A non-systematic review of the published scientific evidence has been carried out on the duration of empirical antibiotic treatment in surgical intra-abdominal infections (IIA) with effective focus control. Given the progressive increase in antibiotic resistance, it is urgent to have strategies to reduce the pressure on the microbiota. The American guidelines made by Mazuski et al. of 20171, as the central axis in the recommendations of the duration of empirical antibiotic treatment in intra-abdominal infections with control of the focus and a bibliographic search of all the articles that contained the keywords in Pubmed and Google Scholar is added. 21 articles referring to the duration of empirical antibiotic treatment in intra-abdominal infection with control of the focus are collected. With the American guidelines and these articles, a proposal is prepared for the duration of empirical antibiotic treatment in patients without risk factors between 24 and 72 h. And in those who present risk factors, it should be individualized with active monitoring every 24 h of fever, paralytic ileus and leukocytosis (FIL), before an early detection of complications or the need for changes in antibiotic treatment. Short treatments are just as effective as those of longer durations and are associated with fewer adverse effects, therefore, daily adjusting and reassessing the duration of empirical antibiotic treatment is essential for better practice.
El aumento progresivo de las resistencias antibióticas apremia el tener estrategias para disminuir la presión sobre la microbiota. La duración del tratamiento antibiótico empírico es variable, a pesar de las recomendaciones de las guías. Se ha realizado una revisión bibliográfica de la evidencia científica publicada sobre la duración del tratamiento antibiótico empírico en las infecciones intraabdominales (IIA) quirúrgicas con control de foco efectivo. Se analizan las guías americanas realizadas por Mazuski et al. de 20171, como eje central en las recomendaciones de la duración de tratamiento antibiótico empírico en infecciones intraabdominales con control del foco y se añade una búsqueda bibliográfica de todos los artículos que contuviesen las palabras claves en Pubmed y Google Scholar. Se recopilan 21 artículos referentes en la duración del tratamiento antibiótico empírico en la infección intraabdominal con control del foco. Con las guías americanas y estos artículos se ha elaborado una propuesta de duración del tratamiento antibiótico empírico en pacientes sin factores de riesgo entre 24 y 72 horas. Y en los que presentan factores de riesgo se habría de individualizar el mismo con monitorización activa cada 24 horas de fiebre, íleo paralítico y leucocitosis (FIL), ante una detección precoz de complicaciones o de necesidad de cambios en el espectro antibiótico. Los tratamientos cortos son igual de eficaces que los de duraciones más prolongadas y se asocian a menos tasa de efectos adversos, por tanto, ajustar y reevaluar diariamente la duración del tratamiento antibiótico empírico es fundamental para una mejor praxis.
Following a review of the published scientific evidence on the duration of empirical antibiotic treatment in surgical intra-abdominal infections with effective focus control commissioned by the 7 VINCat (Surveillance System of nosocomial infections in hospitals in Catalonia), it is recommended that this should be as limited as possible in patients without risk factors and evaluated individually in patients with risk factors. The reduction of the duration of empirical antibiotic treatment in this type of infection is based on scientific evidence that short courses of treatment are equally effective as longer courses and are associated with fewer adverse effects associated with the use of antibiotics. Thus, limiting the duration of antibiotic treatment is a useful strategy to reduce the deleterious effects of antimicrobials, both at the individual patient level (avoiding adverse effects derived from them) and at the collective level (avoiding the selection of resistant strains of bacteria, with the consequent reduction of antimicrobial resistance rates).
In this paper, a non-systematic review of the most relevant scientific articles related to the duration of antibiotic treatment in surgical intra-abdominal infections is carried out, reviewing the scientific evidence in this field.
MethodologyA review of the literature related to the duration of empirical antibiotic treatment in intra-abdominal infections with surgical control of the focus was carried out in Pubmed and Google Scholar sources, both in Spanish and English: Antibiotic therapy, antibiotic treatment, intra-abdominal sepsis, intra-abdominal infection, intra-abdominal infection, antimicrobial treatment's length, prophylactic antibiotics, brief antibiotic prophylaxis and abdominal surgery, antimicrobial stewardship).
Definitions used- 1
Type of infection: Surgically managed intra-abdominal infections were analysed, including: acute appendicitis, acute cholecystitis, acute diverticulitis, pancreatitis, mechanical bowel ischaemia and gastroduodenal perforation.
- 2
What is considered focus control? Complete, incomplete and no focus control
- a)
Complete focus control: a range of physical measures that eliminate the source of infection, limit contamination, block factors that facilitate persistent infection, and correct or control anatomical defects to restore physiological function. This is not limited to surgery, but includes other methods such as endoscopic or percutaneous. The following are considered as situations with control of the focus: exeresis of the affected organ, drainage or exteriorisation.
- b)
No focus control: no focus control manoeuvre has been necessary, e.g. acute diverticulitis with pericolonic phlegmon with no or minimal systemic repercussions.
- c)
Incomplete focus control: due to technical difficulty or surgery that cannot completely isolate the source of infection. This will be the case for biliary fistulas, duodenal stump dehiscences or oesophageal perforations.
- a)
In the guideline developed by Mazuski et al.1 in 2017, on behalf of the American Surgical Infection Society, the following recommendations are set out:
- -
Do not administer empirical antibiotics for severe acute or necrotising pancreatitis or acute uncomplicated sigmoid diverticulitis.
- -
Uncomplicated acute sigmoid diverticulitis.
- -
Do not extend prophylaxis beyond 24 h in case of intra-abdominal contamination.
- -
In uncomplicated intra-abdominal infection, with control of the focus, a duration of antibiotic treatment of less than 24 h is recommended. Thus, in the case of acute appendicitis, cholecystitis without perforation or segmental small bowel ischaemia without perforation, 24 h of treatment is recommended.
- -
In complicated intra-abdominal infection with adequate control of the focus, antibiotic therapy duration of 3–5 days is recommended.
- -
In the case of complicated intra-abdominal infection without control of the focus, the duration is left to the surgeon's choice, based on the resolution of fever, paralytic ileus and leukocytosis (FIL). In these cases, they recommend at least 7 days if bacteraemia is present and no recommendation if septic shock is present.
In these same guidelines, the authors list a series of variables related to patient comorbidity, severity and evolution of the infection, and microbiology; and consider that patients with 2 or more of these risk factors should not be treated with 24-h empirical antibiotic treatment alone, even if the infection is uncomplicated. In these patients, they suggest that the duration of antibiotic treatment should be based on the resolution of the FIL.
In conjunction with this guideline, an exhaustive review of the scientific literature found 21 articles with recommendations and justifications for the duration of empirical antibiotic treatment in intra-abdominal infection. The following table specifies the articles found and analysed that address the duration of treatment (Table 1).
Bibliography by year of articles dealing with duration of empirical antibiotic treatment in intra-abdominal infection.
| Year | Title | Journal | Author |
|---|---|---|---|
| 1980 | Leukocytosis at termination of antibiotic therapy: Its importance for intra-abdominal sepsis | Arch Surg | Lennard et al.2 |
| 1981 | Effect of prophylactic antibiotics in acute non-perforated appendicitis: a prospective, randomized, double-blind clinical study | Ann Surg | Busuttil et al.3 |
| 1982 | Implications of leukocytosis and fever at conclusion of antibiotic therapy for intra-abdominal sepsis | Ann Surg | Lennard et al.4 |
| 1983 | Acute non-perforating appendicitis. Efficacy of brief antibiotic prophylaxis | Arch Surg | Winslow et al.5 |
| 1989 | Antibiotic prophylaxis in acute non-perforated appendicitis. The Danish Multicenter Study Group III | Ann Surg | Bauer et al.6 |
| 1994 | Minimal antibiotic therapy after emergency abdominal surgery: A prospective study | BJS | Schein et al.7 |
| 1995 | Single-dose cefotetan or cefoxitin vs. multiple-dose cefoxitin as prophylaxis in patients undergoing appendectomy for acute non-perforated appendicitis | J Am Coll Surg | Liberman et al.8 |
| 2000 | Complicated appendicitis: Is there a minimum intravenous antibiotic requirement? A prospective randomised trial | Arch Surg | Taylor et al.9 |
| 2004 | The efficacy of postoperative oral antibiotics in appendicitis: A randomised prospective double-blinded study | Am Surg | Taylor et al.10 |
| 2005 | Optimum duration of prophylactic antibiotics in acute non-perforated appendicitis | Anz J Surg | Mui et al.11 |
| 2005 | Antibiotics vs. placebo for prevention of postoperative infection after appendicectomy | Cochrane Database Syst Rev | Andersen et al.12 |
| 2006 | Can we define the ideal duration of atb therapy? | Surg Infect | Hedrick et al.13 |
| 2008 | A prospective, double blind, multicenter, randomized trial comparing ertapenem 3 vs. ≥5d in community-acquired IIA infection. | J Gastrointest Surg | Basoli et al.14 |
| 2010 | Diagnosis and management of complicated intra-abdominal infection in adults and children: Guidelines by the Surgical Infection Society and the Infectious Diseases Society of America | Surg Inf | Solomkin et al.15 |
| 2014 | Duration of antibiotic treatment after appendectomy for acute complicated appendicitis | Br J Surg | Van Rossem et al.16 |
| 2014 | Association of Excessive Duration of ATB Therapy for Intra-Abdominal Infection with Subsequent Extra-Abdominal Infection and Death: A Study of 2552 Consecutive Infections | Surg Infect | Riccio et al.17 |
| 2014 | Effect of postoperative antibiotic administration on postoperative infection following cholecystectomy for acute calculous cholecystitis: A randomised clinical trial | JAMA | Regimbeau et al.18 |
| 2015 | Trial of short course antimicrobial therapy for intraabdominal infection | N Engl J Med | Sawyer et al.19 |
| 2016 | Duration of Antimicrobial Therapy in Treating Complicated Intra-Abdominal Infections: A Comprehensive Review | Surg Infect | Sartelli et al.20 |
| 2017 | The Surgical Infection Society Revised Guidelines on the Management of Intra-Abdominal Infection | Surg Infect | Mazuski et al. 1 |
| 2018 | Short-course antibiotic therapy for critically ill patients treated for postoperative intra-abdominal infection: The DURAPOP randomised clinical trial | Intensive Care Med | Montravers et al.21 |
The review of the articles on this subject first offers us the DURAPOP study, published in 2018 by Montravers et al. in the journal Intensive Care of Medicine21. This is a prospective, multicentre, randomised study in which 21 French hospitals participated and which included Intensive Care Unit patients with intra-abdominal infection, in whom initial control of the focus had been achieved and who had received adequate empirical antibiotic coverage. A total of 249 patients were randomised to short-course (8 days) or long-course (15 days) regimens, with no differences in mortality or need for surgical reintervention between the two arms, so that short-course regimens (8 days) were considered to be equally effective as longer courses (15 days) in critically ill patients.
Another literature review on the subject conducted in 2016 by Sartelli et al. and published in Surgical Infections20, reviewed 29 articles and concluded that patients with effective control of the focus and who do not present criteria for FIL complication should receive between 3 and 5 days of antibiotic treatment. However, in critically ill patients with septic shock it is recommended to individualise treatment to the inflammatory response, which could be monitored analytically, in their case they proposed procalcitonin. He emphasised that antibiotic treatment in the case of uncomplicated intra-abdominal infection, if it was limited to an organ without peritoneal involvement and with effective control of the focus, antibiotic prophylaxis should be sufficient and postoperative antibiotic treatment would not be necessary11,18.
In line with previous studies, a multicentre, randomised, open-label clinical trial (STOP-IT study), published in The New England Journal of Medicine19, enrolled 518 patients with complicated intra-abdominal infection with adequate focus control. Patients were randomised into 2 branches: short-course empirical antibiotic treatment (4 ± 1 day; 258 patients) or the control arm (260 patients), which consisted of receiving antibiotic treatment until 48 h after resolution of symptoms and signs of FIL with a maximum duration of 10 days, the mean duration of treatment in this arm being 8 days. In this study, no differences were observed in surgical site infection (6.6% vs. 8.8%; p = .43), recurrences (15.6% vs. 13.8%; p = .67), or 30-day mortality (1.2% vs. .8%; p = .99).
The idea of reduced post-operative antibiotic treatment has been discussed previously in studies focusing on a single origin of intra-abdominal infection, biliary and appendicular origin, by Regimbeau et al.18 and Mui et al.11, respectively.
Regimbeau et al.18 published the results of a randomised, open-label, multicentre, postoperative, multicentre study of acute cholecystitis in 17 hospitals in France in the journal JAMA in 2014. The total number of patients included was 414 with acute calculous cholecystitis grade I and II, according to the 2007 Tokyo Guidelines Classification, out of an initial 479. Patients received 2 g of amoxicillin-clavulanic acid every 8 h before surgery and after surgery, one group did not continue with this treatment and another group received 5 days. There were no significant differences in surgical site infection after 30 days between the two groups. The postoperative infection rate was 17% (35 out of 207) in the no treatment group and 15% (31 out of 207) in the treatment group.
In the case of the study by Mui et al.11, 269 patients undergoing open appendectomy for acute non-perforated appendicitis were randomised into 3 groups: the first group did not receive antibiotics after surgery, the second received 2 postoperative doses plus preoperative doses, and the third received 5 days of postoperative treatment. Antibiotic treatment was with cefuroxime and metronidazole in all branches. No significant differences were found between the 3 groups in terms of surgical site infection, although the group with the longest duration had lower infection rates than the other 2 (6.5%, 6.4% and 3.6%, p = .64). However, there were differences in antibiotic-related adverse effects, with significantly higher adverse effects in the group of patients receiving longer duration (0%, 1.1% and 4.8%; p = .048).
Even in previous reviews it had been questioned whether or not antibiotic prophylaxis was necessary in patients undergoing appendectomy. In a Cochrane systematic review conducted in 2003 by Andersen et al.12 controlled clinical trials comparing the use of antibiotics vs. placebo in patients undergoing appendectomy for suspected acute appendicitis were evaluated and 45 studies with a total of 9576 patients were included in the review. The main conclusion of this study was that the use of antibiotic prophylaxis was associated with lower rates of postoperative infections, regardless of the type of appendicitis. Therefore, this study demonstrates the need for the use of antibiotic prophylaxis to prevent superficial and deep surgical site and organ space infection and should be routinely administered in these patients.
Going further back in time to 1994, Schein et al. published in the British Journal of Surgery22, a prospective study of 163 patients with any type of intra-abdominal infection. In this study, patients were stratified according to the type of intra-abdominal infection and antibiotic duration protocols were applied according to the degree of intra-abdominal infection, subsequently evaluating postoperative complications according to the protocol received. The aim of protocolising the duration of antibiotic treatment and differentiating between intra-abdominal contamination and established infection was to shorten the antibiotic regimens administered with the hypothesis that longer regimens did not improve patient outcomes and could even be counterproductive by favouring resistance, increasing the adverse effects of antibiotics and potentially favouring nosocomial extra-abdominal infections. Thus, the authors defined that patients with acute phlegmonous appendicitis, acute phlegmonous cholecystitis, uncomplicated diverticulitis, gastroduodenal perforations or traumatic bowel injuries of less than 12 h preoperatively or small bowel ischaemia without perforation would not receive empirical antibiotic treatment. Acute gangrenous appendicitis, acute gangrenous cholecystitis or acute cholecystitis with empyema without free pus would receive 24 h of empirical antibiotic treatment. The duration of antibiotic treatment would be 48 h in intra-abdominal infections with non-diffuse peritonitis and those traumatic intestinal injuries or gastroduodenal perforations lasting more than 12 h. Finally, antibiotic therapy would be between 3 days and 5 days in cases of purulent peritonitis of any origin. With these guidelines, overall mortality was 2% and surgical wound infection 7%.
The concept of persistence of leukocytosis and fever in intra-abdominal sepsis as a guide in the maintenance of empirical antibiotic treatment was presented to the scientific community by Lennard et al.4 in 1982 in their publication in the journal Annals of Surgery. In their study, they compared the evolution of patients who underwent surgery based on the presence or absence of fever and leukocytosis after the end of antibiotic treatment. The study showed that patients with persistent leukocytosis or fever at the time of antibiotic discontinuation had an increased risk of postoperative intra-abdominal infection at 60 days postoperatively, by 33% if there was persistent leukocytosis and by up to 50% in those with persistent fever.
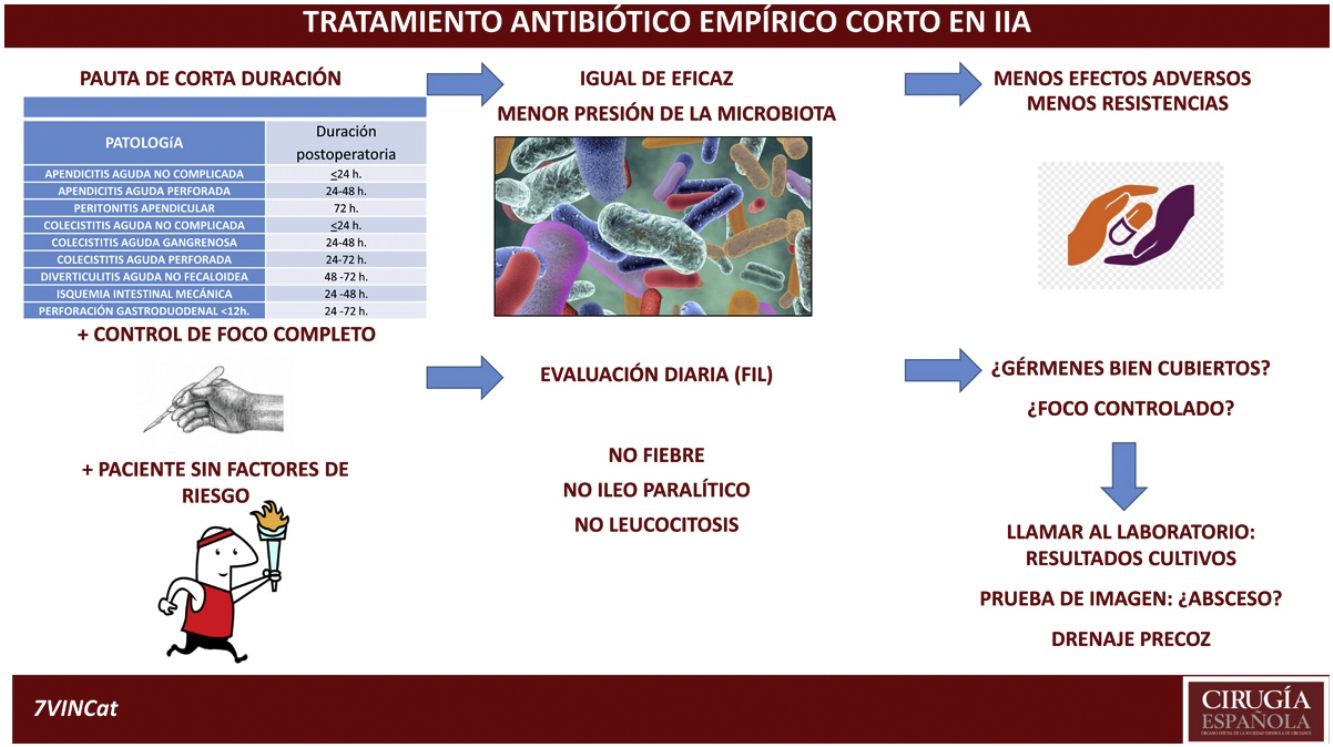
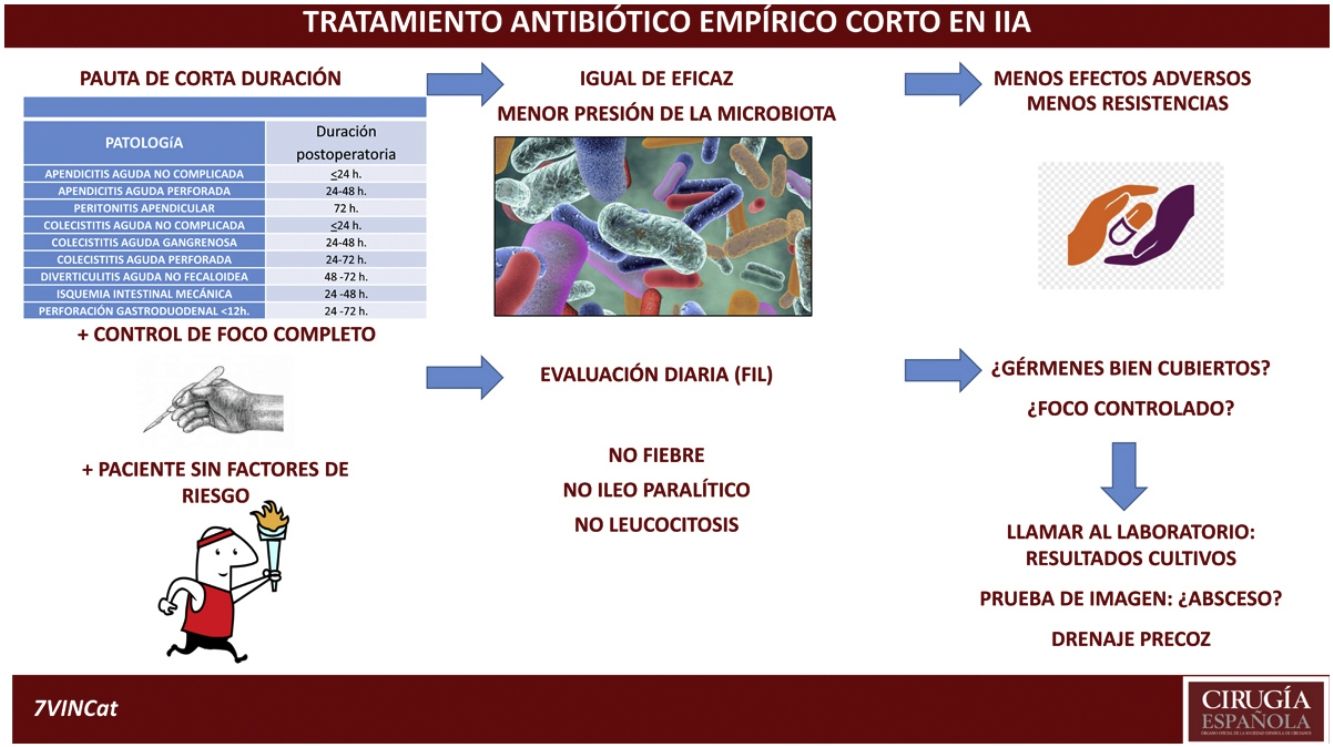
DiscussionThe aim of this review is to highlight the fact that antibiotic treatment is complementary to other actions and that it must be assessed on a case-by-case and daily basis. Following this review and assessing the current situation of emergency surgery in intra-abdominal disease, with empirical antibiotic treatments with a broader spectrum and adapted to the patient's risk factors, with advances in diagnostic and monitoring mechanisms available both analytical and radiological, as well as, percutaneous interventional capacity and the evidence of increasing antibiotic resistance, we propose a duration of antibiotic treatment according to the origin of the intra-abdominal infection similar to that proposed in Table 2, open to the decision of the surgeon responsible and individualising the duration in patients with risk factors for poor evolution (Table 3). In all cases, constant re-evaluation is required in all intra-abdominal infections every 24 h and if the duration of the intra-abdominal infection is longer than the time indicated, the presence of a possible intra-abdominal complication should be evaluated early.
Proposed duration of empirical antibiotic treatment in intra-abdominal infection with complete control of focus.
| Proposed duration of empirical antibiotic treatment in patients without risk factors | |
|---|---|
| Pathology | Postoperative duration |
| Uncomplicated acute appendicitis | ≤24 h |
| Acute perforated appendicitis | 24–48 h |
| Apendicular peritonitis | 72 h |
| Uncomplicated cholecystitis | ≤24 h |
| Acute aggravengenous cholecystitis | 24–48 h |
| Acute perforated acute cholecystitis | 24–72 h |
| Acute non-fecaloid diverticulitis | 48–72 h |
| Mechanical intestinal ischaemia | 24–48 h |
| Gastroduodenal perforation <12 h | 24–72 h |
Exceptions to the duration of this antibiotic treatment. Patients with >2 High-risk items.
| Phenotypic/physiological risk factors |
| Older age >70 years |
| Malignancy |
| Significant cardiovascular involvement |
| Significant liver disease or cirrhosis |
| Significant renal disease |
| Hypoalbuminemia |
| Extensive infection or insufficient control of initial focus |
| Inability or delay in initial focus control |
| Diffuse/generalised peritonitis (4 intra-abdominal quadrants) |
| Elevated Mannheim index |
| Microbiological features with suspicion of resistant germs |
The joint action of the interprofessional epidemiological surveillance teams in each centre is essential to guarantee the monitoring of resistance and the antibiotic pressure exerted by the empirical antibiotic regimens administered, both in terms of dose and duration. These teams are responsible for ensuring the optimisation of the antibiotic treatment used in the different departments and the continuous re-evaluation of these results.23–27
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Membrilla-Fernández E, Gómez-Zorrilla S, González-Castillo AM, Pelegrina-Manzano A, Guzmán-Ahumada J, Prim N, et al. Evidencia científica de la duración del tratamiento antibiótico en las infecciones intraabdominales con control de foco quirúrgico. Cir Esp. 2022;100:608–613.