Thanks to a 30% increase in DCD, a donation rate of 43.4 per million people was achieved in 2017. In absolute numbers, there were 134 liver transplantations (LT) from DCD during this period (92.5% from cDCD).1 Another way to optimise liver grafts is by DLT. In this procedure, the transplant recipient is a living donor of his or her own liver. The liver of a patient with familial amyloid polyneuropathy (FAP) is anatomically and functionally normal aside from the production of amyloid.2 Because 95% of mutated transthyretin is synthesized in the liver and liver function is strictly normal, LT was considered the initial curative treatment.3 Therefore, the FAP liver can be used as a graft.2 The Domino Liver Transplant Registry (DLTR), created in 1999, reports a total of 1187 domino transplantations performed in 63 different hospitals in 21 countries until December 31, 2017.4 The technical challenge of this procedure is the need to share the venous cuffs between the donor and the recipient, because the length of the latter can be compromised.5
A 62-year-old woman with FAP agreed to donate her whole liver after LT. The patient's clinical stage of FAP was “Coutinho 1” (unimpaired ambulation; mostly mild sensory, motor, and autonomic neuropathy in the lower limbs). Initially, we proposed two treatment options: tafamidis at a dose of 20mg once daily (Vyndaqel®; Pfizer Inc., New York, NY, USA) or liver transplantation.2 Her brother had undergone transplantation, so she decided on LT. She consented to donate her whole liver and to carry on a DLT. The donor was a 45-year-old man who had suffered a massive acute myocardial infarction. His family accepted the limited therapeutic efforts. The organ donation was viewed as a process separate from the consent to withdrawal of support. At that moment, we did not have the normothermic regional perfusion device, so all organs were procured by a super rapid recovery technique. The donor functional warm ischemia time (interval between systolic blood pressure <60mmHg until cold flushing of the organs was initiated) was 11min, and the liver was perfused with Celsior (3 L through the aorta and 2 L through the inferior mesenteric vein). The graft weight was 1.114kg, and there were no arterial anatomical anomalies.
She received the cDCD graft after a cold ischemia time (CIT) of 290min. The arterial and portal flow rates were 193ml/min and 754ml/min, respectively. No T-tube was placed. She did not need intraoperative blood products. From an anaesthesiological point of view, the procedure had no incidences. The patient did not develop reperfusion syndrome.6 The hepatic outflow was reconstructed as previously described.7 The recipient of the domino graft was a 66-year-old man who underwent transplantation for alcoholic cirrhosis. For this case, CIT was 255min and the arterial and portal flow rates were 197ml/min and 1800ml/min, respectively. Both procedures were uneventful. The immunosuppressive regimen was similar for the two recipients, consisting of tacrolimus, mycophenolate and steroids.
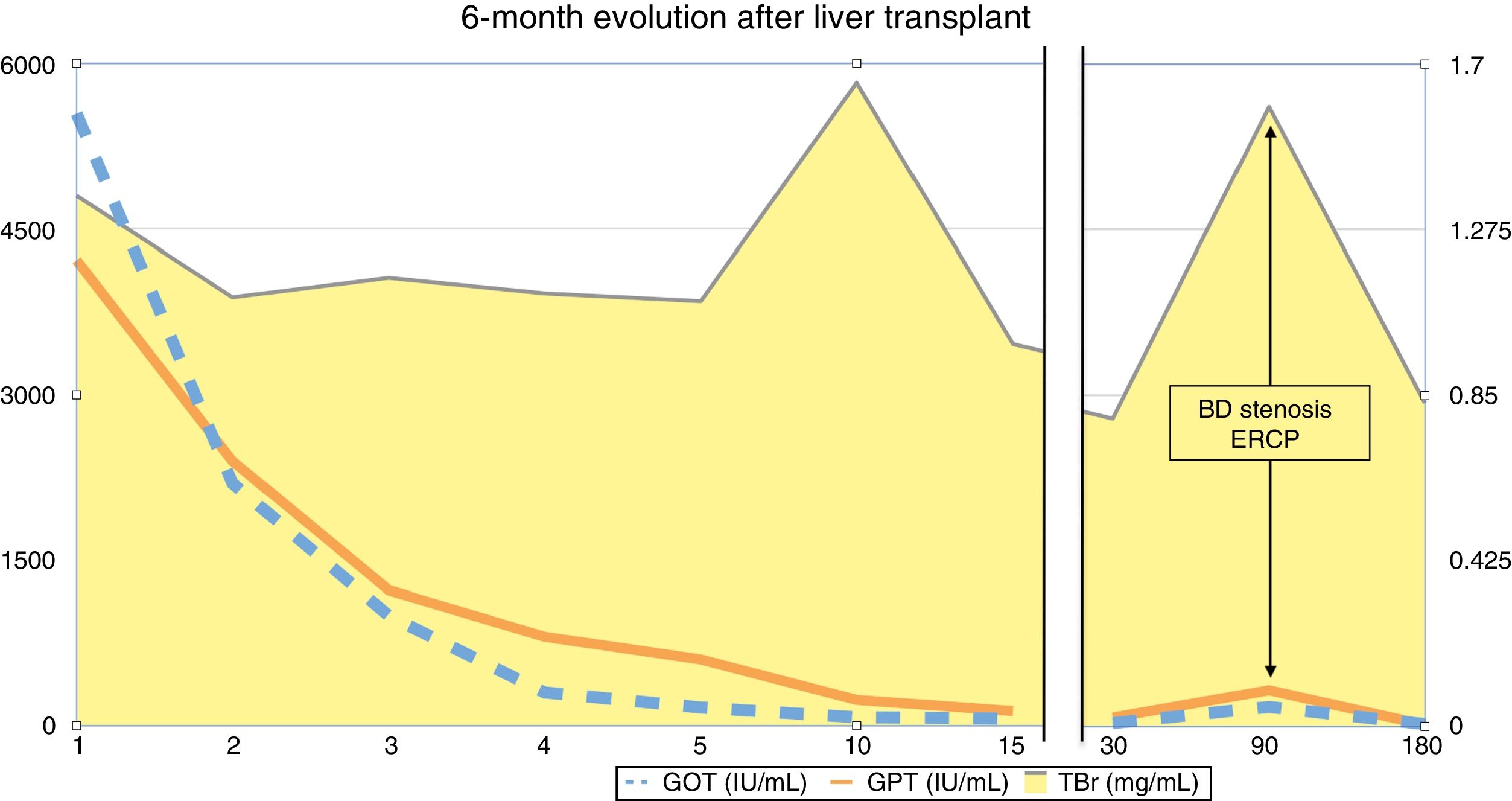
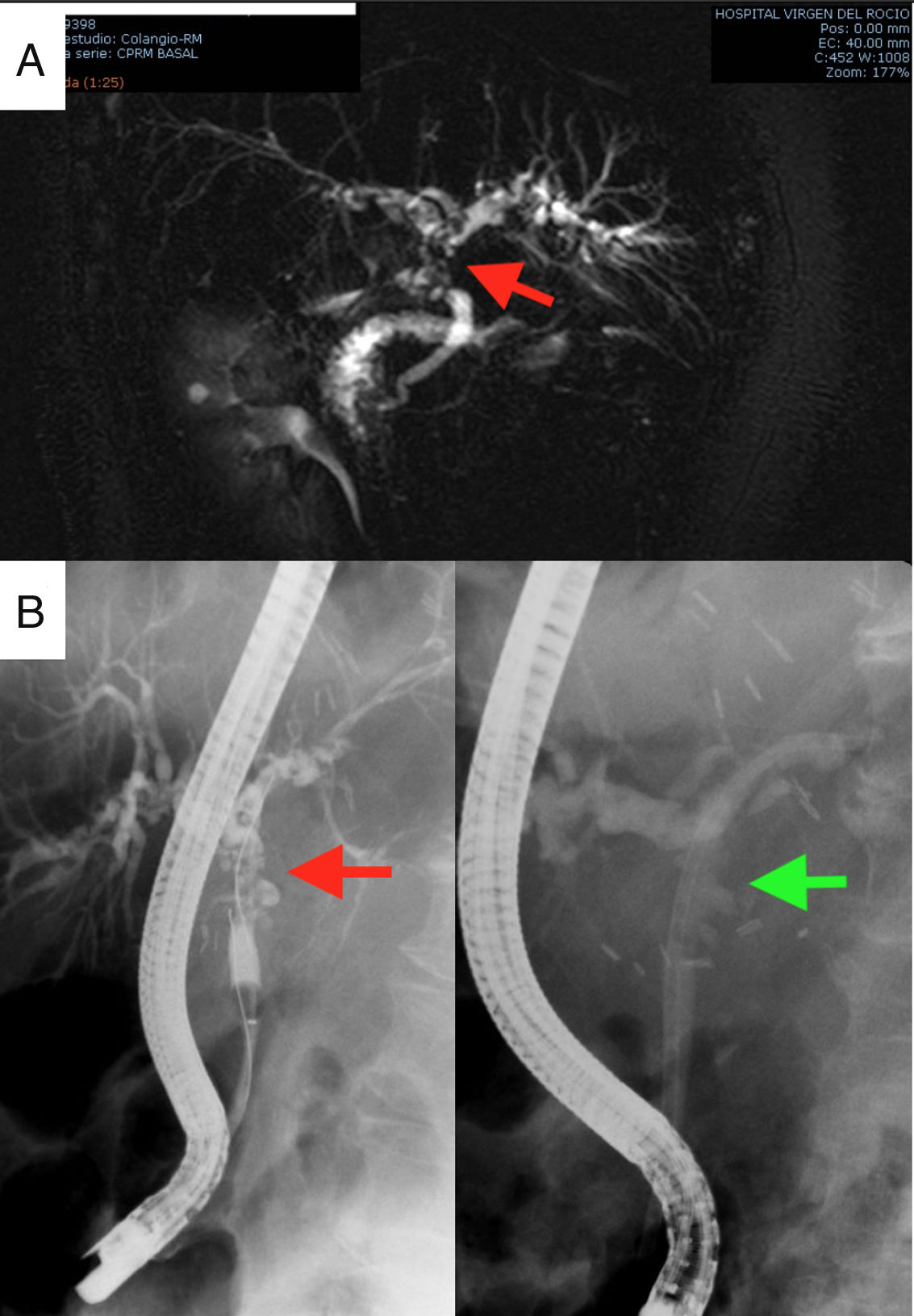
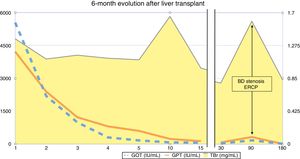
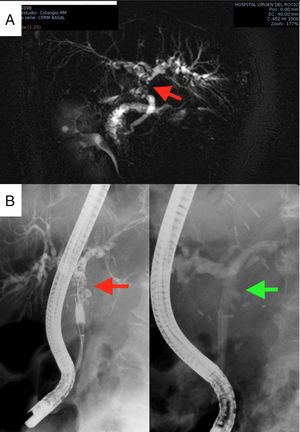
The first postoperative day (POD) she met criteria for primary graft dysfunction.8 The transaminases peak was normalised at 5th POD (Fig. 1). The Doppler ultrasound showed cDCD graft patency (hepatic artery, portal vein and hepatic veins). She was discharged the19th POD. In the third month after transplantation, the patient presented cholestasis and mild jaundice. A magnetic resonance cholangiopancreatography (MRCP) was performed (Fig. 2A). It was informed as an anastomotic biliary stenosis without ischemic cholangiopathy data. A plastic stent was successfully placed by endoscopy (Fig. 2B). At the 6-month follow-up, she had normal graft function (Fig. 1). We did not have repeated the MRCP because she has not presented new data compatible with ischemic cholangiopathy.
Complementary studies: MRCP diagnosing anastomotic stenosis, indicated by the red arrow (2A); and therapeutic endoscopic retrograde cholangiopancreatography for the placement of plastic biliary stents. The arrow in the left image indicates the stenosis, while in the image on the right the stenosis is resolved with the plastic prosthesis (2B).
Thanks to the Real Decreto 1723/2012 (BOE n° 313, de 29 de diciembre de 2012), the rate of LT from cDCD increased from 3% in 2014 to 11.6% in 2017. Most of the Spanish centers have gradually abandoned super rapid recovery technique. The NRP has better results in terms of efficacy (patient and graft survival) and safety (lower rate of ischemic cholangiopathy). Based on the recent scientific evidence available, our group also uses the NRP since 2017 for the cDCD.9,10 To the best of our knowledge, this is the first description of the use of a cDCD for DLT. Controlled DCD is a safe option for DLT under strict selection criteria.
Please cite this article as: Marín-Gómez LM, Suárez-Artacho G, Padillo-Ruiz J, Gómez-Bravo MA. Trasplante hepático dominó con injerto procedente de donante en muerte circulatoria (Maastricht III). Cir Esp. 2019;97:605–607.