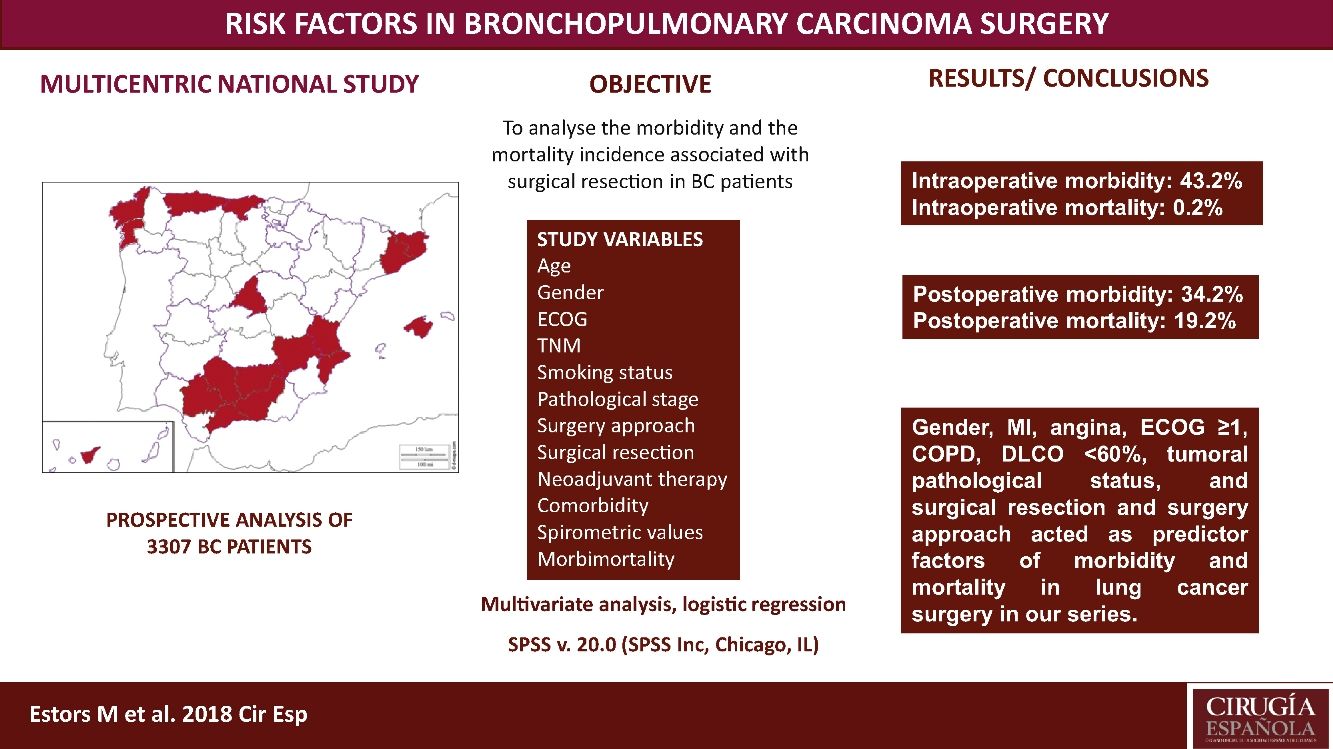
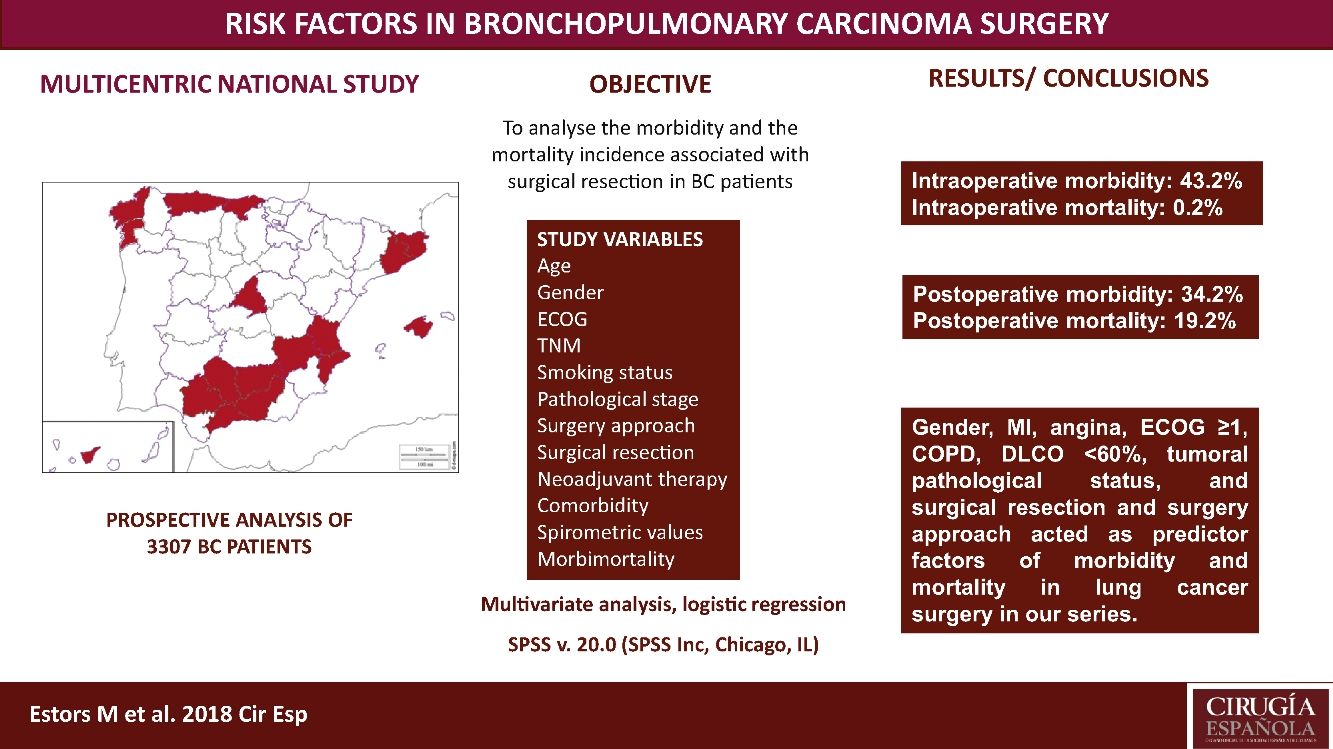
The most suitable treatment in most early-stage lung cancer patients is surgical resection. Despite previously assessing each patient's status being relevant to detect possible complications inherent to surgery, no consensus has been reached on which factors are “high risk” in such patients. Our study aimed to analyze the morbidity and the mortality incidence associated with this surgery in our setting with a multicentre study and to detect risk parameters.
MethodsA prospective analysis study with 3307 patients operated for bronchopulmonary carcinoma in 24 hospitals. Study variables were age, TNM, gender, stage, smoking habit, surgery approach, surgical resection, ECOG, neoadjuvant therapy, comorbidity, spirometric values, and intraoperative and postoperative morbidity and mortality. A multivariate logistic regression analysis of the morbidity and mortality predictor factors was done.
ResultsWe recorded 34.2% postoperative morbidity and 2.1% postoperative mortality. Gender, myocardial infarction, angina, ECOG ≥1, COPD, DLCO <60%, clinical pathological status, surgical resection and surgery approach were shown as morbidity and mortality predictor factors in lung cancer surgery in our series.
ConclusionsThe main variables to consider when assessing the lung cancer patients to undergo surgery are gender, myocardial infarction, angina, ECOG, COPD, DLCO, clinical pathological status, surgical resection and surgery approach.
El tratamiento más adecuado en la mayoría de los pacientes con cáncer de pulmón en estadio inicial es la resección quirúrgica. A pesar de evaluar anteriormente que el estado de cada paciente sea el adecuado para detectar posibles complicaciones inherentes a la intervención quirúrgica, no se ha alcanzado ningún consenso sobre los factores que son de «alto riesgo» en esos pacientes. Nuestro estudio tuvo como objetivo analizar la morbilidad y la incidencia de mortalidad asociada con esta intervención quirúrgica en nuestro entorno con un estudio multicéntrico y descubrir los parámetros de riesgo.
MétodosSe trata de un estudio de análisis prospectivo con 3.307 pacientes operados de carcinoma broncopulmonar en 24 hospitales. Las variables de estudio fueron edad, sistema TNM, sexo, estadio, tabaquismo, abordaje quirúrgico, resección quirúrgica, escala ECOG, tratamiento neoadyuvante, comorbilidad, valores espirométricos y morbimortalidad intra- y postoperatoria. Se realizó un análisis de regresión logística multivariante de los factores pronósticos de morbilidad y mortalidad.
ResultadosRegistramos el 34,2% de morbilidad postoperatoria y el 2,1% de mortalidad postoperatoria. Sexo, infarto de miocardio, angina, ECOG ≥1, EPOC, DLCO <60%, estado clínico patológico, resección quirúrgica y abordaje quirúrgico aparecieron como factores pronósticos de morbilidad y mortalidad en cirugía de cáncer de pulmón en nuestra serie.
ConclusionesLas principales variables que deben tenerse en cuenta al evaluar a pacientes con cáncer de pulmón para realizarles una intervención quirúrgica son sexo, infarto de miocardio, angina, ECOG, EPOC, DLCO, estado clínico patológico, resección quirúrgica y abordaje quirúrgico.
Nowadays, bronchopulmonary carcinoma (BC) is the main cause of death by cancer worldwide. In early stages, surgical resection is still the most suitable treatment for most patients, and assessing each patient's status is of utmost importance before operating and foreseeing the appearance of any complications inherent to this treatment.1 One in every five stage-I non-cell lung cancer (NSCLC) patients is considered to be at high risk for postoperative complications2 and mortality rates of 2.2% have been published for patients undergoing NSCLC resection.3
Several factors have been proved to increase surgical risk for BC resection and impact patient outcomes. They include among others: age, chronic obstructive lung diseases (COPD), former myocardial infarction (MI), smoking and/or carbon monoxide diffusing capacity (DLCO) under 60%.4,5 Different risk staging models have been recently developed trying to propose tools for chest surgeons.3,5,6 To date, however, no consensus has been reached about the definition of “high risks” in such patients, finding only weak recommendations in the literature. This makes selecting the plan to be followed in each patient a difficult task.
This study aimed to analyze the incidence of morbidity and mortality associated with BC resection in our setting by a multicentre study with patients from 24 Spanish departments of Thoracic, and to evaluate the risk factors that impact morbidity and mortality, which must be considered in order to plan the most suitable therapeutic strategies for each patient. This analysis also provides us with a valuable tool to assess the outcomes in our network.
Patients and MethodsPatientsThis study is a prospective analysis that includes 3307 patients with suspected NSCLC undergoing surgery with curative purpose in 24 Spanish departments of Thoracic Surgery from 1 June 2012 to 30 November 2014. Patients with unresectable disease undergoing exploratory thoracotomies were excluded. All the involved hospitals recorded the same data for the disease, treatment and patient progress, being cares reviewed by the individual surgeons. Later, an external data manager collected data in a uniform manner. An auditory ensured the proper collection of data. Histology different from BC were excluded. The European guidelines criteria were followed for the surgery, neoadjuvant therapy and posterior diagnosis.7
The study variables were chosen according to previous literature7,8 and they were defined in advance and agreed by all the authors: age, stage, gender, pathological stage, smoking habit, surgery approach, surgical resection, clinical status, neoadjuvant therapy (chemotherapy or chemo-radiotherapy), comorbidity, spirometric values (DLCO, forced expiratory volume in one second (FEV1), and intraoperative and postoperative morbidity and mortality. Postoperative morbidity and mortality was assessed by considering the first 30 days for the purpose. Tumors were staged using the 7ª Revision in the International System for Staging Lung Cancer (TNM). The Eastern Cooperative Oncology Group (ECOG) scale was used for classifying clinical status (Table 1).
Hospitals That Have Participated in the Study.
| Hospital General de Asturias |
| Hospital General de Valencia |
| Hospital de Jaén |
| Hospital U. N. S. de la Candelaria (Tenerife) |
| Hospital Clínico de Madrid |
| Hospital de Palma de Mallorca |
| Hospital Puerta de Hierro (Madrid) |
| Hospital de Albacete |
| Hospital Clínic (Barcelona) |
| Hospital Sagrado Corazón (Barcelona) |
| Hospital Virgen del Rocío (Sevilla) |
| Hospital Gregorio Marañón (Madrid) |
| Hospital Germans Trias i Pujol |
| Hospital U. Virgen de las Nieves (Granada) |
| Hospital Carlos Haya (Málaga) |
| Hospital de Alicante |
| Hospital de la Ribera (Alzira, Valencia) |
| Hospital La Paz (Madrid) |
| Hospital A Coruña |
| Hospital Islas Cies (Vigo) |
| Hospital Reina Sofía (Córdoba) |
| Hospital U. de Girona Dr. Josep Trueta |
| Hospital Universitario Donostia |
| Hospital de Santiago de Compostela |
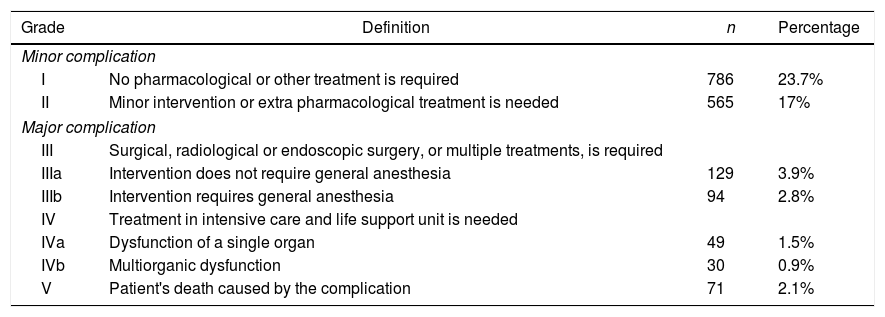
All complications collected in data had clinical transcendence. To analyze morbidity, the systematic classification of operative morbidity into five normalized groups (Table 2), proposed by Dindo et al.,9 was used. It helped to make an objective comparison of surgical procedures and patient series, and also between different surgeons and operating teams. Hemorrhage was considered a complication when it was >200ml/h with hemodynamic instability and/or a total of 1500ml, and air leak when it lasted at least 5 days. A possible occurrence of atelectasis was reviewed after surgery in all patients. Hypoxia was considered when <60 O2mmhg, angina pectoris in the absence of obstructive coronary artery disease and myocardial infarction when coronary artery was obstructed. All myocardial infarction were remote. Ischemic cardiomyopathy was considered when patients suffered simultaneously angina pectoris and myocardial infarction.
Grade Complication of Patients’ Morbidity.
| Grade | Definition | n | Percentage |
|---|---|---|---|
| Minor complication | |||
| I | No pharmacological or other treatment is required | 786 | 23.7% |
| II | Minor intervention or extra pharmacological treatment is needed | 565 | 17% |
| Major complication | |||
| III | Surgical, radiological or endoscopic surgery, or multiple treatments, is required | ||
| IIIa | Intervention does not require general anesthesia | 129 | 3.9% |
| IIIb | Intervention requires general anesthesia | 94 | 2.8% |
| IV | Treatment in intensive care and life support unit is needed | ||
| IVa | Dysfunction of a single organ | 49 | 1.5% |
| IVb | Multiorganic dysfunction | 30 | 0.9% |
| V | Patient's death caused by the complication | 71 | 2.1% |
Each patient was enrolled after acceptance of informed consent document. Study protocol was approved by the ethics committees of the Hospitals involved in the study and conducted in accordance with the Declaration of Helsinki.
Statistical AnalysisCategorical variables were expressed as absolute values and percentages (%). A multivariate logistics regression analysis was done to analyze the correlation between morbidity (including minor and major complications), mortality and clinical characteristics of patients (age, TNM, gender, pathological stage, smoking habit, surgery approach, surgical resection, ECOG, neoadjuvant therapy, comorbidity, spirometric values). The correlation between clinical characteristics and intraoperative mortality was not shown due to limited number of patients in this group (n=3). Variables showing a minimum significance threshold on the univariate analysis were included for the multivariate test. Level of significance was considered for a P-value of ≤.05. Statistical analysis was run with the SPSS package, v. 20.0 (SPSS Inc, Chicago, IL, USA).
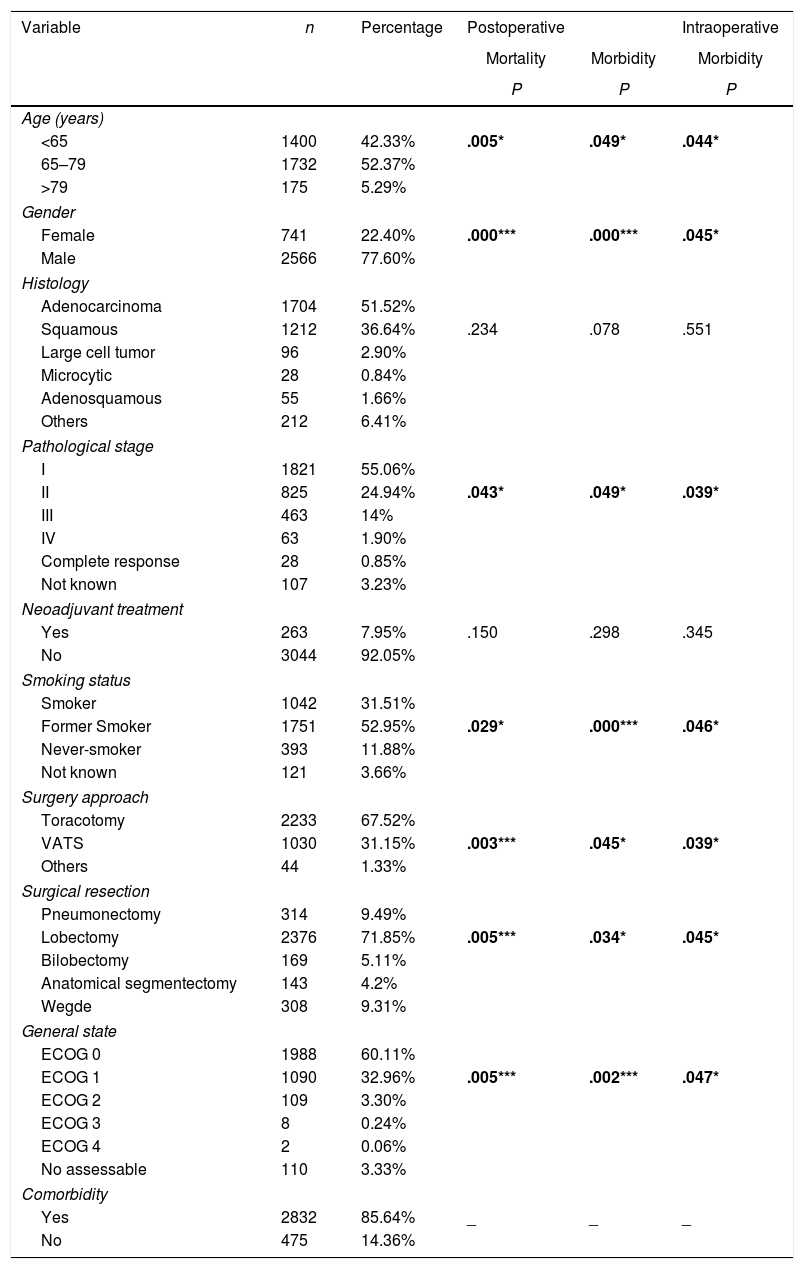
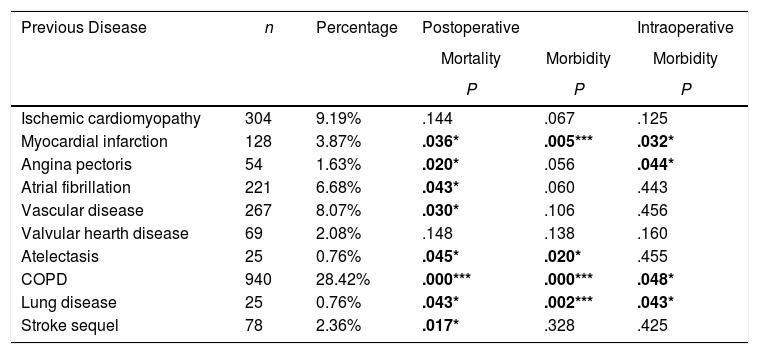
ResultsTable 3 offers the clinical-pathological characteristics of the patients included in our study. From 3307 cases, 77.6% were male, with a median age of 66 years. Adenocarcinoma was the most frequent histological subtype (55.1%). Distribution by stages was as follows: I: 55.1%, II: 24.9%, III: 14%, IV: 1.9%. A total of 263 patients (8%) received neoadjuvant treatment. The most frequent surgery approach was thoracotomy (67%). Lobectomy was the most widely performed surgical resection (71.8%). Regarding comorbidity (Table 4), COPD criteria (28.4%) was the most frequent, followed by ischemic cardiomyopathy (9.2%), vascular disease (8.1%) and atrial fibrillation (6.7%).
Univariate Analysis Results for the Association Between Patients’ Characteristics and Morbidity/mortality.
| Variable | n | Percentage | Postoperative | Intraoperative | |
|---|---|---|---|---|---|
| Mortality | Morbidity | Morbidity | |||
| P | P | P | |||
| Age (years) | |||||
| <65 | 1400 | 42.33% | .005* | .049* | .044* |
| 65–79 | 1732 | 52.37% | |||
| >79 | 175 | 5.29% | |||
| Gender | |||||
| Female | 741 | 22.40% | .000*** | .000*** | .045* |
| Male | 2566 | 77.60% | |||
| Histology | |||||
| Adenocarcinoma | 1704 | 51.52% | |||
| Squamous | 1212 | 36.64% | .234 | .078 | .551 |
| Large cell tumor | 96 | 2.90% | |||
| Microcytic | 28 | 0.84% | |||
| Adenosquamous | 55 | 1.66% | |||
| Others | 212 | 6.41% | |||
| Pathological stage | |||||
| I | 1821 | 55.06% | |||
| II | 825 | 24.94% | .043* | .049* | .039* |
| III | 463 | 14% | |||
| IV | 63 | 1.90% | |||
| Complete response | 28 | 0.85% | |||
| Not known | 107 | 3.23% | |||
| Neoadjuvant treatment | |||||
| Yes | 263 | 7.95% | .150 | .298 | .345 |
| No | 3044 | 92.05% | |||
| Smoking status | |||||
| Smoker | 1042 | 31.51% | |||
| Former Smoker | 1751 | 52.95% | .029* | .000*** | .046* |
| Never-smoker | 393 | 11.88% | |||
| Not known | 121 | 3.66% | |||
| Surgery approach | |||||
| Toracotomy | 2233 | 67.52% | |||
| VATS | 1030 | 31.15% | .003*** | .045* | .039* |
| Others | 44 | 1.33% | |||
| Surgical resection | |||||
| Pneumonectomy | 314 | 9.49% | |||
| Lobectomy | 2376 | 71.85% | .005*** | .034* | .045* |
| Bilobectomy | 169 | 5.11% | |||
| Anatomical segmentectomy | 143 | 4.2% | |||
| Wegde | 308 | 9.31% | |||
| General state | |||||
| ECOG 0 | 1988 | 60.11% | |||
| ECOG 1 | 1090 | 32.96% | .005*** | .002*** | .047* |
| ECOG 2 | 109 | 3.30% | |||
| ECOG 3 | 8 | 0.24% | |||
| ECOG 4 | 2 | 0.06% | |||
| No assessable | 110 | 3.33% | |||
| Comorbidity | |||||
| Yes | 2832 | 85.64% | _ | _ | _ |
| No | 475 | 14.36% | |||
Video-assisted-Thoracic Surgery (VATS).
The Eastern Cooperative Oncology Group scale (ECOG).
Univariate Analysis Results for the Association Between Patients’ Comorbidity and Morbidity/Mortality.
| Previous Disease | n | Percentage | Postoperative | Intraoperative | |
|---|---|---|---|---|---|
| Mortality | Morbidity | Morbidity | |||
| P | P | P | |||
| Ischemic cardiomyopathy | 304 | 9.19% | .144 | .067 | .125 |
| Myocardial infarction | 128 | 3.87% | .036* | .005*** | .032* |
| Angina pectoris | 54 | 1.63% | .020* | .056 | .044* |
| Atrial fibrillation | 221 | 6.68% | .043* | .060 | .443 |
| Vascular disease | 267 | 8.07% | .030* | .106 | .456 |
| Valvular hearth disease | 69 | 2.08% | .148 | .138 | .160 |
| Atelectasis | 25 | 0.76% | .045* | .020* | .455 |
| COPD | 940 | 28.42% | .000*** | .000*** | .048* |
| Lung disease | 25 | 0.76% | .043* | .002*** | .043* |
| Stroke sequel | 78 | 2.36% | .017* | .328 | .425 |
Chronic obstructive lung diseases (COPD).
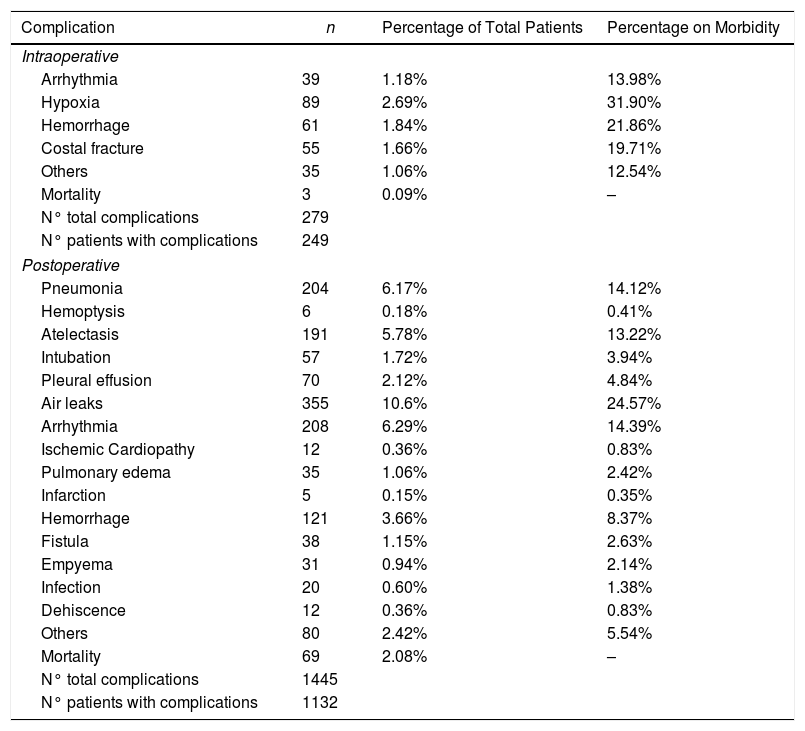
We recorded an intraoperative morbidity of 7.5%. We found 279 complications, being the most frequent hypoxia (<60 O2mmh) (2.7%), hemorrhage (by iatrogenic section of artery or vein) (1.8%) and costal fracture (1.7%). Postoperative morbidity was 34.2%, where air leaks (10.6%), arrhythmia (6.3%) and pneumonia (6.2%) stood out (Table 5). Overall, we found that the majority of patients’ complications were minor (78.4%; Table 2). Intraoperative mortality was 0.1%. 30-days postoperative mortality rate was 2.1% (69 patients). From them, 41 had undergone lobectomy, 19 pneumonectomy, 5 sublobar resection and 4 bilobectomy, which means a higher rate of deaths in pneumonectomy (6.1% pneumonectomy vs 1.7% lobectomy, 2.4% bilobectomy and 1.1% sublobar resection).
Patients’ Morbidity.
| Complication | n | Percentage of Total Patients | Percentage on Morbidity |
|---|---|---|---|
| Intraoperative | |||
| Arrhythmia | 39 | 1.18% | 13.98% |
| Hypoxia | 89 | 2.69% | 31.90% |
| Hemorrhage | 61 | 1.84% | 21.86% |
| Costal fracture | 55 | 1.66% | 19.71% |
| Others | 35 | 1.06% | 12.54% |
| Mortality | 3 | 0.09% | – |
| N° total complications | 279 | ||
| N° patients with complications | 249 | ||
| Postoperative | |||
| Pneumonia | 204 | 6.17% | 14.12% |
| Hemoptysis | 6 | 0.18% | 0.41% |
| Atelectasis | 191 | 5.78% | 13.22% |
| Intubation | 57 | 1.72% | 3.94% |
| Pleural effusion | 70 | 2.12% | 4.84% |
| Air leaks | 355 | 10.6% | 24.57% |
| Arrhythmia | 208 | 6.29% | 14.39% |
| Ischemic Cardiopathy | 12 | 0.36% | 0.83% |
| Pulmonary edema | 35 | 1.06% | 2.42% |
| Infarction | 5 | 0.15% | 0.35% |
| Hemorrhage | 121 | 3.66% | 8.37% |
| Fistula | 38 | 1.15% | 2.63% |
| Empyema | 31 | 0.94% | 2.14% |
| Infection | 20 | 0.60% | 1.38% |
| Dehiscence | 12 | 0.36% | 0.83% |
| Others | 80 | 2.42% | 5.54% |
| Mortality | 69 | 2.08% | – |
| N° total complications | 1445 | ||
| N° patients with complications | 1132 | ||
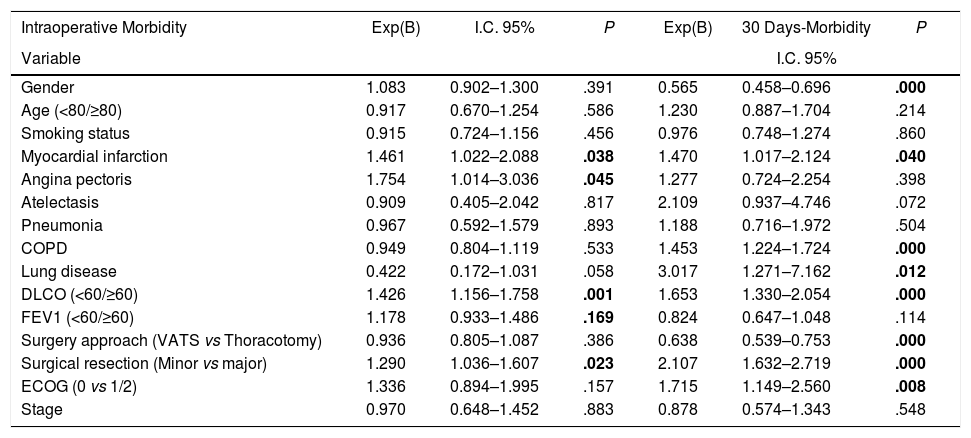
Univariate analysis of the association between patients’ characteristics and morbidity/mortality are shown in Tables 3 and 4. Regarding the pre-surgery factors that influenced intraoperative and postoperative morbidity and mortality (Table 6), the multivariate analysis show that those patients with a background of MI (OR 1.4; 95%CI: 1–2.1), angina (OR 1.7; 95%CI: 1–3), DLCO <60% (OR 1.4; 95%CI: 1.1–1.7) and major resection (OR 1.3; 95%CI: 1–1.6) presented a higher probability of intraoperative morbidity. The patients with a background of MI (OR 1.4; 95%CI: 1–2.1), COPD (OR 1.4; 95%CI: 1.2–1.7), lung disease (OR 3.1; 95%CI: 1.3–7.2), ECOG ≥2 (OR 1.7; 95%CI: 1.1–2.6), DLCO <60% (OR 1.6; 95%CI: 1.3–2.1) and major resection (OR 2.1; 95%CI: 1.6–2.7) presented a higher probability of postoperative morbidity. Females (OR 0.6; 95%CI: 0.4–0.7) and the patients undergoing for Video-assisted-Thoracic Surgery (VATS) (OR 0.6; 95%CI: 0.5–0.7) were less likely to present postoperative morbidity.
Multivariate Analysis for the Association Between Patients’ Variables and Morbidity/Mortality.
| Intraoperative Morbidity | Exp(B) | I.C. 95% | P | Exp(B) | 30 Days-Morbidity | P |
|---|---|---|---|---|---|---|
| Variable | I.C. 95% | |||||
| Gender | 1.083 | 0.902–1.300 | .391 | 0.565 | 0.458–0.696 | .000 |
| Age (<80/≥80) | 0.917 | 0.670–1.254 | .586 | 1.230 | 0.887–1.704 | .214 |
| Smoking status | 0.915 | 0.724–1.156 | .456 | 0.976 | 0.748–1.274 | .860 |
| Myocardial infarction | 1.461 | 1.022–2.088 | .038 | 1.470 | 1.017–2.124 | .040 |
| Angina pectoris | 1.754 | 1.014–3.036 | .045 | 1.277 | 0.724–2.254 | .398 |
| Atelectasis | 0.909 | 0.405–2.042 | .817 | 2.109 | 0.937–4.746 | .072 |
| Pneumonia | 0.967 | 0.592–1.579 | .893 | 1.188 | 0.716–1.972 | .504 |
| COPD | 0.949 | 0.804–1.119 | .533 | 1.453 | 1.224–1.724 | .000 |
| Lung disease | 0.422 | 0.172–1.031 | .058 | 3.017 | 1.271–7.162 | .012 |
| DLCO (<60/≥60) | 1.426 | 1.156–1.758 | .001 | 1.653 | 1.330–2.054 | .000 |
| FEV1 (<60/≥60) | 1.178 | 0.933–1.486 | .169 | 0.824 | 0.647–1.048 | .114 |
| Surgery approach (VATS vs Thoracotomy) | 0.936 | 0.805–1.087 | .386 | 0.638 | 0.539–0.753 | .000 |
| Surgical resection (Minor vs major) | 1.290 | 1.036–1.607 | .023 | 2.107 | 1.632–2.719 | .000 |
| ECOG (0 vs 1/2) | 1.336 | 0.894–1.995 | .157 | 1.715 | 1.149–2.560 | .008 |
| Stage | 0.970 | 0.648–1.452 | .883 | 0.878 | 0.574–1.343 | .548 |
| Intraoperative mortality | 30 days-mortality | |||||
|---|---|---|---|---|---|---|
| Gender | – | – | – | 0.180 | 0.042–0.770 | .021 |
| Age (<80/≥80) | – | – | – | 1.506 | 0.578–3.928 | .402 |
| Smoking status | – | – | – | 1.529 | 0.354–6.609 | .570 |
| Myocardial infarction | – | – | – | 2.121 | 0.858–5.244 | .103 |
| Angina pectoris | – | – | – | 4.009 | 1.286–12.497 | .017 |
| Atelectasis | – | – | – | 1.532 | 0.194–12.094 | .686 |
| Pneumonia | – | – | – | 2.895 | 0.979–8.562 | .055 |
| COPD | – | – | – | 2.124 | 1.246–3.621 | .006 |
| Lung disease | – | – | – | 2.964 | 0.564–15.578 | .199 |
| DLCO (<60/≥60) | – | – | – | 1.066 | 0.537–2.116 | .854 |
| FEV1 (<60/≥60) | – | – | – | 0.871 | 0.426–1.781 | .705 |
| Surgery approach (VATS vs Thoracotomy) | – | – | – | 0.307 | 0.138–0.682 | .004 |
| Surgical resection (Minor vs major) | – | – | – | 2.116 | 0.811–5.517 | .125 |
| Stage | – | – | – | 0.345 | 0.128–0.927 | .035 |
| ECOG (0 vs 1/2) | – | – | – | 2.910 | 1.258–6.731 | .013 |
| Stroke sequel | – | – | – | 3.378 | 1.259–9.064 | .016 |
| Vascular disease | – | – | – | 1.492 | 0.728–3.06 | .274 |
| Fibrillation | – | – | – | 1.549 | 0.678–3.535 | .299 |
Chronic obstructive lung diseases (COPD).
Carbon monoxide diffusing capacity (DLCO).
Forced expiratory volume in one second (FEV1).
Video-assisted-Thoracic Surgery (VATS).
The Eastern Cooperative Oncology Group scale (ECOG).
On the other hand, the patients with a background of angina (OR 4; 95%CI: 1.3–12.5), COPD (OR 2.1; 95%CI: 1.2–3.6), prior stroke with sequelae (OR 3.4; 95%CI: 1.2–9.1) and ECOG ≥1 (OR 2.9; 95%CI: 1.2–6.4) had a higher probability of postoperative mortality, while females (OR 0.2; 95%CI: 0.1–0.8), the patients operated for VATS (OR 0.3; 95%CI: 0.1–0.7), and stage I and II patients (OR 0.3; 95%CI: 0.1–0.9) presented a lower probability of postoperative mortality.
DiscussionSurgical resection continues to be the treatment of choice in early stages of NSCLC, and its results depend on both correct oncological assessments and careful overall perioperative selection, which prevent complications inherent to this treatment from appearing.1–3
In our series, we found an intraoperative morbidity of 7.5% and a postoperative morbidity within the first 30 days of 34.2%. Postoperative and intraoperative mortality were of 2.1% and 0.1%, respectively. These data agree with the figures described in the literature.2,3,10 The decrease in postoperative mortality found in latest studies was particularly significant when comparing to the Sociedad Española de Patología del Aparato Respiratorio (SEPAR) study reporting morbidity and mortality rates of 35.2% and 6.8%, respectively, for patients undergoing thoracotomy between 1993 and 1997.1 Several issues may explain this positive tender in quality of surgery for NSCLC, such as a better patient selection for surgery (thanks to the introduction of PET images within preoperatory clinical staging tools), and the implementation of perioperative programs of respiratory rehabilitation.
In our study, we found that surgical complications resulting from BC resection were mainly minor, highlighting air leaks (10.6%), which were more frequent than those found in other series.1 Cardiac arrhythmia was the most frequently observed complication behind persistent air leaks, and was also the most frequent postoperative medical complication. It is usually described in most similar studies, with rates ranging between 3.8 and 40% after lung cancer surgery.
One of the most important predictors of morbimortality after NSCLC surgical resection is patient clinical status before surgery.11 As expected, this variable was significantly statistical in our series in the patients with ECOG values above or equal to 1, who presented a higher probability of intra- and postoperative morbidity and mortality.
One of the most controversial questions is whether age is a limiting factor for lung surgery, specifically in patients aged over 80.12 In our series, octogenarian patients shown similar morbidity and mortality rates than other age groups. Many studies corroborate this finding,4,13,14 concluding that octogenarian patients can be submitted to lung resection. We ought not to forget that these patients are more fragile and we must always contemplate the sublobar surgery as an alternative, provided it is feasible, and by VATS since this approach is less aggressive.
In recent decades, the proportion of females diagnosed with BC has significantly increased in Spain1 probably related to social smoking habits. As previously reported in literature,5 in our series, females presented lower postoperative morbidity and mortality rates, probably due to lower comorbidity. As it is well-known, a high percentage of patients with BC present clinical COPD criteria, being it considered a risk for lung surgery.2 In our sample, 28.4% of the patients had COPD, whose presence has been statistically related with both mortality and postoperative morbidity. The structural lesions that this pathology causes increase the dead space, imbalanced ventilation-perfusion quotients, hemodynamic changes in lung capillaries and altered diffusion, which increase the efforts made to breath and can lead to respiratory failure, which is the main cause of death during lung resection surgery.15
Low FEV1 values are associated with increased cardiorespiratory morbidity and mortality.16,17 After performing a multivariate analysis in our sample, for which we dichotomised the FEV1 values into higher than or equal to 60% and into lower than 60% according to previous literature,18 we observed that this variable did not act as an independent risk factor for morbidity and mortality. However, as we mentioned, COPD has been statistically related to morbidity and mortality, and we should not forget that FEV1 is one of the clinical criteria used to define obstructive lung disease.
Establishing a cut off of 60% for DLCO, we found that DLCO acted as a risk factor for morbidity, but not for mortality. Preoperative DLCO <60 have been associated with 25% mortality and 40% morbidity in patients who underwent lung resection surgery. Low predicted DLCO values act as a predictor of cardiorespiratory complications, and of mortality even in patients with FEV1 that fall within normality limits. Brunelli et al.19 found a weak correlation between preoperative DLCO values and forced volume expired in the first second (FEV1%) values and systematic postoperative DLCO calculations, which improves predicting the lung resection risk. This is currently the reason why the guidelines of the American College of Chest Physicians8 and those of the European Respiratory Society and the European Society of Thoracic Surgeons20 recommend taking preoperative DLCO measurements in all candidate patients for lung resection surgery. In our study we did not find DLCO as an independent risk factor for mortality but it should be taken into account that DLCO is other of the clinical criteria used to define COPD.
Ischemic cardiomyopathy is a high risk factor for lung resection surgery with an incidence ranging between 11% and 17%. Its presence implies a 3% risk for cardiac complications.21,22 We obtained a similar incidence in our series. Those patients with a background of angina prior to surgery have a higher intraoperative morbidity probability. Similarly, those patients who have suffered MI before surgery present higher postoperative morbidity.
Video-assisted surgery appears to offer the similar results to conventional open approaches, and refers to the possibility of achieve optimal oncological resections.23,24 Several studies13,25,26 have suggested that performing lung resections by VATS in qualified centers is associated with lower morbidity. We coincide with these studies because in our series, the VATS surgical approach was statistically associated with lower morbidity. We also found lower postoperative mortality for those patients who underwent VATS, which also agrees with previous results.5,16
Sublobar resections have obtained excellent oncological results in tumors <2cm, and have shown a lower perioperative morbidity risk.27 The advantages of minor resections include maintaining lung function, a lower risk of perioperative morbidity and mortality, the possibility of patients tolerating future resections if a second primary lung tumor and/or recurring tumors appear/s, and a simple surgical procedure, plus the possibility of carrying it out by less invasive techniques. The current main disadvantage of such operations is the increased risk of loco-regional recurrence. It has been shown that 5-year survival rate for segmentectomy in tumors <3cm is similar to lobectomy.28 However wedge resection is correlated to worse survival.29
It is noteworthy that the pathological status of the patients in our series was also observed to act as a mortality predictor factor. Thus stages I and II patients presented a lower mortality probability. This may be due to the fact that surgical resection and surgery approach employed in early stages are less aggressive, and also because these patients received no neoadjuvant therapy, which could perhaps impact morbidity.
We acknowledge limitations to our analysis. From the different Hospitals that participated in the study, we are unable to assess the effects, if any, that the different surgeons, their work team or the hospital's volume have on the development of patients’ complications. Furthermore, we analyzed complications altogether, rather than evaluating individual complications separately. Finally, other variables not contemplated in the study could have a role in patients’ morbidity and mortality.
Despite these limitations, our study has several strengths. The large numbers of patients from different regions included in the study reflects the current practice patterns across the nation, and it may serve to guide surgeons in appropriate patient selection. Further, these findings may serve to stimulate the implementation of better perioperative protocols designed to reduce the incidence of postoperative complications among BC patients.
In summary, we found that MI, DLCO <60%, major resection, angina, COPD and lung disease are the comorbidities related to intraoperative and postoperative morbidity during BC surgery, causing mainly minor complications, being the most frequent air leaks, arrhythmia, pneumonitis and hypoxia. On the other hand, mortality appears to be related mainly to angina, prior stroke or ECOG ≥1.
ConclusionsWe recorded a postoperative morbidity and mortality of 34.2% and 2.1%, respectively. Gender, MI, angina, ECOG ≥1, COPD, DLCO <60%, tumoral pathological status, and surgical resection and surgery approach worked as predictor factors of morbidity and mortality in lung cancer surgery in our series.
Conflict of InterestNone declared.
FundingThis study was funded by a grant by Sociedad Española de Cirugía Torácica (SECT).
None declared.
Please cite this article as: Estors-Guerrero M, Lafuente-Sanchis A, Quero-Valenzuela F, Galbis-Carvajal JM, Crowley S, Carvajal Á, et al. Factores de riesgo para el desarrollo de complicaciones tras tratamiento quirúrgico del carcinoma broncopulmonar. Cir Esp. 2020;98:226–234.