To analyze the surgical burden of UC care in the last two decades, analyzing the characteristics of the patients, surgical indications along with the short and long-term results.
MethodSingle-center retrospective cohort analysis of UC patients undergoing abdominal and anorectal surgery between January 2000 and December 2020. The care burden, clinical data and results were analyzed according to distribution by decades.
Results128 patients, 37% female, underwent 376 surgical interventions (296 intestinal procedures and 80 anorectal). Mean follow-up for the cohort was 106±64 months. Timing from diagnosis to first surgery was under 5 years in 53.3%. In the second decade of the study there were fewer operated patients (73 vs. 48) as well as the total number of interventions per patient (2.7 vs. 2.0). The proportion between elective and urgent surgery was reversed in the second decade, observing an increase in laparoscopic surgery (70% vs. 8%) together with a decrease in major postoperative morbidity (Clavien-Dindo≥IIIa) (20% vs 8.4%). 80 patients underwent a restorative proctocolectomy, with a failure of 5% at 1 year but 23.7% in the long term. 37 patients required anorectal surgery, of which 26 (71%) were serial interventions, most due to septic complications of the pouches.
ConclusionsThe number of colectomies and interventions per patient decreased in the last decade, while there were improvements in morbidity and surgical approach. The need for sequential surgeries and long-term active instrumental surveillance for possible functional deterioration constitutes a significant clinical burden.
Analizar la carga quirúrgica asistencial por CU en 20 años, analizando las características de los pacientes, indicaciones quirúrgicas y resultados a corto y largo plazo.
MétodoAnálisis retrospectivo unicéntrico de pacientes intervenidos de enero del 2000 a diciembre del 2020. La carga asistencial, los datos clínicos y los resultados se analizaron según distribución por décadas.
ResultadosCiento veintiocho pacientes, 37% mujeres, con 376 intervenciones quirúrgicas (296 procedimientos intestinales y 80 anorrectales). El seguimiento medio de la cohorte fue de 106±64 meses. El lapso entre el diagnóstico y la primera cirugía fue <5 años en el 53,3%. En la segunda década del estudio hubo menos pacientes operados (73 frente a 48) y un menor número de intervenciones por paciente (2,7 frente a 2,0). La proporción entre cirugía electiva y urgente se revirtió en la segunda década, observándose un aumento de la cirugía laparoscópica (70% vs. 8%) junto con una disminución de la morbilidad postoperatoria mayor (Clavien-Dindo≥IIIa) (20% vs 8.4%). Se realizó una proctocolectomía restauradora a 80 pacientes, con un fracaso al año del 5% pero del 23,7% a largo plazo. Treinta y siete pacientes requirieron cirugía anorrectal, de los cuales 26 (71%) fueron intervenciones seriadas, la mayoría por complicaciones sépticas de los reservorios.
ConclusionesEl número de colectomías y de intervenciones por paciente disminuyó en la última década, a la vez que hubo mejorías en la morbilidad y el abordaje quirúrgico. La necesidad de cirugías secuenciales y de una vigilancia instrumental activa a largo plazo por el posible deterioro funcional constituye una importante carga clínica.
Ulcerative colitis (UC) is a chronic inflammatory disease affecting the rectal and, to a various extent, the colonic mucosa. The goal of treatment is to achieve and sustain clinical remission, as confirmed by disappearance of clinical symptoms, and endoscopic mucosal healing.1,2 Most patients with UC have a mild–moderate course, generally most active at diagnosis, however, between 25 and 50% of patients require UC-related hospitalization at some point during disease course.2
Although most UC patients can be treated effectively by a wide variety of medications, in cases in which medical therapies fail or there are severe acute abdominal symptoms, elective or emergent surgical intervention may be necessary. Nevertheless, the cumulative risk of surgery appears to be decreasing over time.3,4
When emergent surgery is required, subtotal colectomy with temporary end ileostomy and rectosigmoid preservation, is advised. The most frequently performed elective intervention is restorative proctocolectomy with ileal pouch-anal anastomosis (IPAA).
The colectomy rate and the results of surgery in UC, elective or urgent, is highly variable according to the countries3–6 and according to whether the studies are population-based,5 hospital-based, or come from reference institutions specialized in IBD.6 The incidence and need for anal surgery in UC are less well documented. The origin of abscesses and fistulas can be related to medical or mucosal complications of UC or its treatment.
In this study, we aimed to analyze the evolution, throughout these first 20 years of the century, of the clinical burden of UC surgery in an exclusively regional tertiary referral Hospital in a southern European country, as well as whether the indications, timing, type and results obtained comply with quality standards.
MethodsRetrospective and observational analysis of a series of consecutive patients who underwent surgery for ulcerative colitis and who required hospital admission in the period from January 1, 2000 to December 31, 2020.
-Setting: This is a University Hospital with a reference population of 730,000 inhabitants.
-Study population: All patients who required intestinal or anorectal surgery for UC were included. Patient records were identified after retrospective review of the Minimum Basic Data Set (a clinical-administrative database of the Spanish National Health Service) of our institution complemented with a monographic maintained database of surgical activity of the Colorectal Surgery Unit.
The collected data included sex, age at diagnosis, age at first surgical intervention (anal or intestinal), preoperative received medical therapies and total cumulative prescribed drugs, time interval between diagnosis and surgery, surgery dates and time, indications for intestinal surgery, surgical methods and surgical approaches (open or laparoscopic), early postoperative surgical morbi-mortality (according to Clavien-Dindo classification),7 last follow up and survival condition.
Long term follow-up, and missing data completion, was completed through a review of patients charts and medical records. The last follow-up date was the latest patient visit or the date of patient death.
For a more detailed analysis of the chronological changes, we divided the study period into two parts of 11 and 10 years each.
- Definition of disease extent and emergency/elective surgery: Montreal classification at preoperative stage as well as Mayo endoscopic subscore were registered. Medications before and after surgery included 5-ASA or sulfasalazine, systemic steroids, immunomodulators (IMMs), calcineurin inhibitors and biological agents.
Emerging operations included acute severe flare related complication (i.e., colonic hemorrhage, perforation or toxic megacolon) or refractory acute severe colitis. Elective procedures included continuous or intermittent chronic refractory UC, colorectal dysplasia (no endoscopically manageable) or carcinoma and restorative or completion surgeries after previous emergency procedures. Restorative surgery (e.g., ileoanal pouch anastomosis – IPAA) was conducted in one, two or three stages. In some patients (due to advanced age, comorbidities or patient's preference) only completion proctectomy was performed resulting in a permanent stoma. Furthermore, ileorectal anastomosis was also considered in selected individual patients with no rectal inflammatory activity or mild proctitis.
-Among the patients who underwent an IPAA, the following specific results were analyzed: fistulas related to the pouch or anastomosis, refractory or rapidly relapsing form of pouchitis, symptomatic inflammation of the distal cuff (e.g., cuffitis), rate of revisional surgery (including redo-pouch) and pouch failure, defined as the creation of a permanent end ileostomy with or without pouch excision.
IPAA survival was estimated as time from pouch creation to pouch failure event.
-Statistical analysis: Continuous quantitative variables are provided as the median and range, since a significant number of variables failed to show a normal distribution. The Shapiro–Wilk test and graphical methods were used to check the normality of quantitative variables. Qualitative variables are expressed as their relative and absolute frequencies. Quantitative variables were compared using Student's T-test for normal distribution and Mann–Whitney U test for non-parametric. For categorical variables, comparison of frequencies was made by the chi-square test for both parametric and non-parametric distribution. Pouch survival analysis was performed using the multiple decrement model for competing risks,8 considering the main event outcome (i.e., pouch survival vs. failure) along with competing secondary events risks (i.e., patient death prior to potential pouch failure) and influence of confounding factors as chronic active medical therapy for pouch inflammation or sepsis. This model estimated the accrual incidence for pouch failure and patient death, during study follow-up period. The Pepe–Mori test was performed to compare pouch failure accrual incidence according to defined confounding variables. Level of significance was set at 5%.
The data were recorded using Microsoft Excel v.21 (Microsoft Office 365, 2021) and all statistical analyses were performed using STATA version 16 (StataCorp, College Station, TX 77845, USA).
The study was reviewed and approved by our institution ethics committee.
Results-Demographic characteristics: During the study period, a total of 128 patients, 37% female, underwent 376 surgical interventions that required hospital admission, of which 296 were intestinal procedures and 80 were anorectal. The median postoperative follow-up was 118±84 months. The mean age at UC diagnosis was 38±15 years and the mean age at the time of first surgical intervention was 46.5±15.4 years, with a mean time-to-surgery from UC diagnosis of less 5 years in 53% of the entire cohort. Additional demographic and preoperative medical treatment data are summarized in Table 1.
Demographic characteristics and preoperative treatment of UC patients study population.
| Sex | Male: 80 (62.7%); female: 48 (37.3%) |
| Age at diagnosis of UC | 38.18 (±15.46) years |
| Age at first abdominal surgery | 46.53 (±16.19) years |
| Interval diagnosis of CU – 1st abdominal surgery (n=121): | |
| <5 years | 65 (53.32%) |
| 5–10 years | 16 (13.33) |
| >10 years | 40 (33.33%) |
| Previous CU medical treatment: | |
| Cumulative medication* | 2.26 (±0.89) drugs |
| Preoperative systemic corticosteroids therapy (oral or iv.). | 66 (55%) |
| Preoperative biologic therapy | 28 (23.33%) |
| 1st anal or abdominal surgery-year distribution (n=128) | |
| 2000–2010 | 78 (61%) |
| 2011–2020 | 50 (39%) |
| Median follow-up& | 149.5 (±82.12) months |
-Chronological changes in intestinal surgery: As can be seen in Table 2, in the second part of the study there were fewer operated patients (73 vs. 48, respectively) as well as the total number of interventions and the number of operations per patient (2.7 vs. 2.0, respectively). The proportion of elective and urgent surgery was reversed in the second decade, with emergent surgery being more frequent in this decade, all in line with the difference in surgical indications between both decades. Obviously, the use of biologicals increased over time. However, the most frequent first intestinal surgery was subtotal colectomy with end-ileostomy (50.8%), with no differences between the two decades. Surgery with and without intention of intestinal reconstruction (i.e., ileoanal or ileorectal anastomosis) was performed in 70% and 30% of the patients, respectively, without appreciable differences between both periods. The increase in laparoscopic surgery and the decrease in major postoperative complications can also be observed (Clavien-Dindo III–IV).
Clinical and surgical characteristics or CU patients undergoing intestinal surgery.
| Group 1(2000–2010) | Group 2(2011–2020) | Total | p-Value | |
|---|---|---|---|---|
| Number of patients (n) | 73 | 48 | 121 | p=0.0013 |
| Total number of abdominal surgical procedures (absolute; n) | 198 | 98 | 296 | p<0.001 |
| Patient-abdominal surgical procedure ratio (“n” surgical procedures – patient): | ||||
| 1 | 13 (17.8%) | 16 (33.3%) | 29 | p=0.435 |
| 2–3 | 42 (57.5%) | 30 (62.5%) | 72 | p=0.0019 |
| 4–5 | 18 (24.6%) | 1 (4.2%) | 20 | p=0.003 |
| First abdominal surgery: | ||||
| Elective surgery | 44 (60.3%) | 19 (39.6%) | 63 | p<0.001 |
| Emergency surgery | 28 (38.4%) | 28 (58.3%) | 56 | |
| (Missing data n=2) | ||||
| Pre-surgical cumulative CU medical treatment (X¯ (SD)) | 2.04 (0.73) | 2.54 (0.87) | 2.31 (0.29) | p=0.0021 |
| Pre-surgical systemic biological treatment: | ||||
| Yes | 4 (5.5%) | 25 (52.1%) | 29 | p<0.0001 |
| No | 69 (94.5%) | 23 (47.9%) | 92 | |
| First abdominal surgery indication: | ||||
| Acute severe “fulminant” colitis | 25 (34.7%) | 21 (44.4%) | 46 | p=0.28 |
| Chronic UC or intolerance to medical therapy | 32 (44.4%) | 15 (32.1%) | 47 | |
| Dysplasia or colorectal adenocarcinoma | 13 (18%) | 11 (23.3%) | 24 | |
| Procedure complication (i.e. endoscopic complication)a | 2 (2.7%) | – | 2 | |
| First abdominal surgical procedure: | ||||
| CT+end ileostomy | 39 (53.4%) | 22 (45.8%) | 61 | p=0.68 |
| CT+ileorectal anastomosis | 4 (5.5%) | 5 (10.4%) | 9 | |
| PCT+end ileostomy | 2 (2.7%) | 3 (6.3%) | 5 | |
| PCT+IPAA (±diverting ileostomy) | 23 (31.5%) | 14 (29.2%) | 37 | |
| Segmental colon or rectal resection | 5 (6.8%) | 4 (8.3%) | 9 | |
| First abdominal surgical procedure approach: | ||||
| Laparotomy | 60 (82.2%) | 17 (35.4%) | 77 | p<0.0001 |
| Laparoscopy | 13 (17.8%) | 31 (64.6%) | 44 | |
| First surgical procedure morbidity–mortality (Clavien-Dindo)b: | ||||
| No surgical morbidity (CD 0) | 15 (20.5%) | 21 (43.7%) | 36 | p=0.16 |
| Minor surgical morbidity (CD I–II) | 37 (50.7%) | 21 (43.7%) | 58 | p=0.003 |
| Major surgical morbidity (CD IIIa–IV) | 20 (27.4%) | 3 (6.3%) | 23 | p<0.0001 |
| Early-postoperative mortality (CD V) | 1 (1.4%) | 1 (2.1%) | 2 | p=NA |
| (Missing data n=2) | ||||
| Follow-up (months) (X¯ (SD)) | 160.47 (62.15) | 56.81 (17.21) | 108.64 (63.99) | |
CT: total colectomy; PCT: panproctocolectomy.
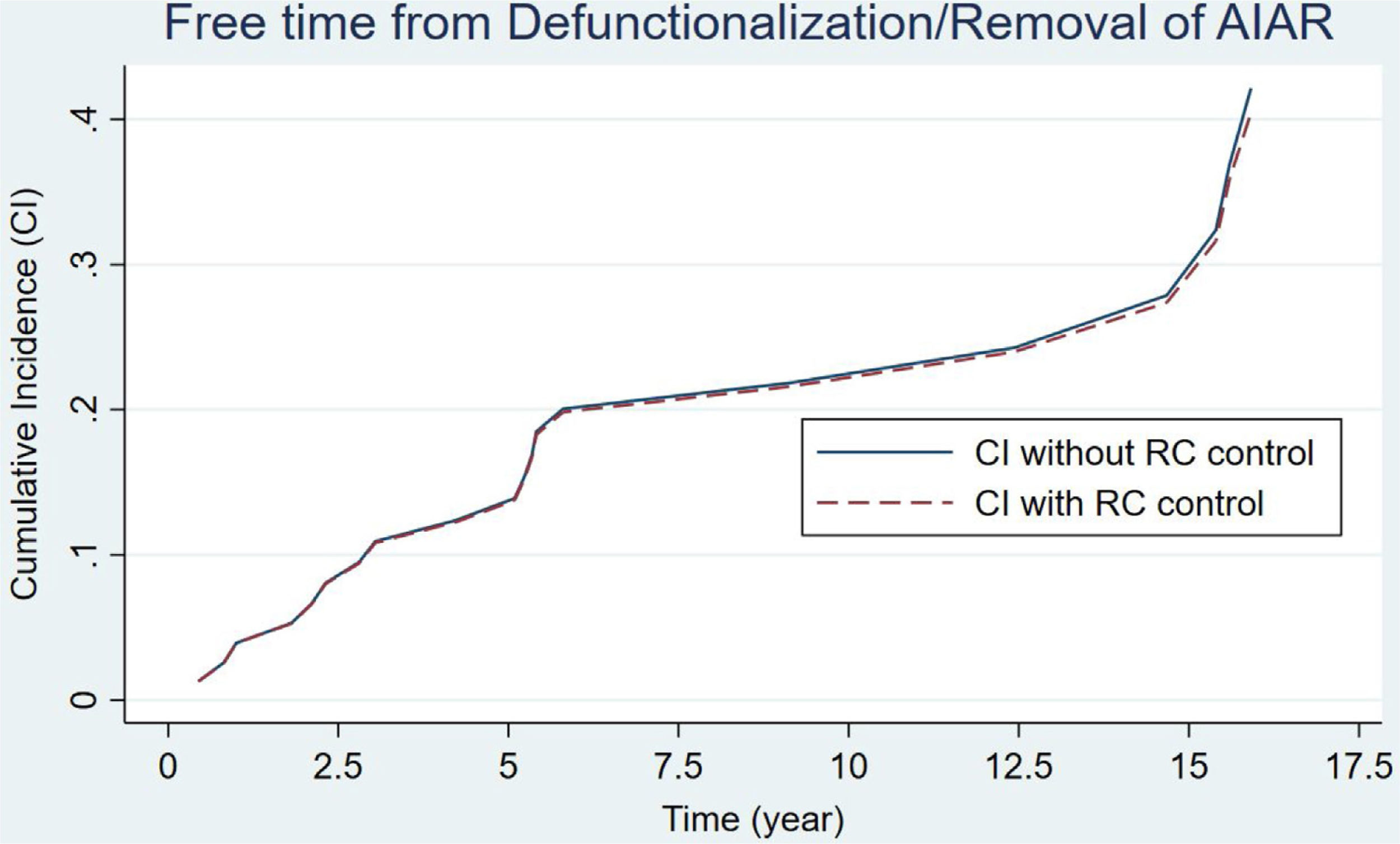
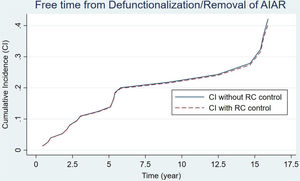
An IPAA was performed in 80 patients. Table 3 presents the main data related to this intervention. As we can see, there was a decrease in the number of IPAAs performed in the second period of the study (a mean of 5 and 2.6 IPAA's procedures per year, respectively). There were hardly any variations regarding the surgical stages, reservoir configuration or type of anastomosis. The same table includes the data regarding the causes of reservoir failures and related reoperations. Pouch dysfunction, associated with refractory pouchitis or cuffitis, and late pelvic sepsis (Crohn's like condition) were the main causes of pouch failure in both periods. 13 pouch excisions with end ileostomy and 10 diverting ileostomies leaving the pouch in situ were performed for pouch failures. At the end of the study, 61 patients (76.2%) had a functional reservoir without the need for a stoma. Estimation of pouch failure accrued incidence curves during follow-up showed no appreciable differences between the two groups, considering the potential influence of patient death (Fig. 1) (p=0.178). IPAA failure risk at 5, 10 and 15 years was 13%, 21% and 25% respectively. Furthermore, no significant differences were found when considering potential confounding factors for IPAA failure (Pepe–Mori test p=0.414).
Performed IPAA (ileo pouch-anal anastomosis) clinical and surgical characteristics.
| Group 1(2000–2010) | Group 2(2011–2020) | Total | p-Value | |
|---|---|---|---|---|
| Total number of IPAA (absolute, n) | 54 (67%) | 26 (33%) | 80 | p<0.0001 |
| IPAA surgery schedulea: | ||||
| One-stage surgery | 3 (5.6%) | – | 3 | p=0.025 |
| Classical two-stage surgery | 12 (22.2%) | 14 (53.8%) | 26 | p=0.583 |
| Modified two-stage surgery | 8 (14.8%) | – | 8 | p=0.001 |
| Three-stage surgery | 31 (57.4%) | 12 (46.2%) | 43 | p=0.001 |
| Pouch configuration: | ||||
| J-pouch | 53 (98.1%) | 24 (92.3%) | 77 (96%) | p=0.19 |
| S-pouch | 1 (1.9%) | 2 (7.7%) | 3 (4%) | |
| Type of IPAA anastomosis: | ||||
| Stapled | 53 (98.1%) | 22 (84.6%) | 75 | p=0.064 |
| Handsewn | 1 (1.9%) | 4 (15.4%) | 5 | |
| Chronic cuffitis | 8 (14.8%) | 8 (30.8%) | 16 (20%) | p=0,19 |
| Chronic reservoritis | 11 (20.3%) | 7 (26%) | 18 (23.6%) | p=0.17 |
| Actual patients with active medical therapy | 7 (13%) | 10 (38.5%) | 17 (22%) | p=0.31 |
| Topical therapy (i.e. 5-ASA or corticosteroids) | 3 | 4 | 8 | |
| Biologic drugs | 4 | 4 | 8 | |
| Antibiotic cycles | – | 1 | 1 | |
| IPAA failure related reintervention (n=19 patients): | p=0.77 | |||
| Exclusive pouch defunctioning | 5 | 1 | 6 (7.5%) | |
| Pouch defunctioning+IPAA excision | 3 | 1 | 4 (5%) | |
| Exclusive IPAA excision | 6 | 3 | 9 (11.2%) | |
| Indication for IPAA failure related re-intervention (n=19 patients with 23 procedures): | p=0.88 | |||
| Refractory chronic reservoritis or cuffitis | 7 | 3 | 10 | |
| Pouch disfunction | 3 | 2 | 5 | |
| Early pelvic sepsis (<1 year) | 2 | – | 2 | |
| Late pelvic sepsis (>1 year) | 3 | 2 | 5 | |
| Pouch dysplasia or carcinoma | 1 | – | 1 | |
| Actual status: | ||||
| Alive with no stoma | ||||
| Alive with stoma | 39 (66.7%) | 18 (69.3%) | 57 | |
| Death | 10 (24.1%) | 6 (23.1%) | 16 | |
| With functioning IPAA | 4 (7.4%) | – | 4 | |
| Non-functioning IPAA | 3 (5.6%) | – | 3 | |
IPAA surgery schedule: “classical two-stage surgery” refers to initial panproctocolectomy with IPAA and diverting ileostomy with subsequent transit restoration (i.e. ileostomy closure). “Modified two-stage surgery” refers to initial total colectomy with end-ileostomy and subsequent completion proctectomy and IPAA without diverting ileostomy.
Curves for the estimation of pouch failure accrued incidence based on the multiple decrement model for competing risks. Patient death prior to pouch failure was considered a potential competing event during follow-up for the estimation of IPAA survival. Two different curves are depicted, continuous linear curve (not controlled according to the competing event) and the discontinuous linear curve (controlled). No significant difference was found between both incidence curves (p=0.078). The Pepe–Mori test showed no significant influence of potential confounding variables (i.e. active medical therapy) (p=0.414).
-During the follow-up 18 (15%) patients died, of whom 6 died due to disease-related complication (i.e., 2 major postoperative complications from severe acute colitis and 4 local recurrence or disseminated colorectal cancer). Only 1 patient was lost in follow-up.
-Chronological changes in anal surgery: Surgical data of patients with anorectal surgery anal is summarized in Table 4. A total of 37 patients required 80 procedures, with a mean of 2.3 and 1.8 interventions per patient during the first and second decade of the study respectively. This endorses the fact that most anal surgical procedures were pouch related or pouch-related surgeries.
Surgical characteristics of UC patients undergoing anoperineal surgery.
| Group 1 | Group 2 | Total | |
|---|---|---|---|
| (2000–2010) | (2011–2020) | ||
| Number of patients (n) | 24 | 13 | 37 |
| Number of anoperineal surgical procedures: | |||
| Total and per-patient (in brackets) | 56 (2.3) | 24 (1.8) | 80 |
| Patient-anal surgical procedure ratio (“n” surgical procedures – patient): | |||
| 1 | 8 (33.3%) | 7 (53.8%) | 15 |
| 2–3 | 11 (45.8%) | 5 (38.5%) | 16 |
| 4–5 | 5 (20.8%) | 1 (7.7%) | 6 |
| Anal surgery timing in UC patientsa: | |||
| Prior to abdominal surgery | 13 (54.2%) | 2 (15.4%) | 15 |
| Posterior to first abdominal surgery | 11 (45.8%) | 11 (84.6%) | 22 |
| Indication for anal surgery (primary surgery and additional procedures)b: | |||
| Perianal fistula with or without local sepsis (abscess) | 22 (64.7%) | 10 (58.8%) | 32 |
| Pouch-vaginal/pouch-perineal fistula | 12 (35.3%) | 8 (47.2%) | 20 |
| Anoperineal surgical procedures (total number)c: | |||
| Surgical drainage (± seton placement) | 39 (69.6%) | 17 (70.8%) | 56 |
| Fistulectomy/fistulotomy | 8 (14.4%) | 1 (4.17%) | 9 |
| FLAP procedures | 8 (14.4%) | 5 (20.8%) | 13 |
| Gracilis muscle transposition | 1 (1.8%) | 0 | 1 |
| Biological glues or fistula plug. | 0 | 1 (4.17%) | 1 |
Anal surgery timing was considered as the timing of first anoperineal surgery in relation to first abdominal surgery.
During the 20 years covered by this study, an average of 5–6 new patients per year underwent intestinal surgery for presenting UC, although each patient required an average of 2–3 surgical interventions directly related to the disease or its surgical treatment, without counting on other possible surgeries aimed at solving complications or postoperative sequelae (i.e., eventrations, intestinal obstruction, etc.). In addition, 1–2 patients a year required hospital admission for anal or perianal surgeries, requiring an average of 2–3 anal/perianal interventions for each patient, related to perianal abscesses in the first period of the study or, especially in the second period of the study, related to septic complications derived from IPAA procedures.
On the other hand, we observed a significant decrease over the time in the rate of colectomy between the two periods in which we divided the study. This same trend has been verified more robustly in population studies.1,3–6 The reported colectomy rate has been highly variable over time and geographic location. In retrospective population-based studies carried out in the Scandinavian countries in the 90s, a high cumulative rate of more than 22% after 10 years of UC diagnosis were described.9 However, more recent studies revealed considerably lower rates of colectomy.4,6 The recent Swiss Inflammatory Bowel Disease Cohort Study showed a crude colectomy rate of 9.2% and a 20-year cumulative rate of 14.4%.5Although the colectomy rate has traditionally been higher in northern Europe than in southern Europe, these figures seem to be leveling off5 and yet a notable difference persists with the reported rate in Asia with a 10-year cumulative rate of less than 3%.10
The GETECCU (Grupo Español de Trabajo para la Enfermedad de Crohn y Colitis Ulcerosa) is currently carrying out a prospective study on the incidence and need for population-based healthcare resources of IBD in Spain (https://geteccu.org/). There are various population-based regional studies in Spain that estimate an annual crude incidence for UC of 9.1 per 105 inhabitants,11 which extrapolating these data to our reference population would indicate a mean crude incidence of colectomy of 9–10% in the 2000–2010 period of the study and 6–7% in the 2011–2020 period. However, it must be considered that these data are nothing more than a crude estimate based on retrospective regional-based data.
The relationship between elective and emergency surgery reversed in the second decade of the study. Consequently, the indication to perform a colectomy changed over time, observing a proportional decrease in the need for colectomy due to steroid dependent or medical refractory disease and a higher relative frequency of colectomies performed for dysplasia/adenocarcinoma, acute severe colitis and intolerance to medical treatment. This trend is consistent with other studies, pointing out that the more frequent and earlier use of immunomodulators and biologics seems to reduce the need for long-term surgery.2,3,5 On the other hand, an early and rapid step-up approach could select patients who will require a colectomy with shorter period of time lost with futile medical therapies.2
Postoperative mortality in the present series was 1.6%, with one death in each decade. Early major postoperative morbidity (i.e., Clavien-Dindo>III) after the first bowel intervention decreased considerably, from an incidence of 28% in the first decade to 9% in the second. These figures are similar (first decade) or compare favorably (second decade) to others published.12,13 This improvement can be explained by various factors, among which is the progressive increase in minimally invasive surgery, the implementation of preoperative pre-habitation programs and postoperative multimodal rehabilitation, obtaining the AECP (Spanish Association of Coloproctology) accreditation and GETECCU certification, and especially the creation of a multidisciplinary team for the evaluation, care and monitoring of patients with IBD.
In most of the operated patients, a subsequent reconstruction of the intestinal transit was attempted, with IPAA being the most widely used procedure for this. However, the number of IPAAs performed has been decreasing over time, coinciding with the decrease in the need for colectomies. We also observed a slight relative increase in reconstruction by ileorectal anastomosis in the second decade compared to the first. At the end of the study, of the 93 living patients, 78% were free of a stoma, including 61 of the 80 patients who underwent an IPAA.
Most of the late complications following elective surgery for UC are related to reconstructive procedures, mainly IPAA. The incidence of symptomatic cuffitis was higher in the second decade of the study. Interestingly, the greater use of laparoscopy has been related to a long retained rectal cuff, probably because cross-stapling of the anal stump remains challenging with current laparoscopic staplers; thus, avoiding the double-stapling anastomotic technique would be desirable.14 The number of chronic or relapsing pouchitis diagnosed in the second decade was higher than in the first, which can be explained not only by the longer follow-up monitoring of pouches but also by a proactive attitude to monitor inflammation and surveillance of the reservoirs recently established. As a result of the above, a third of the patients with an IPAA were under active medical treatment during the second decade.
Pouch failure can be defined as the need for pouch excision or an indefinite desfunctioning ileostomy. We found 5% of reservoir failures in the first year associated with early postoperative pelvic sepsis. However, pouch survival analysis, using the multiple decrement model for competing risks, showed an IPAA failure risk at 5, 10 and 15 years was 13%, 21% and 25% respectively. Early failures are in line with those reported in the literature, but the accumulated failure is higher than 10–15% reported from reference institutions with a large volume of cases.12,13 Late failures are related to pouch dysfunction, mechanical or inflammatory, Crohn's conversion or Crohn's disease-like condition. In the present series, 19 patients required 23 IPAA failure related re-interventions. In addition, a significant number of patients required diagnostic and therapeutic endoscopies and explorations under anesthesia (e.g., dilatations of anastomotic strictures) that we did not collect because they did not require hospital admission.
There are very few recent publications on ulcerative colitis surgery in our country.15 Experience, even in tertiary centers, is scarce given the low number of new patients operated on annually and this affects learning in its management and the allocation of resources. On the other hand, the idiosyncrasy of our health system makes it difficult to refer these patients to referral centers, which in turn are non-existent or difficult to identify, as is the case in neighboring countries. This trend will increase given that the need for colectomy has decreased in parallel with the increase in the use of biological drugs, although it is still premature to know whether or not we will see a rebound in colectomy rates in the future or a decrease or increase in the number of dysplasia or colorectal cancer.
In any case, these are patients who require several surgical interventions for the same process and who, once operated on, require frequent instrumental and active surveillance, which entails a significant clinical burden. If experience is what makes us experts, surgery for ulcerative colitis will become a challenge if we want to maintain low morbidity and mortality and obtain good functional results with reconstructive surgery.
Conflict of interestsThe authors declare that they have no conflict of interest.