predictive values were calculated. The study was authorised by the Ethical Committee for Clinical Research of Galicia.
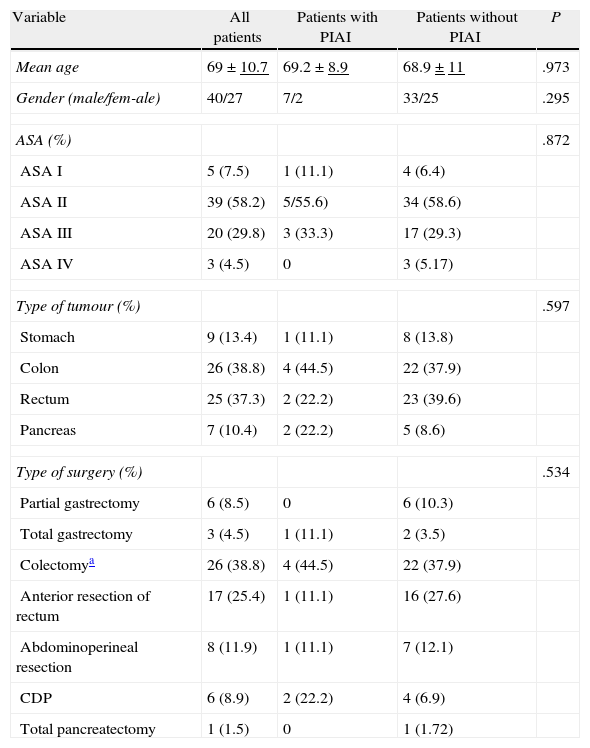
ResultsIn the time period of the study 8 patients were operated who were excluded because of incomplete CRP and PCT values (3 cases), because the preoperative PCT value exceeded 0.5ng/ml (3 cases), because the surgery was palliative (one case) and because the patient refused to participate in the study (one case). Forty patients (59.7%) were male and 27 (40.3%) were female, with an average age of 69 and a median of 68 (range 42–89). Table 1 shows a summary of the data for each type of tumour, surgery performed and ASA classification.
Demographic Variables and Type of Surgery.
| Variable | All patients | Patients with PIAI | Patients without PIAI | P |
| Mean age | 69±10.7 | 69.2±8.9 | 68.9±11 | .973 |
| Gender (male/fem-ale) | 40/27 | 7/2 | 33/25 | .295 |
| ASA (%) | .872 | |||
| ASA I | 5 (7.5) | 1 (11.1) | 4 (6.4) | |
| ASA II | 39 (58.2) | 5/55.6) | 34 (58.6) | |
| ASA III | 20 (29.8) | 3 (33.3) | 17 (29.3) | |
| ASA IV | 3 (4.5) | 0 | 3 (5.17) | |
| Type of tumour (%) | .597 | |||
| Stomach | 9 (13.4) | 1 (11.1) | 8 (13.8) | |
| Colon | 26 (38.8) | 4 (44.5) | 22 (37.9) | |
| Rectum | 25 (37.3) | 2 (22.2) | 23 (39.6) | |
| Pancreas | 7 (10.4) | 2 (22.2) | 5 (8.6) | |
| Type of surgery (%) | .534 | |||
| Partial gastrectomy | 6 (8.5) | 0 | 6 (10.3) | |
| Total gastrectomy | 3 (4.5) | 1 (11.1) | 2 (3.5) | |
| Colectomya | 26 (38.8) | 4 (44.5) | 22 (37.9) | |
| Anterior resection of rectum | 17 (25.4) | 1 (11.1) | 16 (27.6) | |
| Abdominoperineal resection | 8 (11.9) | 1 (11.1) | 7 (12.1) | |
| CDP | 6 (8.9) | 2 (22.2) | 4 (6.9) | |
| Total pancreatectomy | 1 (1.5) | 0 | 1 (1.72) | |
CDP, cephalic duodenopancreatectomy; PIAI, postoperative intra-abdominal infection.
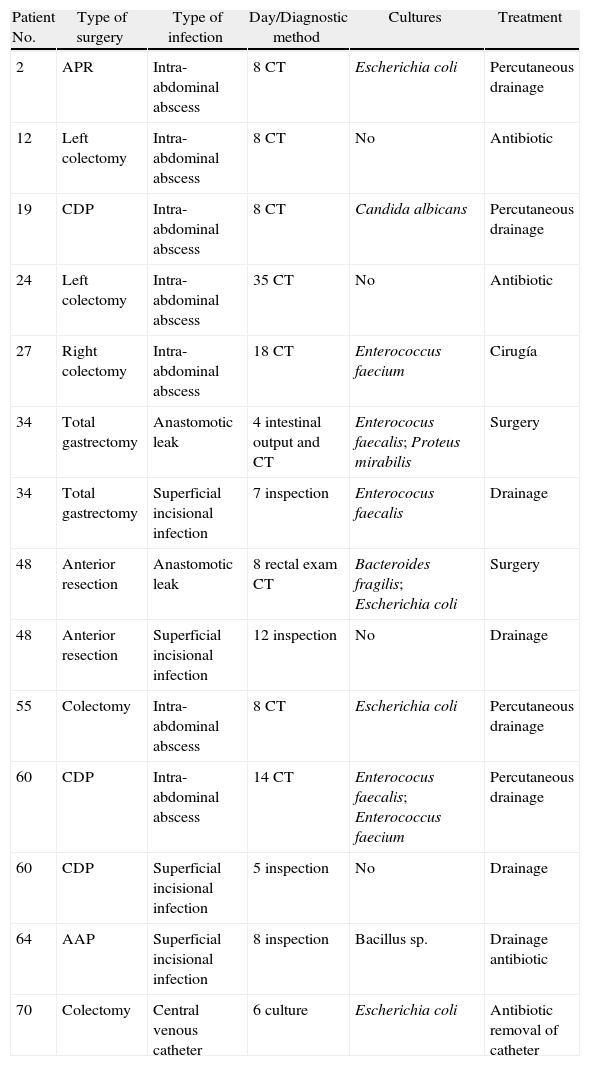
Twenty three patients (34.3%) presented complications. Of the 26 complications found, 14 (53.8%) were infections. Amongst the non-infectious complications, the most common was prolonged ileus, which presented in 5 patients (7.4%). Eleven patients presented some sort of infection in the postoperative period, which represents an incidence of 16.4% (Table 2). Four patients had superficial incisional infection; one, infection associated with a central venous catheter and 9, intra-abdominal infection (13.4%). Of the latter, 7 were abscesses and 2 were diffuse peritonitis. 3 of the abscesses were found to be associated with anastomotic leak (2 in patients who had undergone colectomies and one in a patient after cephalic pancreatectomy). The intra-abdominal abscesses were diagnosed by means of CT between the 8th and 35th postoperative days, with a median of 8 days. There was a positive culture in all the infections, except in 2 intra-abdominal abscesses which were treated using antibiotics alone, and in 2 superficial incisional infections, from which no sample was taken. Five patients (7.4%) were reoperated on, one because of a haemoperitoneum, one because of evisceration, 2 because of anastomotic leak with peritonitis and one because of an intra-abdominal abscess. There was no mortality. When we compared the group of patients with intra-abdominal infection (9) with the group without abdominal infection (58), it was observed that there were no significant differences in terms of age (P=.971), gender (P=.208), ASA classification (P=.665), type of tumour (P=.597) or type of surgery (P=.534).
Postoperative Infections.
| Patient No. | Type of surgery | Type of infection | Day/Diagnostic method | Cultures | Treatment |
| 2 | APR | Intra-abdominal abscess | 8 CT | Escherichia coli | Percutaneous drainage |
| 12 | Left colectomy | Intra-abdominal abscess | 8 CT | No | Antibiotic |
| 19 | CDP | Intra-abdominal abscess | 8 CT | Candida albicans | Percutaneous drainage |
| 24 | Left colectomy | Intra-abdominal abscess | 35 CT | No | Antibiotic |
| 27 | Right colectomy | Intra-abdominal abscess | 18 CT | Enterococcus faecium | Cirugía |
| 34 | Total gastrectomy | Anastomotic leak | 4 intestinal output and CT | Enterococus faecalis; Proteus mirabilis | Surgery |
| 34 | Total gastrectomy | Superficial incisional infection | 7 inspection | Enterococus faecalis | Drainage |
| 48 | Anterior resection | Anastomotic leak | 8 rectal exam CT | Bacteroides fragilis; Escherichia coli | Surgery |
| 48 | Anterior resection | Superficial incisional infection | 12 inspection | No | Drainage |
| 55 | Colectomy | Intra-abdominal abscess | 8 CT | Escherichia coli | Percutaneous drainage |
| 60 | CDP | Intra-abdominal abscess | 14 CT | Enterococus faecalis; Enterococcus faecium | Percutaneous drainage |
| 60 | CDP | Superficial incisional infection | 5 inspection | No | Drainage |
| 64 | AAP | Superficial incisional infection | 8 inspection | Bacillus sp. | Drainage antibiotic |
| 70 | Colectomy | Central venous catheter | 6 culture | Escherichia coli | Antibiotic removal of catheter |
APR, abdomino-perineal resection of the rectum; CDP, cephalic duodenopancreatectomy; CT, computerised tomography.
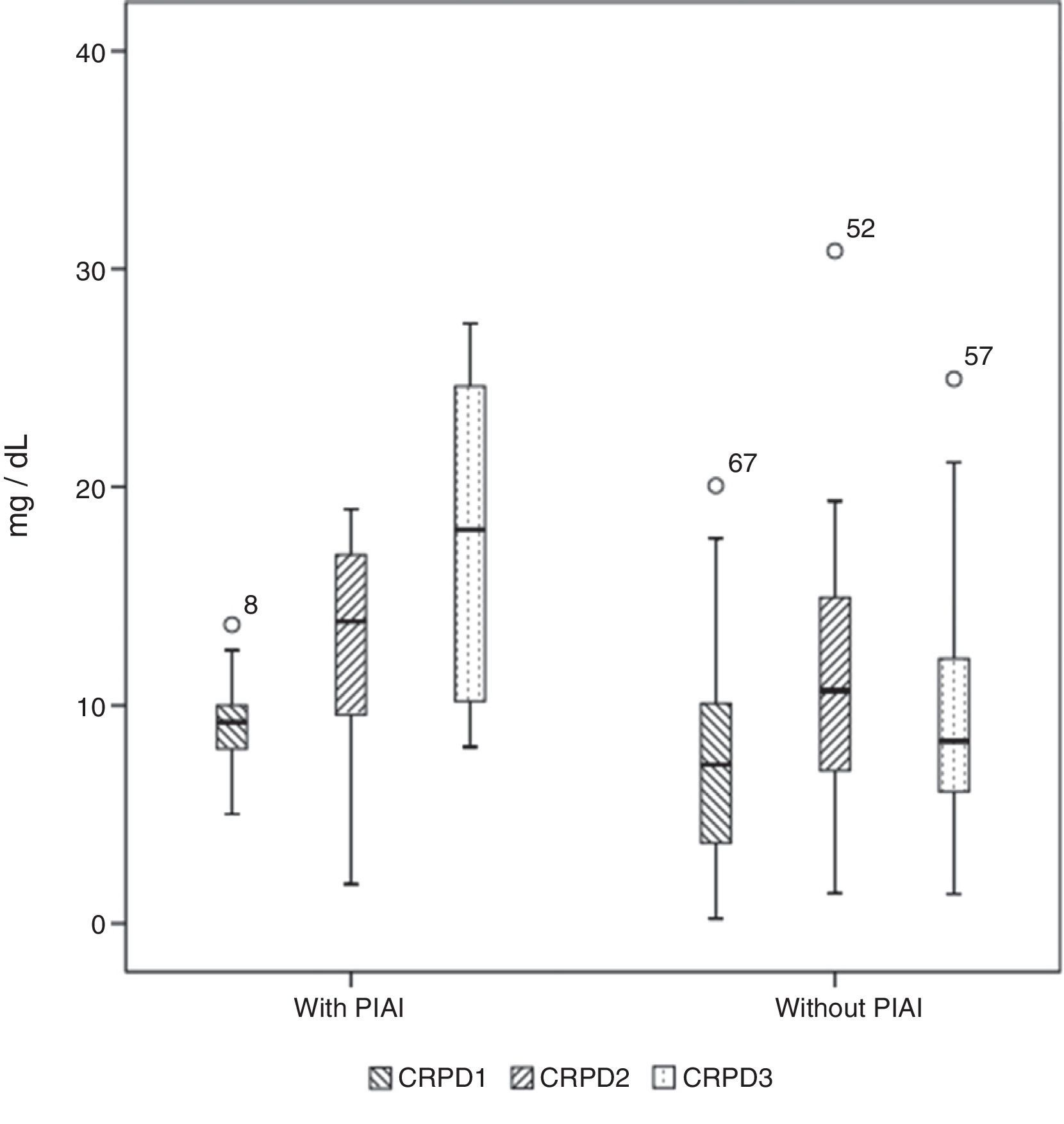
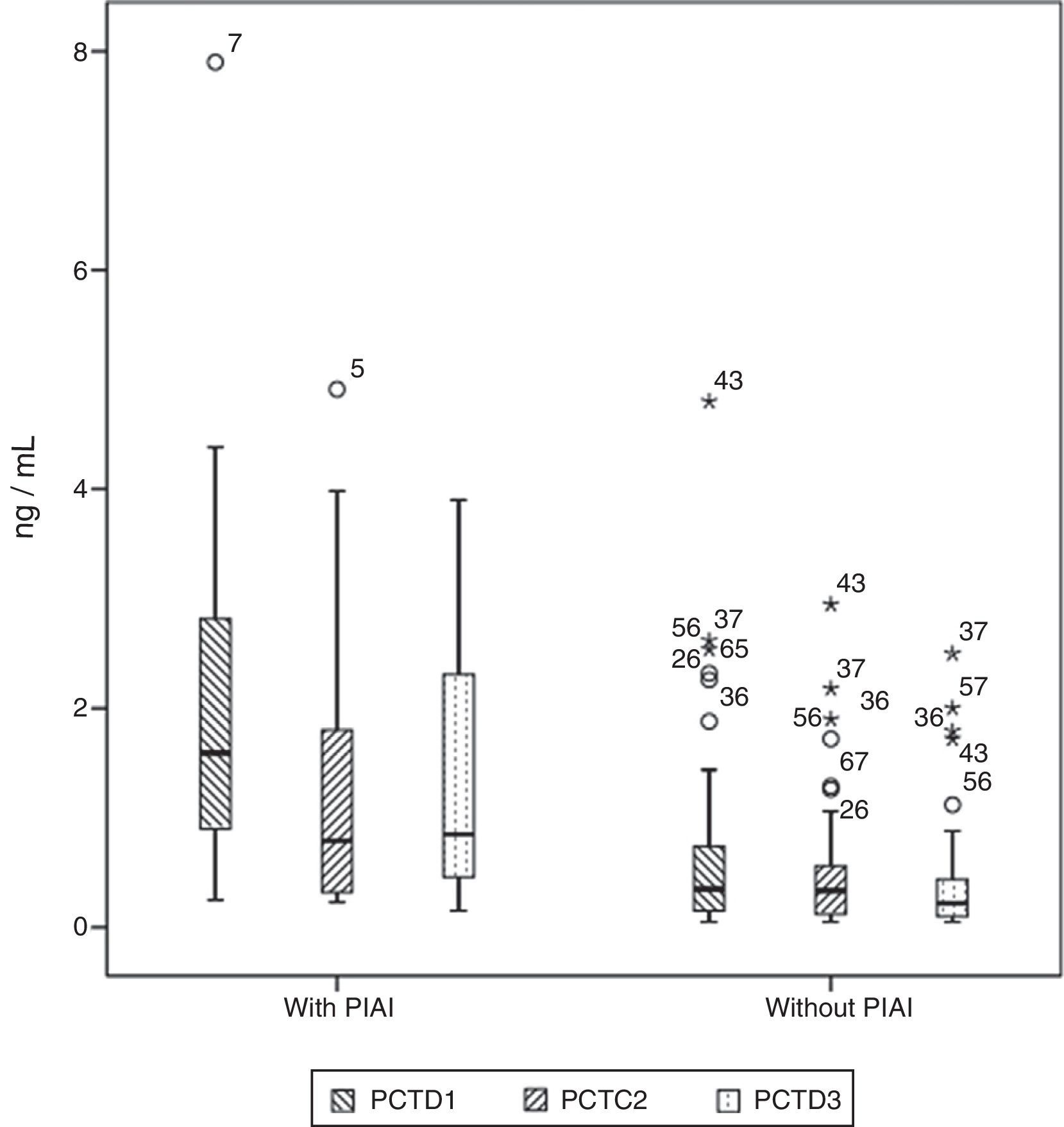
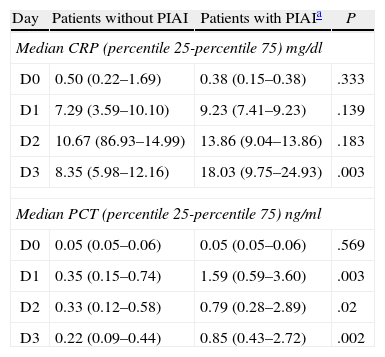
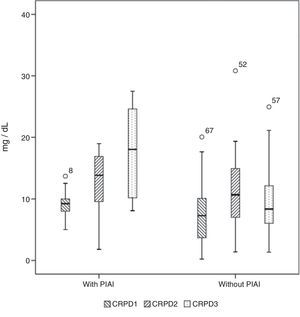
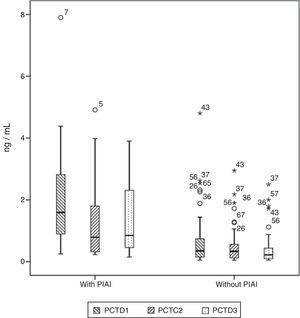
Basal serum levels of CRP and PCT were not different in either group of patients (P=.330 and P=.569, respectively). CRP and PCT levels increased after surgery in 98.5% and 97% of patients, respectively. Both markers increased in 100% of the patients who had PIAI. Over the period of time studied, in patients who did not have PIAI, CRP reached its highest level after 48h, with a median of 10.67mg/d, whereas in patients who did have PIAI the peak was reached after 72h, with a median of 18.03mg/dl (Fig. 1). The highest concentration of PCT, in the first 3 postoperative days was reached after 24h in both groups, with medians of 0.35ng/ml, for patients with no PIAI and 1.59ng/ml for patients with PIAI (Fig. 2). There were no significant differences in PCT levels at 24, 48 and 72h, according to the type of surgery (P=.613, P=.774, P=.764, respectively). Neither were there significant differences in CRP values, in any of the postoperative tests, according to the type of surgery (P=.320; P=.586; P=.741).
Box diagram showing the C-reactive protein (CRP) values in patients with and without postoperative intra-abdominal infection (PIAI). The boxes show percentiles 25, 50 and 75 and the minimum and maximum values. The o symbols represent extreme values. The numbers indicate the order number of the patient.
Box diagram showing procalcitonin (PCT) values in patients with and without postoperative intra-abdominal infection (PIAI). The boxes show percentiles 25, 50 and 75 and the minimum and maximum values. The o symbols represent extreme values. The numbers indicate the order of the patient.
Serum levels of CRP were not significantly different in either group of patients at 24 or 48h after surgery (P=.139 and P=.183, respectively) however 72h postoperatively they were significantly higher in patients with PIAI (P=.003).
Serum levels of PCT were significantly higher in patients with PIAI 24, 48 and 72h after surgery (P=.003; P=.02 and P=.002, respectively). Table 3 shows the CRP and PCT values in both groups of patients.
CRP and PCT Values in Patients With and Without Postoperative Intra-abdominal Infection.
| Day | Patients without PIAI | Patients with PIAIa | P |
| Median CRP (percentile 25-percentile 75) mg/dl | |||
| D0 | 0.50 (0.22–1.69) | 0.38 (0.15–0.38) | .333 |
| D1 | 7.29 (3.59–10.10) | 9.23 (7.41–9.23) | .139 |
| D2 | 10.67 (86.93–14.99) | 13.86 (9.04–13.86) | .183 |
| D3 | 8.35 (5.98–12.16) | 18.03 (9.75–24.93) | .003 |
| Median PCT (percentile 25-percentile 75) ng/ml | |||
| D0 | 0.05 (0.05–0.06) | 0.05 (0.05–0.06) | .569 |
| D1 | 0.35 (0.15–0.74) | 1.59 (0.59–3.60) | .003 |
| D2 | 0.33 (0.12–0.58) | 0.79 (0.28–2.89) | .02 |
| D3 | 0.22 (0.09–0.44) | 0.85 (0.43–2.72) | .002 |
The only quotient which was significantly associated with the appearance of PIAI was CRP D3/CRP D2, with a median of 1.29 for patients with PIAI, and 0.88 for patients without PIAI (P=.003).
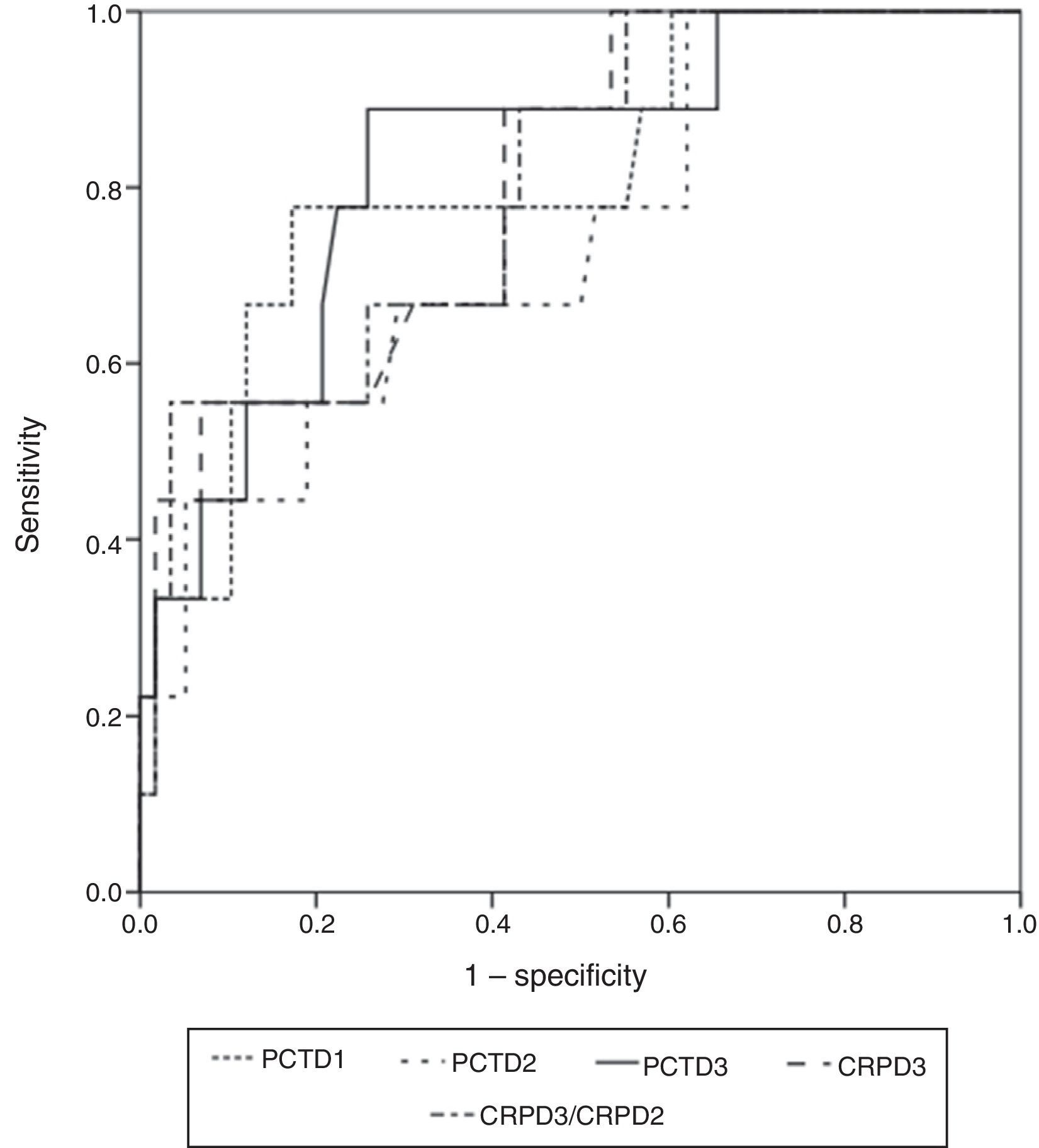
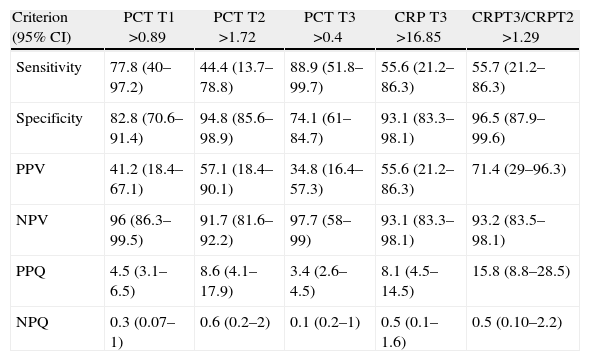
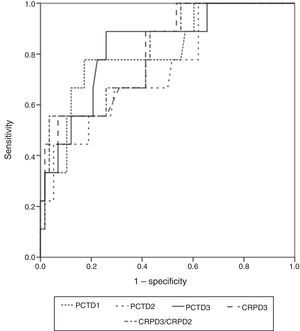
The widest area under the ROC curve corresponded to determination of PCT at 72h (0.829 95%CI: 0.717–0.910; typical error: 0.0742; P=.0001) (Fig. 3). Table 4 shows the best cut-off points, the sensitivity values and the predictive values.
Sensitivity, Specificity Values, PPV, NPV and Probability Quotients.
| Criterion (95% CI) | PCT T1 >0.89 | PCT T2 >1.72 | PCT T3 >0.4 | CRP T3 >16.85 | CRPT3/CRPT2 >1.29 |
| Sensitivity | 77.8 (40–97.2) | 44.4 (13.7–78.8) | 88.9 (51.8–99.7) | 55.6 (21.2–86.3) | 55.7 (21.2–86.3) |
| Specificity | 82.8 (70.6–91.4) | 94.8 (85.6–98.9) | 74.1 (61–84.7) | 93.1 (83.3–98.1) | 96.5 (87.9–99.6) |
| PPV | 41.2 (18.4–67.1) | 57.1 (18.4–90.1) | 34.8 (16.4–57.3) | 55.6 (21.2–86.3) | 71.4 (29–96.3) |
| NPV | 96 (86.3–99.5) | 91.7 (81.6–92.2) | 97.7 (58–99) | 93.1 (83.3–98.1) | 93.2 (83.5–98.1) |
| PPQ | 4.5 (3.1–6.5) | 8.6 (4.1–17.9) | 3.4 (2.6–4.5) | 8.1 (4.5–14.5) | 15.8 (8.8–28.5) |
| NPQ | 0.3 (0.07–1) | 0.6 (0.2–2) | 0.1 (0.2–1) | 0.5 (0.1–1.6) | 0.5 (0.10–2.2) |
NPQ, negative probability quota; PPQ, positive probability quotient; CI, confidence interval; CRP, C-reactive protein; PCT, procalcitonin; NPV, negative predictive value; PPV, positive predictive value.
This study shows that gastrointestinal cancer surgery caused raised serum levels of CRP and PCT in practically all the patients and that the highest concentration was reached earlier for PCT than for CRP. Both proteins are useful markers in predicting postoperative intra-abdominal infection, with a very high negative predictive value (NPV), already in the first 3 postoperative days, although the positive predictive value (PPV) is low. Considering isolated values, PCT is a more valid marker in predicting PIAI than PCR, but if we consider the evolution of the values over time, we can see that the relationship between CRP values at 72h after surgery and CRP values at 48h after surgery has quite a high PPV, higher than that of the isolated determinations of PCR and PCT. The results of our study also show that the raised levels of CRP and PCT preceded the clinical and radiological diagnosis of PIAI by several days, which in most cases was made after the 8th postoperative day. The significant elevation in PCT values in patients with PIAI had already occurred at 24h. The reason that this occurs cannot be deduced from the results of this study, but it could be due to greater intra-operative contamination or bacterial translocation caused by anastomotic ischaemia.
The principal weakness of our study is the small number of patients and that cancers of different types were included. Nonetheless, we believe that the results are still valid, as both in our study and in others published previously,18,20 in patients having undergone gastrointestinal surgery, no significant differences were found in PCT values according to the type of surgery. The clinical relevance of this study lies in the usefulness of PCT and CRP in the early prediction of PIAI, which could potentially enable earlier diagnosis, since radiological examinations can be performed, such as CT with intraluminal and intravenous contrast, in patients with high levels of PCT, before there is clinical suspicion of infection. Thus the morbimortality associated with delayed diagnosis of PIAI could be reduced, especially for cases of anastomotic leak.27 The finding that both CRP and PCT have a high NPV is particularly relevant, making it possible to identify patients very unlikely to develop a PIAI as early as the first 3 days after surgery, and therefore facilitate the safe, early discharge of patients who have undergone gastrointestinal cancer surgery.
Coinciding with the results obtained in our study, other authors have shown that both CRP2,4,7,28 and PCT4,29 levels significantly increase in practically all patients after gastrointestinal surgery. Our study was limited to the first 3 postoperative days and does not show when they normalise, however other authors who have studied the evolution of both proteins over a longer period of time have observed that PCT normalises 5–7 days after surgery and CRP 7–10 days after surgery.29
Although CRP is a non-specific inflammation marker, several authors have shown that persistently high levels from the third postoperative day is a good predictor of postoperative infection.2,7,22,28 Similarly, in our study, CRP only shows a significant relationship with the occurrence of postoperative intra-abdominal infection on the third day after surgery. Ortega-Deballon et al. demonstrated that serum levels of CRP on the fourth postoperative day are a good predictor of anastomotic leak in colorectal surgery; for a cut-off point of 125mg/l, sensitivity was 81.8%, specificity was 64.4% and the NPV 95.8%.2 García-Granero et al. obtained similar results for the values on the third, fourth and fifth postoperative days.22 Our study also shows that CRP levels on the third postoperative day are a good predictor of postoperative intra-abdominal infection, with similar sensitivity, specificity and NPV values, but for a cut-off point which is somewhat higher than those to which these authors refer.
The usefulness of PCT as a marker of postoperative infection has also been demonstrated previously by other authors in different types of surgery; cardiothoracic30,31 and abdominal.4–6,18–24 In the abovementioned García-Granero et al.22 study it was observed that serum levels of PCT between the third and fifth postoperative days were a good predictor of major anastomotic dehiscence, with a sensitivity greater than 90%, an NPV above 99% and a PPV of less than 17%, but not of minor dehiscence (not requiring percutaneous drainage or surgery) in patients undergoing colorectal cancer surgery. However, in our study we considered all PIAI, regardless of whether they required drainage or not, and PCT levels on the third postoperative day are still a good predictor of infection, with very similar sensitivity and NPV values. Another recent study, which included 114 patients who had undergone colorectal surgery, and which covered all surgical site infections, shows similar results to our work, although with somewhat lower sensitivity and NPV values.23 Almost all studies published to date agree with ours in that PCT is a better predictor of postoperative infection than CRP,5,22–24 but a study has recently been published, on 100 patients operated for colorectal cancer, which concludes that PCT is not a better predictor of postoperative infection than CRP.25 Both our study and studies published previously coincide in that the PPV of PCT is low22,23 and, therefore, PCT on its own is not valid for diagnosing PIAI. However, it can enable patients to be selected for imaging studies such as CT with contrast, before infection is clinically suspected. An interesting finding from our study, which has not been published before, is that the quotient between CRP levels at 72h after surgery and CRP levels at 48h after surgery is also significantly associated with the occurrence of PIAI; with a greater PPV, above 70%, although this finding should be interpreted with caution, as there is a very wide confidence interval, which detracts from the validity of this data.
In conclusion, our study suggests that serum levels of PCT in the first 3 postoperative days are a good marker of PIAI, with a very high NPV, which makes it possible to safely predict that there is no PIAI. The quotient between CPR values at 72h after surgery and CPR values at 48h after surgery combine high NPV with high PPV.
However, these results need to be corroborated with studies that include a greater number of patients and usefulness of both proteins needs to be confirmed with randomised prospective studies in which early diagnostic tests are indicated in a group of patients, such as CT, according to serum levels.
FundingPartially funded by a grant from the José Luis Castaño Foundation.
Conflict of InterestsThere is no conflict of interests to declare.
Please cite this article as: Domínguez-Comesaña E, López-Gómez V, Estevez-Fernández SM, Mariño Padín E, Ballinas-Miranda J, Carrera-Dacosta E, et al. Procalcitonina y proteína C reactiva como marcadores precoces de infección intraabdominal postoperatoria en pacientes operados de cáncer gastrointestinal. Cir Esp. 2014;92:240–246.