Total gastrectomy is a surgery with significant perioperative morbidity and mortality, being considered the treatment of choice in proximal gastric cancer. First described in 1980, our group reported and standardized totally laparoscopic 95% gastrectomy in 2014. This technique aims to reduce the complications of total gastrectomy while maintaining oncological radicality. We present the initial results from a cohort of consecutive cases after performing the technique for 4 years at 2 hospital centers.
MethodsA prospective observational study was carried out in 67 patients with laparoscopic 95% gastrectomy between 2014 and 2017. The main objective has been to detect complications (Clavien Dindo>IIIa), focusing on anastomotic leaks as the most important. The secondary objective was to assess the quality of oncological surgery.
ResultsSixty-seven consecutive patients were included, in whom 95% totally laparoscopic gastrectomy was performed. There was no case of anastomotic leak. Two patients (2.98%) had one or more Clavien Dindo complications equal to or greater than IIIa. The total hospital stay was 6 (3–13) days. R0 radical resection was performed in all patients.
Conclusions95% gastrectomy allows selected patients to meet the oncological standards of resection in proximal gastric cancer in a reproducible and safe manner, reducing perioperative risks such as anastomotic leakage. It is a non-comparative observational prospective study, so more studies are needed to assess the standardization of the technique.
La gastrectomía total es una cirugía con importante morbimortalidad perioperatoria que es considerada el tratamiento de elección en el cáncer gástrico proximal. Descrita por primera vez en 1980, nuestro grupo describió y estandarizó la gastrectomía 95% totalmente laparoscópica en 2014. Esta técnica pretende disminuir las complicaciones de la gastrectomía total sin descuidar la radicalidad oncológica de la misma. Se presentan los primeros resultados de una cohorte de casos consecutivos tras 4 años realizando la técnica en 2 centros hospitalarios.
MétodosSe ha llevado a cabo un estudio prospectivo observacional en 67 pacientes con gastrectomía 95% laparoscópica realizadas entre 2014 y 2017. El objetivo principal ha sido la detección de complicaciones (Clavien Dindo>IIIa), centrándose en la fuga anastomótica como la más importante. Objetivo secundario fue valorar la calidad de la cirugía oncológica.
ResultadosSe incluyeron 67 pacientes consecutivos en los que se realizó gastrectomía 95% totalmente laparoscópica. No existió ningún caso de fuga anastomótica, 2 pacientes (2.98%) presentaron una o más complicaciones Clavien Dindo≥IIIa. La estancia total fue de 6 (3–13) días. Se realizó resección radical R0 en todos los pacientes.
ConclusionesLa gastrectomía 95% permite en pacientes seleccionados cumplir los estándares oncológicos de resección en el cáncer gástrico proximal de manera reproductible y segura, disminuyendo los riesgos perioperatorios como la fuga anastomótica. Se trata de un estudio prospectivo observacional no comparativo, por lo que son necesarios más estudios para valorar la estandarización de la técnica.
Total gastrectomy is the treatment of choice in proximal gastric cancer and, according to some authors, in cancer with diffuse infiltration. It is an aggressive surgery with significant perioperative morbidity that alters future quality of life. The objective of this intervention is to perform oncologically satisfactory surgery, or R0. Described in 1980, 95% gastrectomy aims to reduce the complications of total gastrectomy without affecting its oncological radicality. Our group described the technique in 2014.1 We present the initial results from a cohort of consecutive cases after 4 years of performing the technique at 2 medical centers.
MethodsA prospective observational study was conducted in patients with laparoscopic 95% gastrectomy performed at 2 hospitals, CHL hospital in Luxembourg and Galdakao Ospitalea in Bizkaia (Spain), between January 1, 2014 and December 31, 2017. Included in the study were all patients with gastric cancer (cT1-4 N0-3M0) located in the antrum or body of the stomach, in whom laparoscopic curative gastrectomy was proposed with an R0 objective. Patients with tumors infiltrating the esophagogastric junction, those under 18 years of age or those who had received palliative surgery were excluded from the study. All patients were treated by the same 3 surgeons.
ObjectivesThe main objective was to detect complications (Clavien Dindo), focusing mainly on anastomotic leaks. The secondary objective was the quality of the oncological surgery, considering whether resection margins were free (longitudinal and radial), the number of resected lymph nodes, and early local recurrence. Data were collected for all complications occurring in the first 60 postoperative days, as well as the results from the pathology study, including the number of resected and affected lymph nodes, tumor differentiation, Lauren classification type and pTNM stage.
Tumor StagingBased on international clinical guidelines, patients in whom gastric cancer was suspected were studied by gastroscopy and thoracoabdominal CT. Endoscopic ultrasound was performed in patients with suspected infiltration of the esophagogastric junction or other neighboring organs. In advanced T3-4 N–1+ tumors, neoadjuvant treatment was performed by chemotherapy.
TreatmentAll operable patients (cT1-4 N0-3M0) were included for totally laparoscopic gastrectomy with curative intent. All patients with disease above cT1N0 received preoperative chemotherapy with 4 cycles of 5-fluorouracil, oxaliplatin, and taxotere, unless the patient or tumor conditions discouraged it due to obstruction or bleeding.
Surgical TechniqueBetween 4 and 6 weeks after neoadjuvant treatment, patients underwent totally laparoscopic 95% gastrectomy and D2 lymphadenectomy following the technique previously described in another publication.1 After the resection, a Roux-en-Y reconstruction was conducted. In the first 20 cases, total omentectomy was done and analyzed independently, finding no affected lymph nodes or tumor implantation in the greater omentum. In subsequent cases, partial omentectomy was performed, initiating the resection 4cm distal to the gastroepiploic vessels.
Statistical AnalysisBeing an observational cohort, only a descriptive analysis of the data was done, collected prospectively in Microsoft Excel databases and analyzed with SPSS® version 22.0. The continuous variables were reported as means (range).
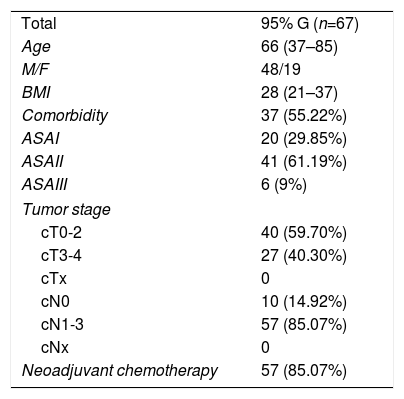
ResultsDuring the study period, 67 consecutive patients were included with gastrectomies with curative intent for gastric cancer. The clinical characteristics of the patients are shown in Table 1. Sixteen patients were treated in 2014, 21 in 2015, 19 in 2016 and 11 in 2017; of these, 10 cases were early gastric cancer and 57 advanced gastric cancer. The follow-up duration was between 4 and 48 months. Six patients were lost to follow-up, 3 of whom died from another cause not related to cancer.
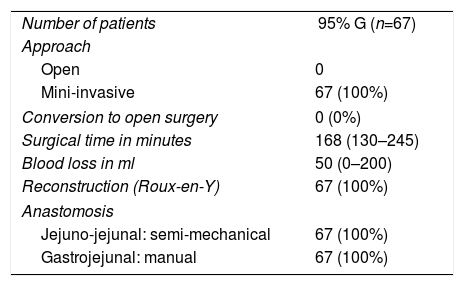
The intraoperative results are summarized in Table 2. 95% gastrectomy was performed in 67 patients (67; 100%) and Roux-en-Y reconstruction with manual anastomosis between the gastric stump and the jejunum using barbed suture and semi-mechanical side-to-side jejuno-jejunal anastomosis in 67 patients (67; 100%). All patients were treated laparoscopically (67; 100%), with no conversions. The surgical piece was extracted protected in a plastic extraction bag and through a Pfannenstiel incision and double-ring wound protector in all cases.
Characteristics of Surgeries.
| Number of patients | 95% G (n=67) |
| Approach | |
| Open | 0 |
| Mini-invasive | 67 (100%) |
| Conversion to open surgery | 0 (0%) |
| Surgical time in minutes | 168 (130–245) |
| Blood loss in ml | 50 (0–200) |
| Reconstruction (Roux-en-Y) | 67 (100%) |
| Anastomosis | |
| Jejuno-jejunal: semi-mechanical | 67 (100%) |
| Gastrojejunal: manual | 67 (100%) |
There were no deaths in the first 60 postoperative days. Two patients (2.98%) presented one or more complications that were Clavien Dindo≥IIIa (Table 3). One patient was re-admitted on the 10th postoperative day due to gastrointestinal bleeding, which was treated by endoscopy, and the other required reoperation due to bowel obstruction caused by internal hernia on the 52nd postoperative day. Four (5.97%) patients presented Clavien Dindo complications≥IIIa: 2 cutaneous infections, one case of self-limited gastrointestinal bleeding and one respiratory infection. The mean stay in the resuscitation unit was one day in the 6 patients who required intensive care due to associated relevant diseases. The total stay was 6 (3–13) days. The last 31 patients were included in the “fast-track” multimodal rehabilitation protocol followed at our hospital, so they did not have drain tubes, nasogastric tubes, or bladder catheters and had immediate mobilization with the adapted use of analgesia and antiemetics.2
Postoperative Data.
| Number of patients | 95% G (n=67) |
| Postoperative complications (CD≥IIIa) | 2 (2.98%) |
| • Anastomotic leak | 0 |
| Postoperative complications (CD<IIIa) | 4 (5.97%) |
| Duodenal stump leak | 0 |
| Digestive hemorrhage | 1 (1.49%) |
| Skin infection | 2 (2.98%) |
| Medical complications | 1 (1.49%) |
| 60-day mortality | 0 |
| Hospital stay | 6 (3–13) |
| ICU stay | 6 patients (1 day) |
| Readmission within 60 days | 2 (2.98%) |
| Postoperative adjuvant chemotherapy | 48 (71.63%) |
The results of the pieces analyzed are shown in Table 4. Radical R0 resection was performed in 67 patients (67; 100%) and neither the proximal nor the distal margins were positive in any of the patients (67; 0%). An average of 41 (26–58) lymph nodes were analyzed, 5 (0–21) of which were positive. No lymph nodes or peritoneal implants were found in the surgical pieces of the first 20 patients in whom total omentectomy was performed.
Pathology Data and Tumor Stage.
| Number of patients | 95% G (n=67) |
| Radical resection (R0) | 67 (100%) |
| R1 | 0 (100%) |
| Positive proximal margin | 0 |
| Positive distal margin | 0 |
| Radial margin involvement | 0 |
| Complete pathological response | 0 |
| Tumor location | |
| Proximal third | 4 (6%) |
| Body of the stomach (middle third) | 21 (31.34%) |
| Distal third | 42 (62.69%) |
| Histologic subtype (Lauren classification) | |
| Intestinal adenocarcinoma | 54 (80.6%) |
| Diffuse or mixed carcinoma | 13 (19.4%) |
| Tumor differentiation grade | |
| G1 | 14 (29.25%) |
| G2 | 36 (53.73%) |
| G3 | 7 (10.45%) |
| Local tumor extension | |
| (y)pT1–2 | 27 (40.23%) |
| (y)pT3–4 | 40 (59.70%) |
| Lymph node extension | |
| (y)pN0 | 6 (8.95%) |
| (y)pN1 | 23 (34.33%) |
| (y)Pn2 | 26 (38.81%) |
| (y)Pn3 | 12 (17.91%) |
| Metastatic disease (y)pM1 | 0 |
| No of resected lymph nodes | 41 (26–58) |
| No of positive lymph nodes | 5 (0–21) |
| Lymph nodes in the omentum | 0/20 |
| Tumor implants in the omentum | 0/20 |
| Tumor stage (TNM 8th Edition) | |
| Ia | 0 |
| Ib | 5 (7.46%) |
| IIa | 17 (25.37%) |
| IIb | 29 (43.28%) |
| IIIa | 13 (19.40%) |
| IIIb | 3 (4.48%) |
| IIIc | 0 |
| IV | 0 |
The duration of the follow-up was between 4 and 48 months. Six patients were lost to follow-up, 3 of which died from another cause not related to cancer. Seven (11.47%) patients presented widespread dissemination of the disease in this period of time, 2 of which died (3.28%). No cases of isolated local recurrence were detected.
DiscussionCurrently, the treatment of gastric cancer is a multidisciplinary, but surgery as a fundamental pillar of the treatment with curative intent, supported by adjuvant treatments such as chemotherapy and radiotherapy.3 In addition to the magnitude of the lymphadenectomy, one of the key points in said surgical treatment is the extension of the gastric resection in each case to either total or subtotal gastrectomy.4 International clinical guidelines recommend total gastrectomy for the treatment of proximal gastric cancer and, in the case of diffuse gastric cancer, with the aim of achieving a safety margin of 5–8cm.5,6
Total gastrectomy presents a high morbidity and mortality even in very experienced hands and can be an independent factor for mortality compared to other gastrectomies.7 The most feared complication is esophagojejunal anastomosis leakage, which the most recent and extensive series reports at between 5 and 15%.8,9 Meanwhile, in subtotal gastrectomy, the gastrojejunal leak occurs between 0.5 and 3% of cases.10 In addition, the mortality associated with anastomotic leakage is high, causing death in up to one of every 3 patients.11
Almost total gastrectomy was described in 1954 and subsequently underwent different descriptions and modifications. In 2013, it was described in Japan as laparoscopic 95% gastrectomy, used in cases of early gastric cancer.12 As previously described, our group described and standardized the modified technique in 2014.1 Subsequently, the indication was extended to selected cases of advanced gastric cancer in which the esophagogastric junction is not affected.
In addition to reducing the complications of total gastrectomy, this gastrectomy aims to maintain oncological radicality by associating, unlike 7/8 gastrectomy, complete resection of the gastric fundus by preserving a small gastric stump of only 1–2cm. The gastric remnant allows for a reproducible manual intracorporeal anastomosis to be performed laparoscopically and safely, for which barbed suture is used. The surgical results obtained with this technique are comparable to those of subtotal gastrectomy in terms of number of complications, recovery and quality of life after surgery. On the other hand, no positive proximal margin or local recurrence was detected until the follow-up date.
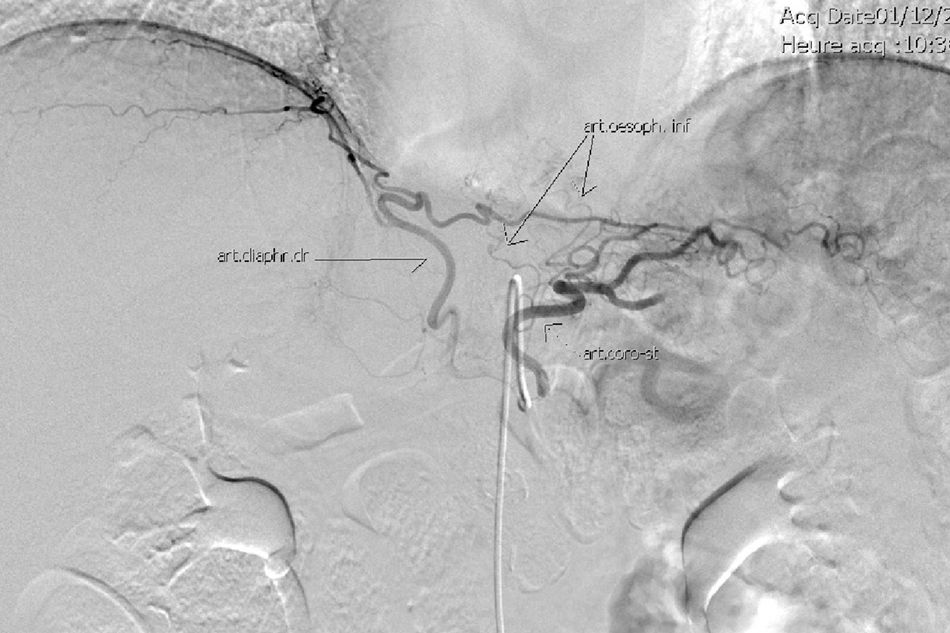
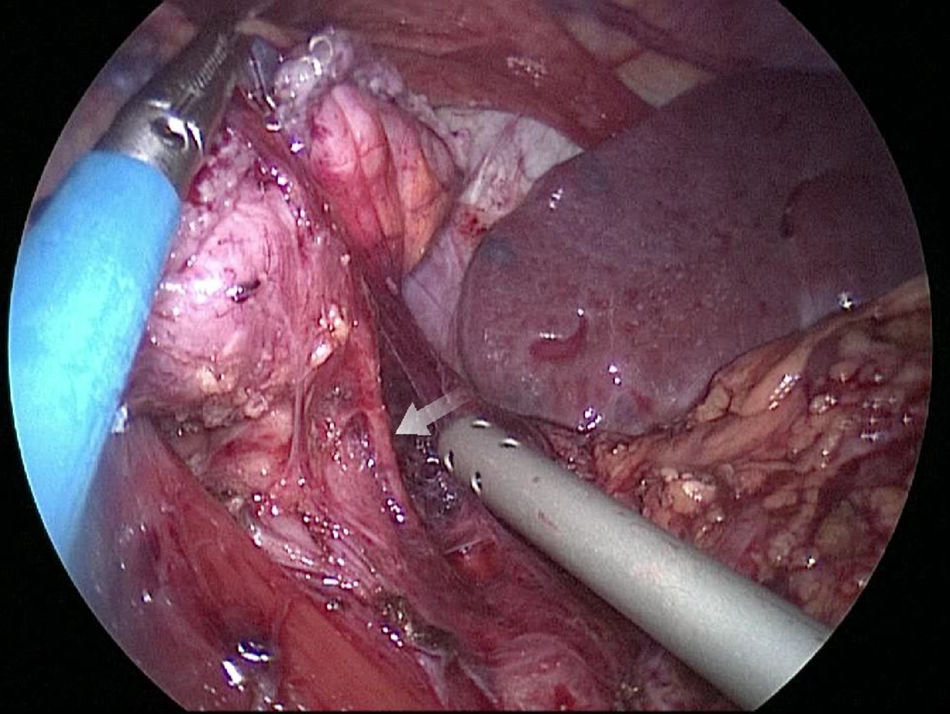
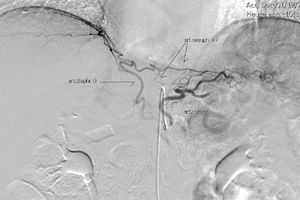
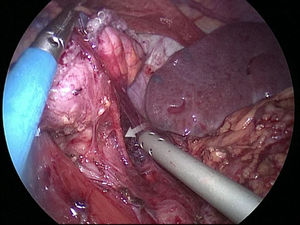
The gastric stump presents vascularization through subserosal vessels of the esophageal metameric vascularization (lower branch of the esophageal artery) and from the diaphragm vessels and the splenic artery, as shown in different arteriographies (Fig. 1) and in the surgical procedures themselves (Fig. 2).13 This enables the creation of a well-vascularized anastomosis between the gastric stump and jejunum with no risk of ischemia, which achieves optimal surgical results, and anastomosis-related complications are practically non-existent.
The lymphadenectomy recommended in advanced proximal gastric cancer is D2.3–6 Using this gastrectomy, it is possible to carry out extensive laparoscopic lymphadenectomy, including the pericardial groups (No. 1 and 2), with no risk of devascularization of the gastric stump.
Last of all, the minimization of complications allows for a greater number of patients to complete a multimodal rehabilitation program and maintain a better functional and nutritional status.14 Thus, more patients can benefit from completing the postoperative adjuvant treatment, with which the prognosis of these patients can improve.15
Therefore, we conclude that totally laparoscopic 95% gastrectomy allows selected patients to meet the oncological standards for resection in proximal gastric cancer in a safe, reproducible manner, while reducing perioperative risks like anastomotic leaks. At the same time, it provides better postoperative recovery and nutritional status, which means that a higher percentage of patients will be able to receive adjuvant chemotherapy. This is a non-comparative prospective observational study, with the biases that such studies entail. Its only aim is to demonstrate the safety of the procedure, which did not compromise the oncological results. 95% gastrectomy is a technique that has been described and accepted for the treatment of proximal gastric cancer, but randomized prospective studies with longer follow-up periods are necessary to evaluate its standardization.16,17
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Sarriugarte A, Arru L, Makai-Popa S, Goergen M, Ibañez-Aguirre FJ, Azagra JS. Resultados a corto plazo de la gastrectomía casi total (95%gastrectomy) laparoscópica. Cir Esp. 2018;96:634–639.