The aim of this study was to review and to assess the quality of the scientific articles regarding early and late anastomotic leak (AL) after colorectal surgery and their risk factors.
An electronic systematic search for articles on Colorectal Surgery, AL and its timing was undertaken using the MEDLINE database via PubMed, Cochrane and Embase. The selected articles were thoroughly reviewed and assessed for methodological quality using a validated methodology quality score (MINCIR score). This review was registered in the PROSPERO registry under ID: CRD42022303012. 9 articles were finally reviewed in relation to the topic of early and late anastomotic leak.
There is a lack of consensus regarding the exact cut-off in time to define early and late anastomotic leak, but it is clear that they are two differentiated entities. The first, occurring in relation to technical factors; whereas the latter, is related to impaired healing.
El objetivo de este estudio fue revisar y evaluar la calidad de los artículos científicos sobre la dehiscència anastomótica temprana y tardía después de cirugía colorrectal y sus factores de riesgo.
Se realizó una búsqueda sistemática electrónica de artículos sobre Cirugía colorrectal, dehiscència de anastomoiis colorectal utilizando la base de datos MEDLINE a través de PubMed, Cochrane y Embase. La calidad metodológica de los artículos seleccionados se revisó minuciosamente y se evaluó mediante una puntuación de calidad metodológica validada (puntuación MINCIR). Este estudio fue registrado en PROSPERO con el ID: CRD42022303012. Despues de una seleccion basada en los criterios de búsqueda, finalmente se revisaron 9 artículos en relación al tema la revisión.
Se pudo observar que existe una falta de consenso en cuanto al tiempo de corte exacto para definir la fuga anastomótica temprana y tardía, pero está claro que son dos entidades diferenciadas. La primera, ocurriendo en relación a factores técnicos; mientras que la segunda situación clinica se relaciona con una cicatrización alterada.
Surgical colorectal resection and performance of an anastomosis is the standard procedure for curative treatment of several colorectal pathologies, such as colorectal cancer, diverticular disease or inflammatory bowel disease.
Anastomotic leakProbably, the greatest challenge and one of the most feared complications in colorectal Surgery is anastomotic leakage (AL). Its incidence, according to the literature, varies from 1.5% to 28% and it has remained stable for the last decades despite technical advances in surgery. AL can account for up to one-third of all postoperative mortality1–3.
There is no unanimity regarding the definition of AL. Most authors agree that AL is an escape of content or a communication between intra- and extraluminal compartments at the anastomotic site, but they differ in other aspects such as clinical and radiological signs that lead to an AL diagnosis2–5.
Most of the articles evaluated in this review, use the definition of colorectal AL proposed in 2010 by the ISREC (International Study Group of Rectal Cancer)6 which consists in a defect of the intestinal wall’s integrity at the anastomotic site leading to a communication between intra- and extraluminal compartments. A pelvic abscess adjacent to the anastomosis is also considered an AL (with or without demonstrated communication)2,4,7. Despite the fact that this definition was developed for low anterior resections (LAR) in rectal cancer, it is useful for all colorectal anastomosis2. The consensus published by Helsdingen et al.2 recommends the association of a grading scale such as Clavien-Dindo or the ISREC grading system to complete the definition. Other authors have also postulated as a definition of AL, the discharge of feces from the pelvic drain and the signs of acute peritonitis8,9.
Early and late anastomotic leakMoreover, there is a clear interest differentiating between two concepts in relation to the timing of the occurrence of the AL: early and late anastomosis leak. We hypothesize that these situations must be regarded as different events because there is a significant variability in the risk factors and clinical presentation, as well as, in the different clinical and surgical management required. In addition, mortality has also been described to differ significantly between these two clinical scenarios10, which can derive into a high clinical impact, since specific and targeted therapies for each entity might be needed.
Despite all these implications, there is a notable dispersion regarding the cut-off point in time that can define the frontier between early and late AL. Precisely this controversy drives the basis of this review. Therefore, we analyzed these concepts comprehensively in order to define and establish the two categories -early and late- AL in colorectal Surgery. We also aim to describe the main risk factors of anastomotic failure in colorectal Surgery related to early AL and the ones that predispose to late AL.
MethodsThis systematic review was developed using a protocol with pre-specified and detailed methods of analysis and eligibility criteria according to the Prisma checklist11. This review was registered in the PROSPERO registry under ID: CRD42022303012.
Search strategy and information sourcesA search in the literature published between September 2001–September 2021 using the MEDLINE database via PubMed, Embase, and Cochrane library was conducted. The search terms used were: Colorectal, anastomosis, leak, early and late. Also relevant articles from reviewed citations were retrieved. Two reviewers (C.G, A.V) evaluated the articles, and a third reviewer (D.P) determined the final decision if any discrepancies about inclusion were considered.
InterventionRelevant studies were identified through title and abstract information. Consequently, a full-text evaluation of the selected articles was carried out.
Inclusion and exclusion criteriaIncluded articles, had the main endpoint or focus in the terms of early and late anastomotic leak in colorectal surgery procedures. Articles reporting other surgical specialties and those that did not describe the timing of the leak were excluded from the analysis. Articles in a language other than English were also excluded.
OutcomesThe primary endpoint of interest was to evaluate the definitions of early and late anastomotic leak in colorectal surgery. Additional secondary goals included the highlight of risk factors that influence both types of anastomotic leak, its clinical impact, management and prognosis.
Quality methodology assessmentThe quality of the retrieved articles was assessed by using a validated methodology quality score (MINCIR score)12.
The MINCIR score is a scale composed of three items: the first is related to the study design, the second to the sample size in the study and the third part is related to the methodology used in the reporting paper. According to this, a score which represents the sum of the three items is generated, with a final score that can vary between 6 and 36 points, with 6 points being the worst methodological quality study and 36 points being the best12.
Two investigators (AV and CG) completed the quality assessment independently and blinded to each other’s result. Discrepancy between evaluations was solved by discussion and, if there was a lack of agreement, by the final decision of the senior author (DP).
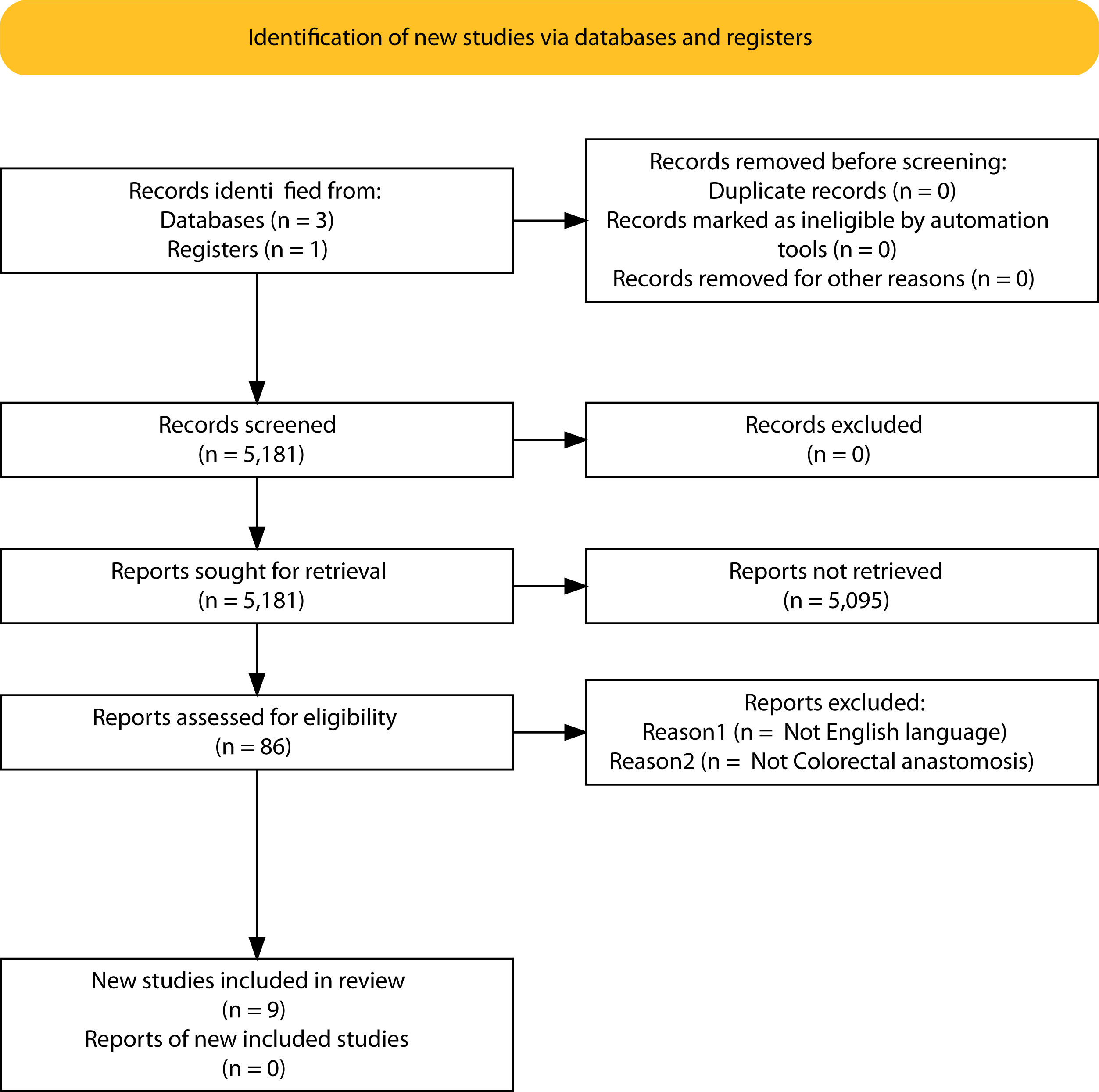
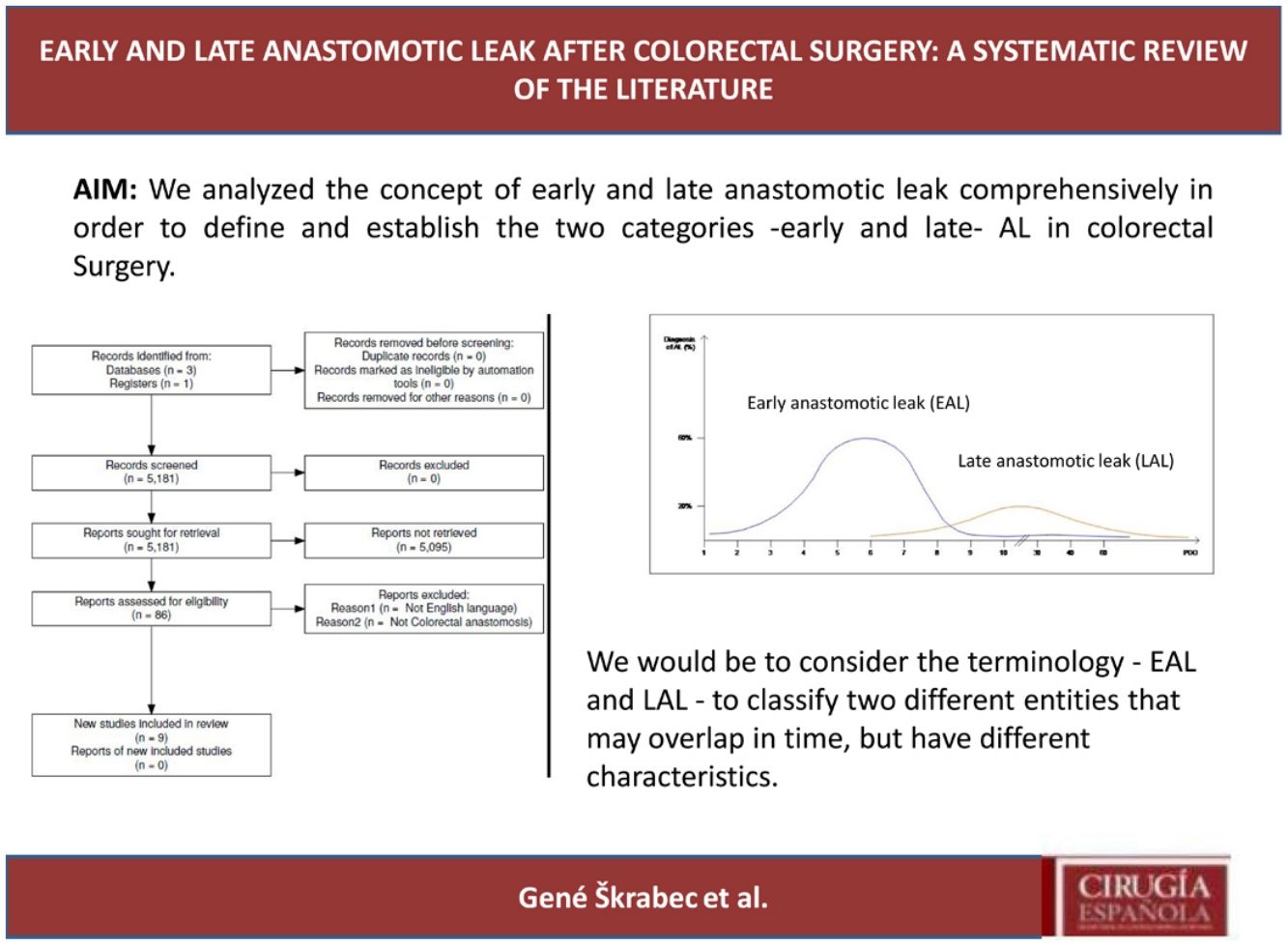
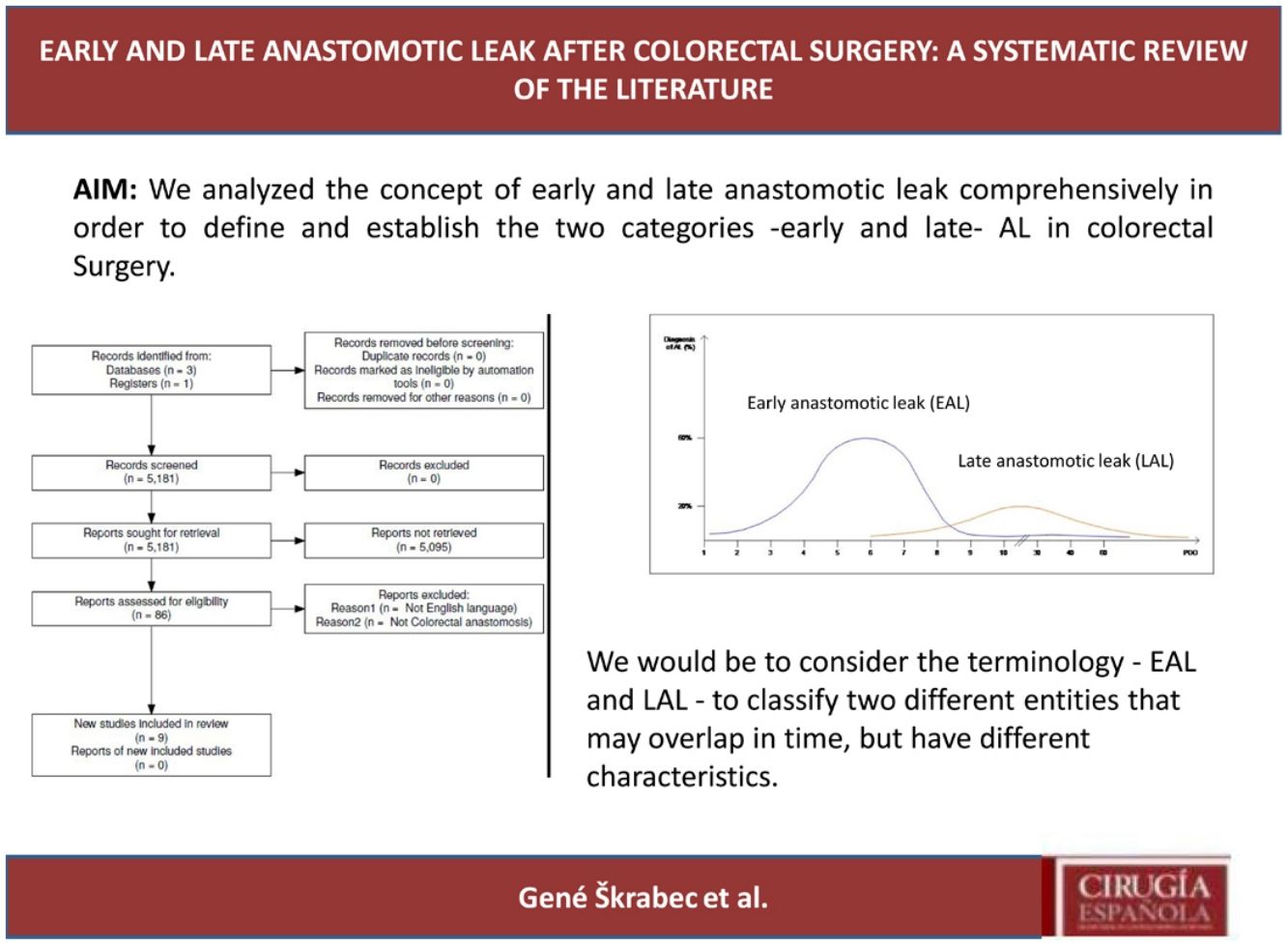
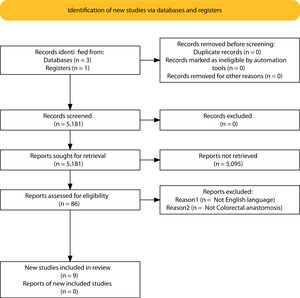
ResultsSelection of articlesBetween January 2001 and December 2021, 5.181 articles were published and appeared in PubMed using the search terms “Colorectal Surgery”, “anastomotic leakage” and “early or late”.
From the initial 5.181 articles retrieved using our search strategy, 5.095 were excluded as they were focused on other topics other than anastomotic leak or regarded other surgical specialties different from Colorectal Surgery.
Therefore, 86 articles were eligible for first assessment and were analyzed in detail (Fig. 1). Of these articles, 9 were finally selected for review as they focused on Colorectal anastomosis leakage and its timing description. The MINCIR score as a quality indicator of retrieved articles ranges from 13 to 23 out of 36 points (see Table 1). Table 1 summarizes the characteristics of the included articles and their quality assessment. Most of the articles were published in the last 5 years, predominantly in Asian countries.
Study design and characteristics.
| Article | MINCIRa score | Sample size (AL/total) | Study design | Year | Country |
|---|---|---|---|---|---|
| Shin et al.9 | 18 | 79/1838 | Retrospective cohort | 2010 | Korea |
| Morks et al.4 | 15 | 28/141 | Retrospective cohort | 2013 | The Netherlands |
| Floodeen et al.8 | 26 | 45/234 | Randomized multicenter trial (RECTODES) | 2013 | Sweden |
| Lim et al.13 | 17 | 141/3204 | Retrospective cohort | 2016 | Korea |
| Li et al.10 | 13 | 101/? | Retrospective cohort | 2017 | China |
| Chen et al.14 | 10 | 43/? | Retrospective cohort | 2018 | Taiwan |
| Sparreboom et al.1 | 23 | 1537/36929 | Retrospective cohort | 2018 | The Netherlands |
| Yang et al.7 | 18 | 262/1903 | Retrospective cohort | 2020 | South Korea |
| Helsdingen et al.2 | Not applicable | Expert panel consensus study | Systematic review | 2020 | The Netherlands |
No uniform definition exists for early and late AL (see Table 2). Four of the analyzed articles define a failure of the anastomosis as early if it is diagnosed until the 30th postoperative day (POD), whereas they name late AL those that occur after day 30 from surgery4,7,13. Shin et al.9 described early AL within the first 3 PO weeks. Tzu-Lianf Chen et al.14, and Sparreboom et al.1 propose cut-off points in time closer to the surgery such as the 5 and 6 POD, respectively. It has also been described in relation to the day of discharge from the hospital: early (during hospitalization) and late (after discharge) AL4,8.
Definitions of anastomotic leakage and early and late AL.
| Authors | Definition of ALa | Timing of early and late AL |
|---|---|---|
| Shin et al.9 | No clear general definition for AL. | Early: first 3 weeks PO. |
| Early AL: development of clinical signs of leakage, such as purulent discharge from the drainage catheter with peritonitis and pyrexia (>38 °C), abdominal pain or leukocytosis. | Delayed: after the first 3 weeks PO. | |
| Delayed AL: AL detected > 3 weeks POa, having resumed normal diet and defecatory function, without signs of peritonitis (leukocytosis, pyrexia, abdominal tenderness or ileus) and no local recurrence. | ||
| Morks et al.4 | ISREC a,b | Early: within 30 days PO. |
| Late: after 30 days PO. | ||
| Floodeen et al.8 | Peritonitis caused by leakage, pelvic abscess, or discharge of faeces from the pelvic drain, including leakage from any stapler line at the anastomosis at any time postoperatively. | Early: AL diagnosed during initial hospital stay. |
| Late: AL diagnosed after hospital discharge. | ||
| Lim et al.13 | Generalized peritonitis, localized peritonitis with or without abscess around the anastomosis site, and any type of fistula or chronic sinus associated leakage. | Early: developed within 30 days PO. |
| Late: developed after 30 days PO. | ||
| Li et al.10 | ISREC | Very early: within the first 5 days after surgery. |
| Early: within 30 PO days. | ||
| Late: after 30 PO days. | ||
| Chen et al.14 | No clear definition (Even though the definition of AL proposed by the ISREC is mentioned) | Early: before the first 5 days after surgery. |
| Late: after and including the 5th day PO. | ||
| Sparreboom et al.1 | Clinically relevant AL that requires radiological or surgical reintervention. | Early: AL until day 6 PO. |
| Late: AL after day 6 PO. | ||
| Yang et al.7 | ISREC | Early: AL until day 30 postoperative. |
| Late: AL after day 30 PO. | ||
| Helsdingen et al.2 | ISREC +/- Grading system (ISREC or Clavien-Dindo) c | No (They conclude that more research is needed to prove this difference and to define the optimal cutoff point). |
a AL: anastomotic leakage; PO: postoperative; ISREC: International Study Group of Rectal Cancer.
b Definition and grading system by ISREC: Defect of the integrity of intestinal wall at the anastomotic site leading to a communication between the intra- and extraluminal compartments.
1) Apparent discharge of gas/pus/feces from abdominal or pelvic drainage tube.
2) Anastomotic defect confirmed by proctoscopy, CT scan using contrast medium or rectal examination (only for lower rectal anastomosis).
3) Confirmed during relaparotomy.
Grade A, anastomotic leakage requiring no active therapeutic intervention.
Grade B anastomotic leakage requiring active therapeutic intervention but manageable without relaparotomy.
Grade C anastomotic leakage requiring relaparotomy, usually associated with takedown of anastomosis followed by end stoma or salvage of anastomosis with ileostomy.
c An international 19-member expert panel achieved consensus on the definition of AL employing a modified Delphi method. The panel recommends to use the ISREC definition as the general definition of colorectal anastomotic leakage (CAL). And when defining CAL, the ISREC grading system should be complemented with the Clavien-Dindo classification.
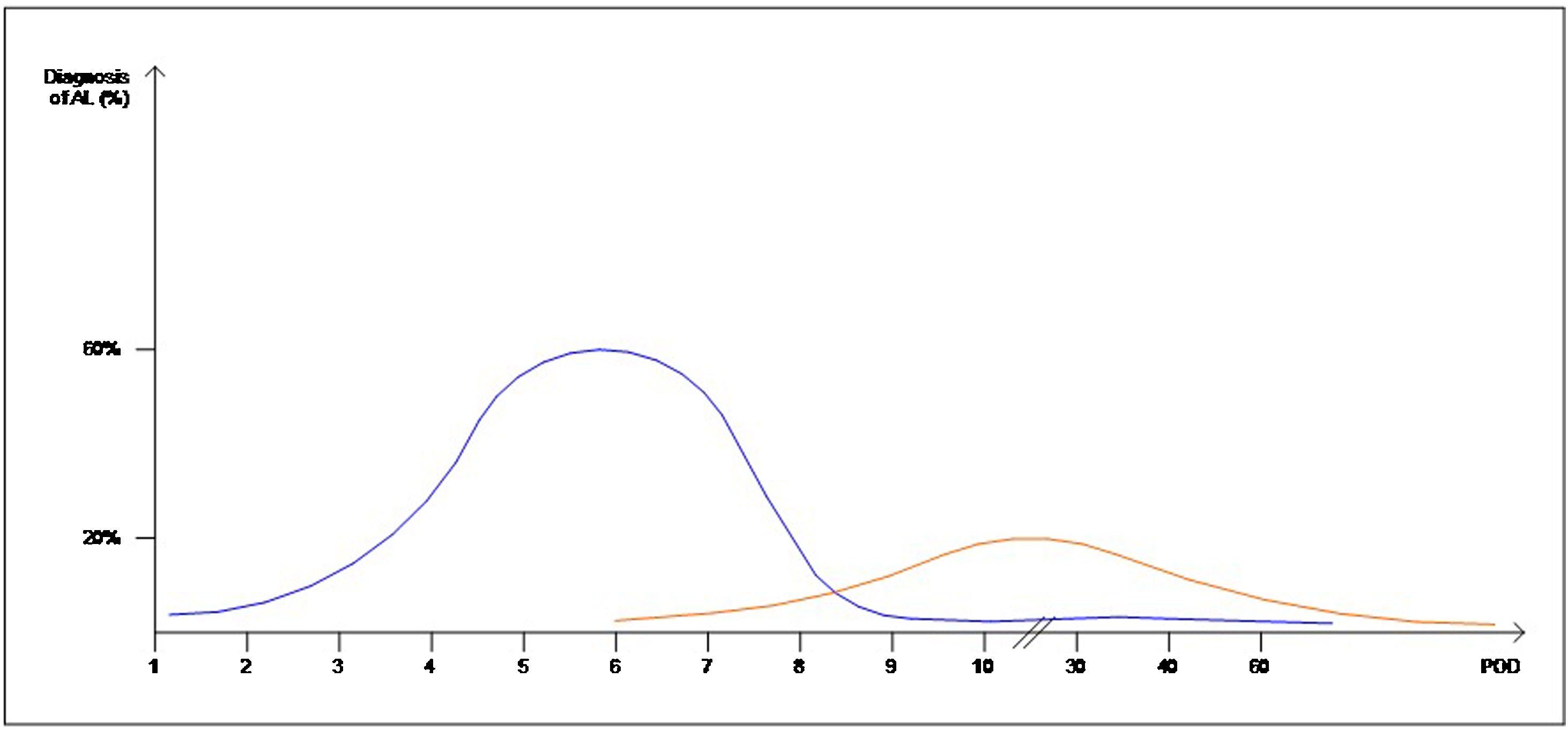
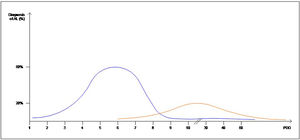
Interestingly, the mean day of diagnosis of EAL ranged between 3,5 to 10 POD, whereas LAL was diagnosed with a mean between 7,5 and 210 POD. This is theoretically represented in Fig. 2. In some articles, EAL had a two time higher incidence than LAL4,8,9.
Risk factors, management and prognosis and quality assessmentTable 3 summarizes the risk factors for each type of AL – early or late – as reported in the assessed articles, specifying which articles focused only on rectal surgery. EAL was consistently related to factors that could be associated with a more cumbersome procedure, and no protection of the anastomosis with an ileostomy. LAL, on the other hand, was related to previous radiotherapy and comorbidities.
Proposed risk factors for early anastomotic leakage and late anastomotic leakage.
| Article | Type of Surgery | EAL | LAL |
|---|---|---|---|
| Shin et al.9 | Rectal cancer | Male gender | Female gender |
| Lower anastomosis location (<4 cm) | |||
| nCRT | |||
| Morks et al.4 | Colorectal surgery (benign and malignant) | Not defined | Preoperative RDT |
| Floodeen et al.8 | Rectal cancer (LAR) | Male gender | Female gender. |
| Longer operation time | Lower BMI | ||
| Increased intraoperative blood loss | |||
| Lim et al.13 | Rectal cancer (LAR) | No diverting stoma | Lower anastomosis location |
| nCRT | |||
| Diverting stoma | |||
| Li et al.10 | Colorectal surgery | For VE-AL: | Not defined |
| No protective stoma | |||
| No anastomotic reinforcement | |||
| nCRT | |||
| No reconstruction of post-peritoneum | |||
| Chen et al.14 | Colorectal surgery | Higher tumour location (median of 7 cm). | Lower tumour location (median of 3 cm). |
| Lower anastomosis location (<5 cm from anal verge). | |||
| Presence of stoma. | |||
| Sparreboom et al.1 | Colorectal surgery | Male sex (+++) | Male sex (+) |
| Rectal cancer | Diverting ileostomy | ||
| Younger age | Rectal cancer | ||
| Increased BMI | Preoperative RDT (Rectum) | ||
| Laparoscopic surgery | Charlson > II | ||
| Emergency surgery | ASA > III-IV | ||
| No diverting ileostomy | Preoperative complications | ||
| Additional resection because of tumour growth | |||
| Yang et al.7 | Rectal cancer | Lower tumour location. | nCRT |
| Alcohol and smoking history. | Lower tumour location | ||
| More intraoperative blood loss (≥200 mL). | Smoking history | ||
| No protective ileostomy during initial operation. | More intraoperative blood loss (≥200 mL) | ||
| Protective ileostomy | |||
| Helsdingen et al.2 | Colorectal surgery | Younger age | High Charlson Comorbidity Index |
| Increased BMI | High ASA Score. | ||
| Laparoscopically performed anastomosis | Preoperative complications | ||
| Emergency operation | Preoperative RDT | ||
| No diverting ileostomy |
RDT: Radiotherapy; LAR: Low anterior resection; BMI: Body Mass Index; ASA: American Society of Anaesthesiologists Score; nCRT: Neoadjuvant chemoradiotherapy; CLS: Colon Leakage Score.
Summary proposal of our definition of early and late AL and the factors involved in each category as well as their consequences are shown in Table 4.
Proposed characteristics for early anastomotic leakage and late anastomotic leakage.
| EAL | LAL | |
|---|---|---|
| CAUSE | TECHNICAL FAILURE | DEFICIENT HEALING |
| PRESENTATION | Higher peritonitis rate10 | Pelvic abscess8,9 |
| Higher rate of Grade C AL4 | Fistula7–9,13 | |
| Stenosis7 | ||
| TIMING (mean day of diagnosis in the assessed articles)1,2,4,7–10,13,14 | 3,5-10 POD a | 7,5-210 POD |
| Risk factors for AL in COLON* surgeries | Anatomical difficulties: Male gender1,8,9, increased BMIa,2 | Denutrition: low BMI8 |
| Difficulties in surgery: Emergency setting1,2, Increased blood loss7,8, longer operation time8 | Patient comorbidities: High Charlson Index1,2 | |
| Insufficient vascularization: Smoking7, no IFAa | High ASAa score1,2 immunosuppression | |
| No reinforcement10 | Microbiome No ERASa implementation | |
| Low volume centers | ||
| Risk factors for AL in RECTUM (LARa) surgeries | In addition to*: No ileostomy 1,2,7,10,13 | In addition to*: nCRDTa,7,9,13 |
| Laparoscopy 1,2 | Preoperative RDTa,1,2,4 | |
| Female8,9 Protective stoma1,13,14 | ||
| Low level anastomosis9,13,14 | ||
| CONSEQUENCES | Higher relaparotomy rate4,7,10,13 | Higher permanent stoma rate7,9,13 |
Despite the importance of AL in colorectal surgery, there is still important heterogeneity in the terminology used to describe this troublesome complication. As noted in Heldingen’s consensus2, the distinction between early and late AL should be made, as there may be relevant clinical implications because of the involved risk factors and different treatment approaches.
Classically AL has been described before the 14th postoperative day (POD)2. Most studies that describe risk factors for colorectal AL describe its diagnosis, at a median between the 6th and 12,7 POD15–19. Sala Hernandez described that 13.4% of the cases of AL were identified before the 4th POD, 69.4% were identified between the 4th and 10th POD, and 17.2% were detected after the 10th POD5.
The definitions and occurrence of EAL and LAL vary greatly in time. Early AL has heterogeneous definitions, ranging from before day 5 to day 60 as Placer et al.20 described. Other authors have opted for defining EAL or LAL in relation to the day of discharge from the hospital as Floodeen et al.4,8,21, but it can be an easily biased variable, due to distinct discharge policies. Regarding the dispersion in time we have graphically summarized these two events in Fig. 2. It shows that early anastomotic leak has a much more concentrated pic incidence at the beginning of the PO period, whereas heterogeneity among the mean day for LAL is much higher, indicating a longer and less pronounced curve.
As Sparrebroom1 on behalf of the Dutch ColoRectal Audit group pointed out, apart from the cut-off point in time, what classifies EAL is the fact that it is due to technical failure and that LAL is related to healing deficiencies (see Table 3).
When considering a technically successful anastomosis, tension free suturing with a precise adaptation of the tissue, and adequate blood perfusion are indispensable requirements. Recently, with the aim of improving technical factors, in the immediate postoperative leaks, intraoperative use of ICG22–24 or even the application of anastomotic sealers25,26 that have been introduced on a daily practice, are promising maneuvers that can diminish AL. The PILARIII trial27, a multicentre randomized controlled trial that aimed to assess the efficacy of intraoperative fluorescence angiography (IFA) in preventing AL, has shown no differences in anastomotic leak rates using ICG’s perfusion assessment. However, the study might be underpowered28. The ongoing IntAct trial (Trial registration: ISCRN: 13334746) compares IFA against standard care (surgery with no IFA) to determine the effect on anastomotic leak in patients undergoing elective anterior resection for rectal cancer, might shed an extra light on the topic29.
Technical failures are considered seminal in the occurrence of anastomosis leak in the first postoperative days. Technical difficulties can appear in relation to patient-dependent factors such: an increased Body Mass Index (BMI) and male sex (narrow pelvis)1,2,8,9. Other factors highlighting the procedure’s difficulty such as: long operation time and extensive intra-operative bleeding, laparoscopic surgery or emergency surgery have also been related to EAL1,2,8,13. Also the Hospital volume can play a role as it implies the expertise of the surgeons. EAL often implies a reintervention and consequently permanent stoma rates are higher.
LAL is generally slower, multifactorial and related to an impaired healing. Understanding anastomotic healing is crucial. All of the four layers of the bowel (mucosa, submucosa, muscularis propria and serosa) seem to play a role in anastomotic healing3,30–32. Otherwise, the serosa seems to be important in providing a matrix for fibroblasts, while the interactions between bacteria, mucus and the mucosal layer also seem important to maintain homeostasis in which anastomotic healing can occur33. In the GI tract collagen is synthesized by fibroblasts and smooth muscle cells and healing is generally faster3. Gut microbiota changes play an interesting role in anastomosis healing34,35. It is believed that one of the mechanisms through which bacterial infections contribute to AL development is through matrix metalloproteinase (MMP) activation and collagenolytic substances. Other factors such as ischemia or severe local inflammation can impair or slow down the healing process. Failure to resolve the initial inflammatory response can lead to anastomotic leakage or development of fistula, whereas uncontrolled collagen accumulation leads to excess scarring and stenosis. This evolution might explain why in LAL it is more frequent to have chronic consequences such as fistula, and chronic abscesses that are often managed conservatively14.
Other patient related factors, such as comorbidities (ASA, Charlson) and the nutritional status, among others, play a key role in the healing process. LAL should not be considered as a continuous process from EAL, but as a distinct entity. Higher American Society of Anesthesiologists Score (ASA), high Charlson Comorbidity Index, lower albumin levels, smoking history preoperative complications and higher anastomotic prediction index (CLS) have been proposed as independent risk factors for LAL, which are all related to clinical characteristics of the patient2,7,8,20. Also, the last decades more clinical experiences demonstrated the clinical benefits of enhanced recovery programs (ERAs) on all morbidity including AL, highlighting that preconditioning the patients no only avoids general postoperative complication, but it may also play a role in healing9,13,36–39.
Most of the assessed articles analyze colon and rectum AL together, but after our revision we concluded that they should be studied separately as they differ in some key points such as the neoadjuvant scheme. Exposure to preoperative radiotherapy (RDT) is also an independent variable that has been related to LAL in some studies1,7,9,13. When taking into account Colon and Rectal Surgery as different entities, it is shown that open surgery is a risk factor for EAL in Colon Surgery while laparoscopic technique is a risk factor for EAL in Rectal Surgery. Possibly, nowadays that most of the colonic procedures are laparoscopic, open surgery is reserved for high-risk patients or technically difficult procedures probably explaining why it is being related to worse outcomes.
Mathieseen40 recommended ileostomy in low anterior resection for rectal cancer as it decreased the rate of symptomatic anastomotic leakage. Performing a diverting ileostomy during the initial operation and a lower anastomosis location (<5 cm from anal verge) have also been postulated as an independent risk factors for LAL in rectal cancer1,2. Probably, due to an asymptomatic leakage that is under-diagnosed. LAL in rectal cancer is usually diagnosed after stoma closure14.
This study has some limitations. Even with the inclusion of important databases with a great number of patients, currently, there are no articles clarifying these two clinical situations with a high MINCIR score (see Table 2). Therefore, there is no high quality evidence available on the specific definitions for these two situations. AL is, nevertheless, a low incidence event that challenges the design of prospective randomized clinical trials (RCT) so the topic is usually retrospectively assessed, dragging along an inevitable bias. However, we believe this is the first systematic review about the topic and that it clarifies the differences about these two entities.
In conclusion, our proposal would be to consider the terminology – EAL and LAL – to classify two different entities that may overlap in time, but have different characteristics. The first occurs during the immediate postoperative period (typically before the 10th POD) in which technical factors play a key role. Its incidence can be decreased by improving technical aspects of the anastomosis construction, such as ICG usage or its reinforcement.
The latter can occur during hospitalization or during the follow-up postoperative period and is related to healing issues. Apart from the factors that may tamper the healing process such as the gut microbiota, it is highly influenced by the patient's frailty or basal status, highlighting the uttermost importance of surgical prehabilitation and implementation of the ERAS program.
Registration and protocolThis review was registered in the Prospero database with the CRD42022303012 registration number; full search protocol can be accessed in Prospero database.
Conflict of interestThe authors declare no competing interests.