The appearance of liver metastases during the follow-up of a patient with a skin melanoma has classically been considered a sign of a very poor prognosis. There are limited therapeutic options, since these lesions are non-resectable and form part of a disseminated disease in several organs.
In certain cases, in those where the disease is restricted to the liver or accompanied by a resectable extra-hepatic disease, hepatectomy can be useful, obtaining acceptable survivals of about 25% at 5 years, although hepatic or skin recurrence is usually early.
The limited number of patient cases published, the absence of randomised studies, and the heterogeneity of the series, make it difficult to reach conclusions so as to recommend which patients may benefit from liver resection, with an acceptable level of scientific evidence, and thus define its real usefulness. There are also no action plans defined as to when and what type of adjuvant therapy we should use.
La aparición de metástasis hepáticas (MH) durante el seguimiento de un paciente con melanoma cutáneo se ha considerado clásicamente un signo de muy mal pronóstico. Las opciones terapéuticas son escasas ya que habitualmente estas lesiones son irresecables y forman parte de una enfermedad diseminada en varios órganos.
En ciertos casos, en los que la enfermedad está restringida al hígado o acompañada de enfermedad extrahepática resecable, la hepatectomía puede ser útil obteniendo supervivencias aceptables, cercanas al 25% a los 5 años, aunque la recidiva hepática o cutánea suele ser temprana.
El escaso número de pacientes publicados, la ausencia de estudios aleatorizados y la heterogeneidad de las series no permite extraer unas conclusiones para poder recomendar que pacientes se benefician de la resección hepática con un nivel de evidencia científica aceptable y así definir su utilidad real. Tampoco están definidas las pautas de actuación en cuando y que tipo de terapia adyuvante debemos emplear.
Malignant melanoma (MM) can metastasise haematogenously to any organ.1–3 30% of MM patients develop distant metastases and survival rates are approximately 6% after 5 years.3–10 The liver is the third solid organ most frequently affected by MM metastases after the lungs and brain. When distant metastases are located in the liver, they are classified as stage IV (M1c).11 The rate of liver metastases (LM) caused by MM was thought to be low, since only 10%–20% of patients with metastatic MM have LM, a rate much lower than metastasis caused by other tumours such as colon adenocarcinoma, which is close to 50%. Studies in autopsies of patients with metastatic MM increase the figure of melanoma LM (MLM) to 55%–75%.1,4–6,8,12 After the diagnosis of MLM, the short-term prognosis is poor, because there is usually bilobar involvement and it is often associated with other metastatic foci. The mean survival rate in these patients varies between 4 and 6 months.1,3–8,13
The detection of distant MM metastases is performed by periodic studies including the determination of serum LDH, a CT of the chest, abdomen and pelvis, brain MRI and/or PET scan.9,14,15 The PET-CT scan has a sensitivity of 85%, specificity of 100% and a predictive value of 98%.14,15
The existence of MLM usually presents non-specific symptoms. There are a small number of patients who have shown symptoms of fulminant liver failure due to extensive bilobar involvement resulting from MLM.2 Three cases of MLM rupture requiring urgent liver surgery have been described.12,16
Several treatments are currently used for MLM: medical (chemotherapy, immunotherapy, and biological agents), ablative (radiofrequency, arterial infusion, chemoembolisation), and liver surgery.3,5–7,11–13 The NCCN guidelines on MM offer several treatment options for minimal, resectable metastatic disease: resection, observation or systemic therapy and reassessment.9 The results obtained with all treatments are mediocre and there are no randomised studies comparing treatment options.8,11,12,17
Chemotherapy has limited effectiveness on metastatic MM and response rates are below 20%.4,9,11 The most commonly used drugs are: ipilimumab, vemurafenib, dacarbazine, temozolomide, and paclitaxel with or without cisplatin or carboplatin.9 Dacarbazine in patients with stage IV MM, presents a response rate of between 12% and 28% with survival rates of 4–12 months.11 Biochemotherapy or a combination of chemotherapy and biological agents (interleukin 2, interferon and chemotherapy), occasionally (15%–20%) results in a complete clinical response.6,9
Surgical resection of MM metastases can be useful as it leads to a reduction in tumoral immunosuppressive factors, allows histological confirmation of the existence of BRAF mutations, and has a clinical response of 25% after 5 years.8,12,18 If we refer exclusively to MLM, the applicability of liver resection is usually low since these patients often have multiple, technically unresectable LM or LM associated with multiple metastatic foci.
The indication of resection in patients with non-colorectal, non-neuroendocrine (NCRNNE) LM tumours, which includes MLM patients among others, is as yet not fully accepted by the medical community and the number of patients undergoing surgery is small, despite a mean survival rate of 5%–25% after 5 years in published series.18–25
Existing data on MLM resection are found in 2 types of articles: series on NCRENNE LM which include LM of several types of tumours, including MLM.19,22,25 and a very limited number of articles devoted exclusively to MLM, where patients with cutaneous and ocular melanoma are often mixed.5,11,12,19–25
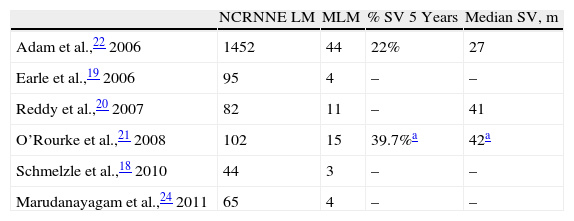
Among the NCRNNE LM series with MLM (Table 1), there is the French multicentre study by Adam et al., which includes 148 MLM (104 choroidal MM and 44 cutaneous MM) of 1452 NCRNNE LM in which resection of MLM obtained a 5-year survival rate of 21% in choroidal MM and 22% in cutaneous MM, with a median survival of 27 months.22 Adam et al. noted that choroidal MM more frequently display multiple intrahepatic tumours while cutaneous MM are associated mostly with extrahepatic disease.22 Reddy et al. published 11 MLN with a median survival of 41 months, but only 5 disease-free months.20 O’Rourke presented a total of 20 melanomas (15 cutaneous MM and 5 ocular)21 and Earle et al., 4 MLM of unspecified origin.19 In all series on NCRNNE LM, it is highlighted that liver resection in these patients should be seen as an integral part of cancer treatment (chemotherapy, radiotherapy and surgery) and should be considered cytoreductive and non-curative.20 The main problem in evaluating these articles is that their main objective is to assess the outcome of resection of NCRNNE LM in general, not of MLM, and as such, information is scarce.18–26
Series of Non-Colorectal, Non-Neuroendocrine Liver Metastases.
| NCRNNE LM | MLM | % SV 5 Years | Median SV, m | |
| Adam et al.,22 2006 | 1452 | 44 | 22% | 27 |
| Earle et al.,19 2006 | 95 | 4 | – | – |
| Reddy et al.,20 2007 | 82 | 11 | – | 41 |
| O’Rourke et al.,21 2008 | 102 | 15 | 39.7%a | 42a |
| Schmelzle et al.,18 2010 | 44 | 3 | – | – |
| Marudanayagam et al.,24 2011 | 65 | 4 | – | – |
LM: liver metastases; MLM: melanoma liver metastases; SV: survival.
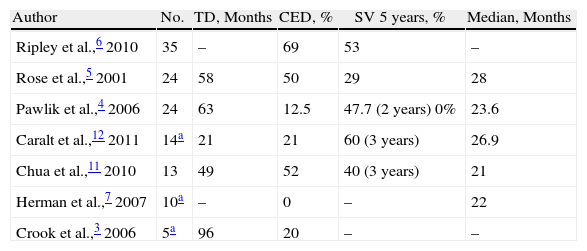
Published series devoted exclusively to MLM are very rare and usually include all types of melanomas (ocular and cutaneous), making it more difficult to draw conclusions (Table 2). We highlight some series.
Series of Cutaneous Melanoma Liver Metastases.
| Author | No. | TD, Months | CED, % | SV 5 years, % | Median, Months |
| Ripley et al.,6 2010 | 35 | – | 69 | 53 | – |
| Rose et al.,5 2001 | 24 | 58 | 50 | 29 | 28 |
| Pawlik et al.,4 2006 | 24 | 63 | 12.5 | 47.7 (2 years) 0% | 23.6 |
| Caralt et al.,12 2011 | 14a | 21 | 21 | 60 (3 years) | 26.9 |
| Chua et al.,11 2010 | 13 | 49 | 52 | 40 (3 years) | 21 |
| Herman et al.,7 2007 | 10a | – | 0 | – | 22 |
| Crook et al.,3 2006 | 5a | 96 | 20 | – | – |
CED: concurrent extrahepatic disease; SV: survival; TD: time between diagnosis of melanoma and melanoma liver metastases.
Ripley et al. present a series of 35 patients (29 cutaneous MM) treated by a combination of surgery, chemotherapy, radiation therapy and therapy with tumour-infiltrating lymphocytes (TIL). 43% of patients had a single MLM. 69% had extrahepatic disease. The 3-year survival rate was 53% with a median of 36 months, rising to 80% in patients with all the disease resected. The positive prognostic factors in the univariate analysis were a negative margin of the liver resection, single LM and an absence of extrahepatic disease. Their recommendation is that patients with single LM and without extrahepatic disease should be evaluated for resection. In patients with residual disease after surgery, the results obtained with the combination of surgery and TIL are spectacular (survival rate of 65% after 3 years) and very poor without TIL.6
Pawlik et al. performed a multicentre study of 40 patients who underwent MLM (24 cutaneous MM and 16 ocular MM). The age of the patients with cutaneous MM was lower (40 years) than those of ocular MM. The time between the onset of MM and MLM was 63 months. 67% were single MLM. In 60% a major hepatectomy was performed. Patients received multiple and varied adjuvant treatment. Recurrence occurred in 75% of patients and occurred at an early stage (5 months after liver resection), with cutaneous recurrence being the most common, or in another extrahepatic location but not in the liver. The clinicopathological features of MM had no prognostic value. Patients with unilobar LM under 5cm had a lower recurrence rate. Those with MLM diagnosed simultaneously with a skin lesion, or who had extrahepatic disease, had higher rates of recurrence. The survival was 23 months, reaching 47% after 2 years and 0% after 5 years. Patients treated with some form of adjuvant treatment achieved better survival than those who underwent surgery alone. Pawlik et al. recommend not performing liver resection if extrahepatic disease exists, but to use some line of chemotherapy and observe clinical evolution in these cases.4
The series of Rose et al., exclusively dedicated to cutaneous MM, includes 1750 patients with MM, of which 34 (2% of the total) were operated on for resection of MLM. 75% had a single MLM. In 10 cases, there was extensive intra-abdominal disease and only exploratory laparotomy was performed. The remaining 24 patients, representing 1.4% of all patients with MM, underwent hepatectomy, and 18 had a complete tumour resection (hepatic and extrahepatic), since 50% had associated extrahepatic disease. In the 24 resected patients, median survival was 28 months compared to 4 months in those who only underwent laparotomy. The 5-year survival rate was 29%. 72% had recurrent disease and the liver was the most common location. Two patients underwent a second liver resection due to recurrence. Patients with a negative hepatic histological margin (R0) who underwent resection of all metastatic foci had better survival rates.5
Chua et al. present a series of 23 patients with MM who have MLM and gastrointestinal metastases, of which 17 are cutaneous MM. Only 15 were operated on, 13 of them with MLM, and 8 were discarded because they were considered unresectable. The other 52% had metastatic foci in addition to liver lesions. 12 out of 13 patients underwent major hepatic resections. The 3-year survival rate was 30%. The poor prognostic factors were the existence of more than one metastatic foci and non-resection. The disease-free period before the appearance of the MLM (>2 years) was not statistically significant due to sample size but the difference is nonetheless very marked (37 vs 9 months).11
Herman et al. propose criteria to resect an MLM: resectable liver disease, absence of extrahepatic disease shown by PET, absence of serious medical comorbidities and a 24-month interval between diagnosis of MM and MLM.7 Crook et al. are unable to identify risk factors that are useful to decide which patients benefit from hepatectomy; they postulate that the number of LM does not matter, but the presence of extrahepatic disease is what matters. They carry out a review of the literature (1978–2000) which includes a total of 60 reported cases, noting that median survival varies between 10 and 51 months, and the 5-year survival varies between 0 and 33%.2
Other MLM treatment options when surgery is not technically feasible are: hepatic artery chemoembolisation, which can provide better results than systemic chemotherapy, although it only stabilises disease since a partial or complete clinical response is exceptional.1 It has been demonstrated that a nodular pattern on angiography is associated with higher survival rates,1 as well as arterial infusion chemotherapy, which achieves some type of remission in only a third of patients11,27–29; radiofrequency may be another option treatment option.7
In conclusion and summarising the series published on hepatic resection of MLM, we can say that its applicability is very limited because when there is metastatic liver involvement by MM, it is usually unresectable and it is part of a disease that has metastasised to several organs.
The series published are very heterogeneous and difficult to compare, and they often combine patients with ocular and cutaneous MM. The time between diagnosis of MM and MLM is usually high (49–96 months), the rate of extrahepatic disease is extremely variable (0%–69%), the rate of major hepatectomies is high (50%–60%), the morbidity and mortality from hepatectomy due to MLM are negligible, there are many adjuvant treatments and the results of 5-year survival rates are extremely variable (0%–53%). Recurrence is usually early, in some series recurrence is in the liver and in others, it is in the skin. The absence of extrahepatic disease or its complete resection, a negative hepatic histological margin, a long time between MM and MLM and a single MLM have been identified as positive prognostic factors.4–7,11
The small number of reported cases, the absence of randomised trials and the heterogeneity of the series do not allow recommendations to be drawn on which patients will benefit from surgical resection and which adjuvant therapies should be used with an acceptable level of scientific evidence. Some general recommendations are:
- -
PET-CT has a high sensitivity in the diagnosis of extrahepatic disease and should be performed if a hepatectomy is planned for MLM.
- -
Hepatectomy is indicated for MLM if the disease is restricted to the liver or, for some authors, it is acceptable if the extrahepatic disease is resectable. The number of MLM is not a determining factor but if there is a single MLM, better results are obtained. Resection should be R0.
Since recurrence is usually early, adjuvant treatment should be used after resection of MLM but it is currently the best regimen has not been defined.
Conflicts of InterestThe authors have no conflicts of interest to declare.
Please cite this article as: Ramia JM, et al. Tratamiento quirúrgico de las metástasis hepáticas de melanoma. Cir Esp. 2013;91:4–8.