Currently, there is growing interest in analyzing the results from surgical units and the implementation of quality standards in order to identify good healthcare practices. Due to this fact, the Spanish Association of Coloproctology (AECP) has developed a unit accreditation program that contemplates basic standards. The aim of this article is to evaluate and analyze the specific quality indicators for the surgical treatment of colorectal cancer, established by the program. Data were collected from colorectal units during the accreditation process.
MethodsWe analyzed prospectively collected data from elective colorectal surgeries at 18 Spanish coloproctology units during the period 2013–2017. Three main and four secondary quality indicators were considered. Colon and rectal surgeries were analyzed independently; furthermore, results were compared according to surgical approach.
ResultsA total of 3090 patients were included in the analysis. The global anastomotic leak rate was 7.8% (6.6% colon vs 10.6% rectum), while the surgical site infection rate was 12.6% (11.4% colon vs 14.8% rectum). Overall 30-day mortality was 2.3%, and anastomotic leak-related mortality was 10.2%. There were higher surgical site infection and mortality rates in the patients operated by open approach, however there was no difference in the anastomotic leak rate when compared with minimally invasive approaches.
ConclusionsThe evaluation of these results has determined optimal quality indices for the units accredited in the treatment of colorectal cancer. Furthermore, it allows us to establish realistic references in our country, thereby providing a better understanding and comparison of outcomes.
Actualmente existe un creciente interés por analizar los resultados de salud en las unidades quirúrgicas, implementando estándares de calidad que permitan dilucidar buenas prácticas asistenciales. Con este motivo la Asociación Española de Coloproctología desarrolló un programa de acreditación de unidades, teniendo en cuenta unos estándares básicos. El objetivo de este artículo es evaluar y analizar los indicadores de calidad específicos del tratamiento quirúrgico del cáncer colorrectal establecidos en el programa, en varias unidades en proceso de acreditación.
MétodosSe analizaron los datos recogidos de forma prospectiva de la cirugía programada colorrectal en 18 unidades de coloproctología durante los años 2013 a 2017. Se consideraron 3 indicadores de calidad principales y 4 secundarios, analizando de forma independiente la cirugía de colon y de recto. Además se compararon los resultados según el abordaje quirúrgico.
ResultadosSe incluyeron para el análisis un total de 3.090 pacientes. La tasa global de fuga anastomótica fue de 7,8% (6,6% colon vs 10,6% en el recto), mientras que la de infección de herida quirúrgica fue de 12,6% (11,4% colon vs 14,8% en el recto). La mortalidad global a los 30 días fue de un 2,3%, siendo la relacionada con fuga anastomótica de un 10,2%. Se evidenció una mayor incidencia de infecciones y muertes en los pacientes con abordaje abierto, pero no hubo diferencias en la tasa de dehiscencia con respecto a abordajes mínimamente invasivos.
ConclusionesLos resultados de este estudio determinan índices de calidad óptimos de las unidades acreditadas en el tratamiento del cáncer colorrectal, y además nos permite establecer referencias realistas en nuestro país, que ayudarán a una mejor comparación de resultados.
In 2010, the Spanish Association of Coloproctology (Asociación Española de Coloproctología, AECP) initiated a pioneering program designed to recognize the excellence of coloproctology units and promote their improvement. Based on the identification of characteristics that these units are required to possess and proper practice of the professionals working in these units, a series of specific criteria were established for their evaluation.1
Certain parameters are used to classify units as basic or advanced, thereby explicitly and publicly recognizing the activity that is carried out. One of the variables that is audited and carries the most weight in the certification process is related with the quality of treatment of patients with colorectal cancer. Due to the high frequency of this disease and its importance in healthcare, the implementation, identification and measurement of indicators is undoubtedly essential.2–6
The objective of this article was to evaluate and analyze the quality of care of program-certified units by analyzing indicators that evaluate the surgical treatment results of colorectal cancer.
MethodsWe analyzed the data collected prospectively during the certification process of several coloproctology units in Spain. The units of the participating hospitals voluntarily initiated the ACREDITA accreditation program, created specifically by the AECP. As part of this program, the clinical units were required to create a database with the information of patients consecutively treated for colon cancer or rectal cancer in order to analyze and evaluate the results of the previously defined quality indicators. All units included a minimum of 80 patients (60 cases of colon cancer and 20 cases of rectal cancer).
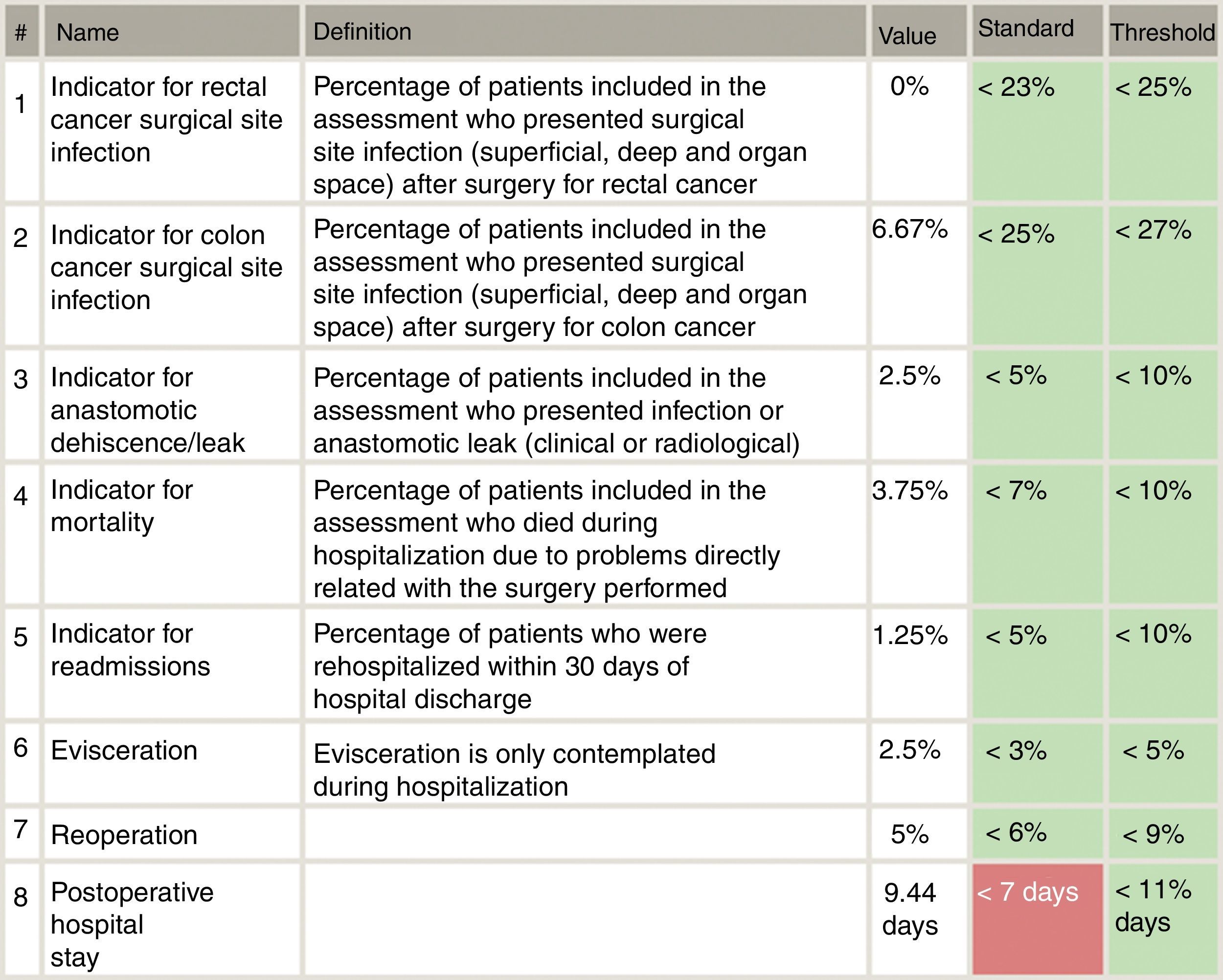
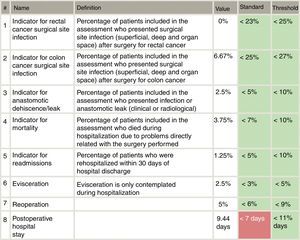
Included in the study were all patients who had consecutively undergone surgery for primary colon or rectal cancer from each of the 18 Spanish coloproctology units that were eligible for AECP certification during the 2013–2017 period (Table 1). Excluded from the study were patients treated with an emergency procedure, those lost to follow-up and those with incomplete data. The follow-up of all patients was at least 30 days post-op, in accordance with the AECP accreditation manual. The quality indicators used evaluate the results of colorectal cancer treatment, and their values were determined by taking into account data published in each section of the international literature, as well as others established by the Quality Division of the AEC7 (Fig. 1). Taking this into consideration, the following were defined as main variables: percentage of infection of the surgical wound, percentage of anastomotic dehiscence/leak, postoperative mortality (30 days). Other secondary variables included in the analysis were: hospital stay, reoperation, conversion to open surgery from laparoscopy or robotic procedure, and lastly, the number of isolated lymph nodes in the surgical piece.
Participating Units.
| Coloproctology Unit, Consorcio Hospital General Universitario de Valencia |
| Coloproctology Unit, Hospital Universitario Donostia, San Sebastián |
| Coloproctology Unit, Complejo Hospitalario de Santiago Compostela |
| Coloproctology Unit, Hospital Universitario Clínico, Valencia |
| Coloproctology Unit, Hospital Universitario de Fuenlabrada |
| Coloproctology Unit, Hospital Universitario Josep Trueta, Girona |
| Coloproctology Unit, Hospital Universitario de Gran Canaria Dr. Negrín |
| Coloproctology Unit, Hospital Universitario Joan XXIII, Tarragona |
| Coloproctology Unit, Hospital Universitario La Fe, Valencia |
| Coloproctology Unit, Complejo Hospitalario de Vigo (CHUVI) |
| Coloproctology Unit, Hospital Universitario Virgen del Rocío, Sevilla |
| Coloproctology Unit, Hospital Universitario de Bellvitge, Barcelona |
| Coloproctology Unit, Complejo Hospitalario de Torrecárdenas, Almería |
| Coloproctology Unit, Hospital Universitario Marqués de Valdecilla, Santander |
| Coloproctology Unit, Hospital Universitario Reina Sofía, Murcia |
| Coloproctology Unit, Hospital NISA 9 Octubre, Valencia |
| Coloproctology Unit, Hospital Universitario Vall d’Hebron, Barcelona |
| Coloproctology Unit, Hospital Universitario San Cecilio, Granada |
Anastomotic dehiscence/leak was defined as leakage of intestinal contents from a surgical union between 2 hollow viscera8 diagnosed clinically and radiologically in the first 30 postoperative days. The remaining variables related to postoperative complications were evaluated during admission and 30 days after surgery in the ambulatory follow-up of the patient.
Statistical AnalysisThe descriptive analysis initially included the overall results and the evaluation of the quality indicators from all the surgeries conducted. Then, cases of colon and rectal surgery were analyzed independently, comparing the results according to the type of procedure and surgical approach. Quantitative variables are presented with their mean and standard deviation (SD) or median and range. The qualitative variables are expressed as number of cases and percentages. The association between qualitative variables was determined by the Chi-squared or Fisher's exact tests. The differences between quantitative variables were analyzed by the Student's t test or ANOVA for independent groups. If, after performing the Kolmogorov–Smirnov test, normal distribution was not verified, the nonparametric Mann–Whitney U or Kruskal–Wallis tests were used when appropriate. The statistical analysis was carried out with the Statistical Package for the Social Sciences (SPSS), version 23 (IBM SPSS statistics, IBM Corporation, Armonk, NY).
ResultsBetween 2013 and 2017 a total of 3157 patients who had undergone elective surgery were included in the database, 67 of whom were excluded from the study due to lack of complete data; therefore, the final number of patients analyzed was 3090. The mean number of patients included in the database per hospital was 125.5 (76–789), and 61.8% were male. Mean age was 71.10±11.5 years. The patients who had undergone open surgery were older on average than patients who had undergone minimally invasive procedures (P<.001).
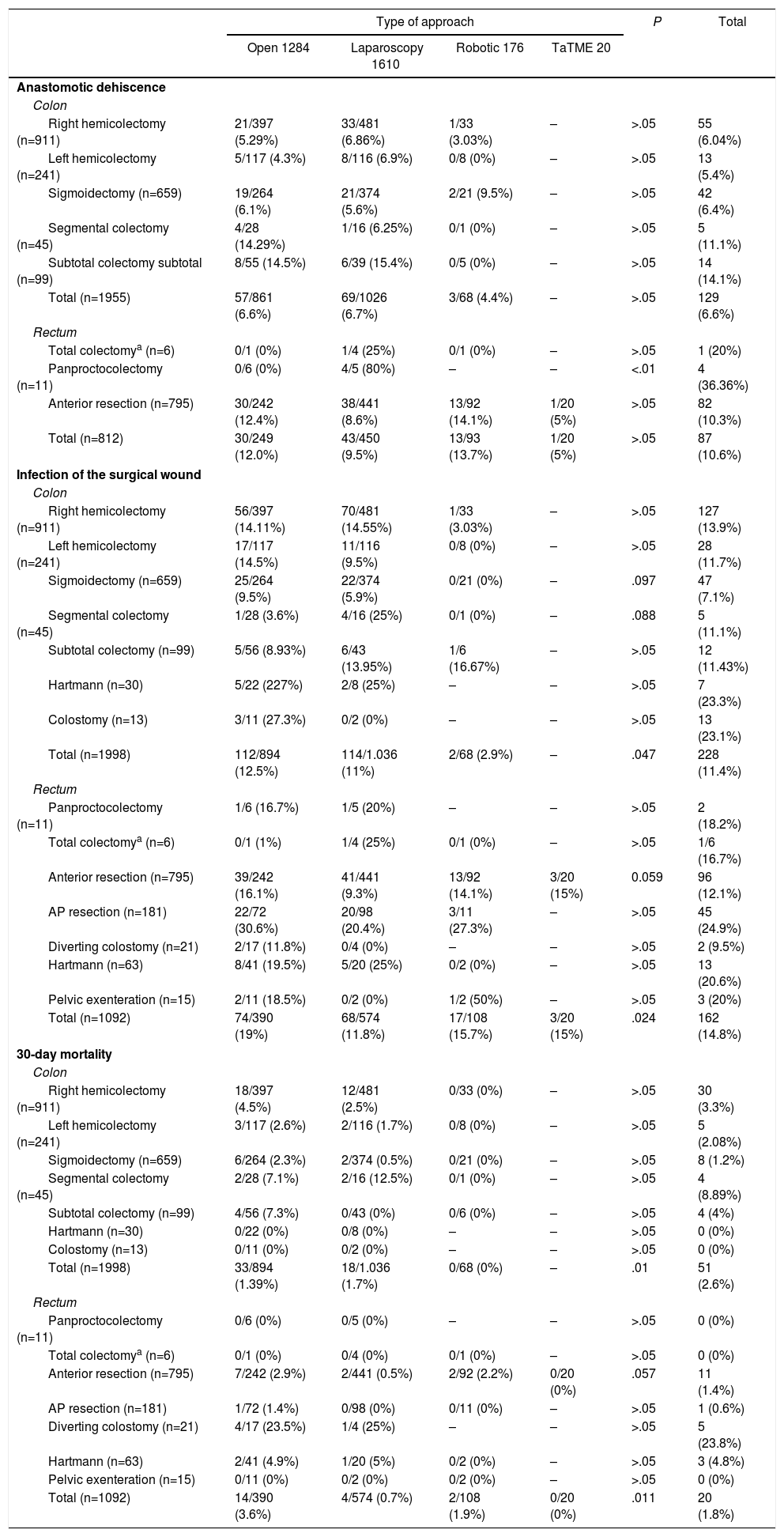
As can be seen in Table 2, the most frequent procedure in the series was right hemicolectomy (29.5%), which constituted 45% of colon cancer surgeries, followed by anterior resection (25.2%), used in 72% of rectal cancer surgeries. The laparoscopic approach was the most frequent in all groups and in most interventions, except in segmental colectomy, Hartmann's procedure and in diversion colostomy, where open surgery was the first choice. The overall rates of anastomotic leakage and surgical wound infection were 7.8% and 12.6%, respectively, while overall 30-day mortality was 2.3%. Table 2 shows these variables according to the main surgical procedure and the type of approach. The incidence of dehiscence in rectal cancer was slightly higher than in the colon (10.6% vs 6.6% respectively), although this was not statistically significant (P<.01).
Main Variables According to Type of Neoplasm, Approach and Procedure.
| Type of approach | P | Total | ||||
|---|---|---|---|---|---|---|
| Open 1284 | Laparoscopy 1610 | Robotic 176 | TaTME 20 | |||
| Anastomotic dehiscence | ||||||
| Colon | ||||||
| Right hemicolectomy (n=911) | 21/397 (5.29%) | 33/481 (6.86%) | 1/33 (3.03%) | – | >.05 | 55 (6.04%) |
| Left hemicolectomy (n=241) | 5/117 (4.3%) | 8/116 (6.9%) | 0/8 (0%) | – | >.05 | 13 (5.4%) |
| Sigmoidectomy (n=659) | 19/264 (6.1%) | 21/374 (5.6%) | 2/21 (9.5%) | – | >.05 | 42 (6.4%) |
| Segmental colectomy (n=45) | 4/28 (14.29%) | 1/16 (6.25%) | 0/1 (0%) | – | >.05 | 5 (11.1%) |
| Subtotal colectomy subtotal (n=99) | 8/55 (14.5%) | 6/39 (15.4%) | 0/5 (0%) | – | >.05 | 14 (14.1%) |
| Total (n=1955) | 57/861 (6.6%) | 69/1026 (6.7%) | 3/68 (4.4%) | – | >.05 | 129 (6.6%) |
| Rectum | ||||||
| Total colectomya (n=6) | 0/1 (0%) | 1/4 (25%) | 0/1 (0%) | – | >.05 | 1 (20%) |
| Panproctocolectomy (n=11) | 0/6 (0%) | 4/5 (80%) | – | – | <.01 | 4 (36.36%) |
| Anterior resection (n=795) | 30/242 (12.4%) | 38/441 (8.6%) | 13/92 (14.1%) | 1/20 (5%) | >.05 | 82 (10.3%) |
| Total (n=812) | 30/249 (12.0%) | 43/450 (9.5%) | 13/93 (13.7%) | 1/20 (5%) | >.05 | 87 (10.6%) |
| Infection of the surgical wound | ||||||
| Colon | ||||||
| Right hemicolectomy (n=911) | 56/397 (14.11%) | 70/481 (14.55%) | 1/33 (3.03%) | – | >.05 | 127 (13.9%) |
| Left hemicolectomy (n=241) | 17/117 (14.5%) | 11/116 (9.5%) | 0/8 (0%) | – | >.05 | 28 (11.7%) |
| Sigmoidectomy (n=659) | 25/264 (9.5%) | 22/374 (5.9%) | 0/21 (0%) | – | .097 | 47 (7.1%) |
| Segmental colectomy (n=45) | 1/28 (3.6%) | 4/16 (25%) | 0/1 (0%) | – | .088 | 5 (11.1%) |
| Subtotal colectomy (n=99) | 5/56 (8.93%) | 6/43 (13.95%) | 1/6 (16.67%) | – | >.05 | 12 (11.43%) |
| Hartmann (n=30) | 5/22 (227%) | 2/8 (25%) | – | – | >.05 | 7 (23.3%) |
| Colostomy (n=13) | 3/11 (27.3%) | 0/2 (0%) | – | – | >.05 | 13 (23.1%) |
| Total (n=1998) | 112/894 (12.5%) | 114/1.036 (11%) | 2/68 (2.9%) | – | .047 | 228 (11.4%) |
| Rectum | ||||||
| Panproctocolectomy (n=11) | 1/6 (16.7%) | 1/5 (20%) | – | – | >.05 | 2 (18.2%) |
| Total colectomya (n=6) | 0/1 (1%) | 1/4 (25%) | 0/1 (0%) | – | >.05 | 1/6 (16.7%) |
| Anterior resection (n=795) | 39/242 (16.1%) | 41/441 (9.3%) | 13/92 (14.1%) | 3/20 (15%) | 0.059 | 96 (12.1%) |
| AP resection (n=181) | 22/72 (30.6%) | 20/98 (20.4%) | 3/11 (27.3%) | – | >.05 | 45 (24.9%) |
| Diverting colostomy (n=21) | 2/17 (11.8%) | 0/4 (0%) | – | – | >.05 | 2 (9.5%) |
| Hartmann (n=63) | 8/41 (19.5%) | 5/20 (25%) | 0/2 (0%) | – | >.05 | 13 (20.6%) |
| Pelvic exenteration (n=15) | 2/11 (18.5%) | 0/2 (0%) | 1/2 (50%) | – | >.05 | 3 (20%) |
| Total (n=1092) | 74/390 (19%) | 68/574 (11.8%) | 17/108 (15.7%) | 3/20 (15%) | .024 | 162 (14.8%) |
| 30-day mortality | ||||||
| Colon | ||||||
| Right hemicolectomy (n=911) | 18/397 (4.5%) | 12/481 (2.5%) | 0/33 (0%) | – | >.05 | 30 (3.3%) |
| Left hemicolectomy (n=241) | 3/117 (2.6%) | 2/116 (1.7%) | 0/8 (0%) | – | >.05 | 5 (2.08%) |
| Sigmoidectomy (n=659) | 6/264 (2.3%) | 2/374 (0.5%) | 0/21 (0%) | – | >.05 | 8 (1.2%) |
| Segmental colectomy (n=45) | 2/28 (7.1%) | 2/16 (12.5%) | 0/1 (0%) | – | >.05 | 4 (8.89%) |
| Subtotal colectomy (n=99) | 4/56 (7.3%) | 0/43 (0%) | 0/6 (0%) | – | >.05 | 4 (4%) |
| Hartmann (n=30) | 0/22 (0%) | 0/8 (0%) | – | – | >.05 | 0 (0%) |
| Colostomy (n=13) | 0/11 (0%) | 0/2 (0%) | – | – | >.05 | 0 (0%) |
| Total (n=1998) | 33/894 (1.39%) | 18/1.036 (1.7%) | 0/68 (0%) | – | .01 | 51 (2.6%) |
| Rectum | ||||||
| Panproctocolectomy (n=11) | 0/6 (0%) | 0/5 (0%) | – | – | >.05 | 0 (0%) |
| Total colectomya (n=6) | 0/1 (0%) | 0/4 (0%) | 0/1 (0%) | – | >.05 | 0 (0%) |
| Anterior resection (n=795) | 7/242 (2.9%) | 2/441 (0.5%) | 2/92 (2.2%) | 0/20 (0%) | .057 | 11 (1.4%) |
| AP resection (n=181) | 1/72 (1.4%) | 0/98 (0%) | 0/11 (0%) | – | >.05 | 1 (0.6%) |
| Diverting colostomy (n=21) | 4/17 (23.5%) | 1/4 (25%) | – | – | >.05 | 5 (23.8%) |
| Hartmann (n=63) | 2/41 (4.9%) | 1/20 (5%) | 0/2 (0%) | – | >.05 | 3 (4.8%) |
| Pelvic exenteration (n=15) | 0/11 (0%) | 0/2 (0%) | 0/2 (0%) | – | >.05 | 0 (0%) |
| Total (n=1092) | 14/390 (3.6%) | 4/574 (0.7%) | 2/108 (1.9%) | 0/20 (0%) | .011 | 20 (1.8%) |
According to the type of approach, there were no significant differences in the percentage of dehiscence in colon surgery. In rectal surgery, the incidence of dehiscence was slightly higher in the open (12%) and robotic (13.7%) approaches than in the laparoscopic (9.5%) approach, particularly in anterior resection (12.4% and 14.1% vs 8.6%); however, this was not statistically significant. The percentage of surgical wound infections was slightly higher in the group of patients with open surgery (14.4% open surgery vs 11.2% laparoscopic+robotic) and very close to reaching statistical significance (P=.065). Infections were more frequent in patients who were treated for rectal neoplasia (P=.006), being especially frequent in the group of patients who underwent open surgery (19% open vs 11.8% in laparoscopy and 15.7% robotic; P=.024). There was higher postoperative 30-day mortality in the open surgery group, and this was statistically significant (3.6% in open surgery, 1.3% in laparoscopic surgery and 1.1% in robotic surgery; P<.01). 10.2% of patients with anastomotic leak died within the first 30 days.
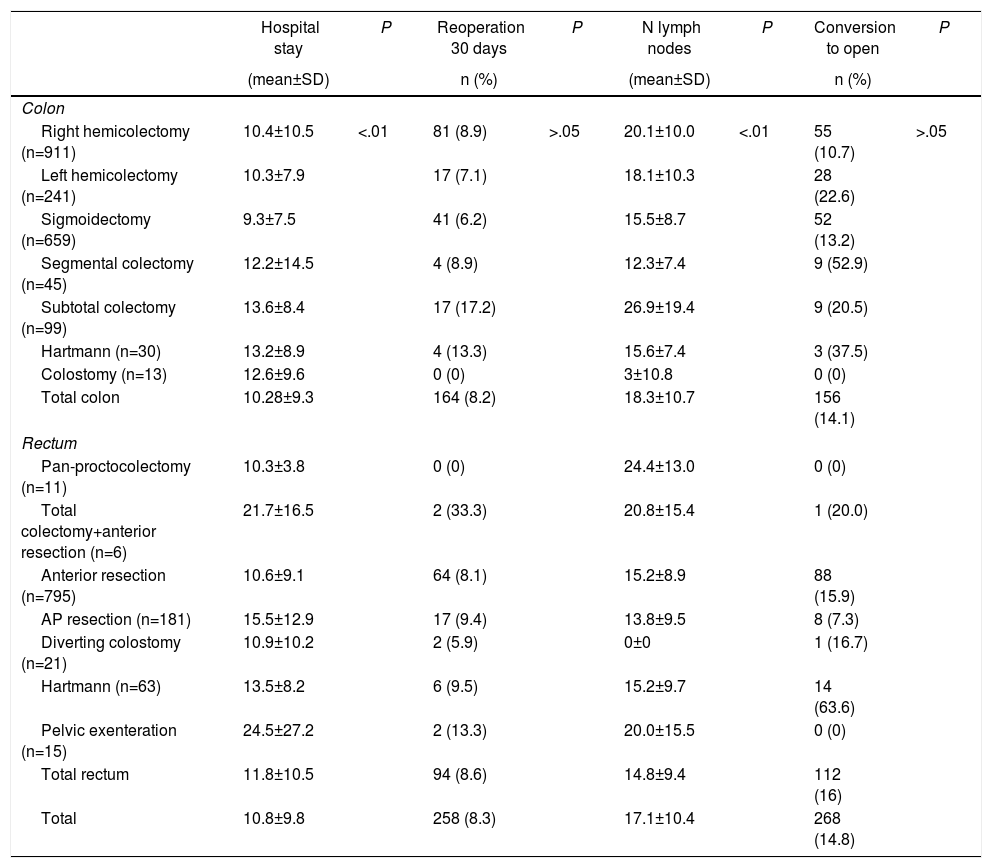
The mean global postoperative hospital stay was 10.8±9.8 days, with significantly longer admissions in patients with open surgery (P<.01). The rate of reoperations within the first 30 days was slightly higher for patients treated for rectal cancer (8.2% vs 8.6%) (P=.686). There were no significant differences in the conversion rate to open surgery (Table 3). The mean number of isolated lymph nodes in the surgical specimen was 18.32±10.7 and 14.8±9.4 for colon and rectal surgeries, respectively. Overall, in 71.2% of the patients, more than 12 lymph nodes were isolated.
Secondary Variables According to Type of Neoplasm and Approach.
| Hospital stay | P | Reoperation 30 days | P | N lymph nodes | P | Conversion to open | P | |
|---|---|---|---|---|---|---|---|---|
| (mean±SD) | n (%) | (mean±SD) | n (%) | |||||
| Colon | ||||||||
| Right hemicolectomy (n=911) | 10.4±10.5 | <.01 | 81 (8.9) | >.05 | 20.1±10.0 | <.01 | 55 (10.7) | >.05 |
| Left hemicolectomy (n=241) | 10.3±7.9 | 17 (7.1) | 18.1±10.3 | 28 (22.6) | ||||
| Sigmoidectomy (n=659) | 9.3±7.5 | 41 (6.2) | 15.5±8.7 | 52 (13.2) | ||||
| Segmental colectomy (n=45) | 12.2±14.5 | 4 (8.9) | 12.3±7.4 | 9 (52.9) | ||||
| Subtotal colectomy (n=99) | 13.6±8.4 | 17 (17.2) | 26.9±19.4 | 9 (20.5) | ||||
| Hartmann (n=30) | 13.2±8.9 | 4 (13.3) | 15.6±7.4 | 3 (37.5) | ||||
| Colostomy (n=13) | 12.6±9.6 | 0 (0) | 3±10.8 | 0 (0) | ||||
| Total colon | 10.28±9.3 | 164 (8.2) | 18.3±10.7 | 156 (14.1) | ||||
| Rectum | ||||||||
| Pan-proctocolectomy (n=11) | 10.3±3.8 | 0 (0) | 24.4±13.0 | 0 (0) | ||||
| Total colectomy+anterior resection (n=6) | 21.7±16.5 | 2 (33.3) | 20.8±15.4 | 1 (20.0) | ||||
| Anterior resection (n=795) | 10.6±9.1 | 64 (8.1) | 15.2±8.9 | 88 (15.9) | ||||
| AP resection (n=181) | 15.5±12.9 | 17 (9.4) | 13.8±9.5 | 8 (7.3) | ||||
| Diverting colostomy (n=21) | 10.9±10.2 | 2 (5.9) | 0±0 | 1 (16.7) | ||||
| Hartmann (n=63) | 13.5±8.2 | 6 (9.5) | 15.2±9.7 | 14 (63.6) | ||||
| Pelvic exenteration (n=15) | 24.5±27.2 | 2 (13.3) | 20.0±15.5 | 0 (0) | ||||
| Total rectum | 11.8±10.5 | 94 (8.6) | 14.8±9.4 | 112 (16) | ||||
| Total | 10.8±9.8 | 258 (8.3) | 17.1±10.4 | 268 (14.8) | ||||
SD: standard deviation.
Recently, there has been growing interest in analyzing health outcomes in surgical units by implementing quality standards to define good care practices. In Spain until now, there has been limited experience in hospital and medical unit certification. Four autonomous communities have regulations and official accreditation programs based on external and voluntary evaluation. The National Healthcare System, in strategy 7 of the Quality Plan, addresses the certification and auditing of hospitals, departments and units, with the aim of establishing common basic requirements as well as guarantees for safety and quality.
The AECP unit accreditation program aims to observe and measure different fundamental characteristics that units must have. It is a voluntary process through which professionals of the units systematically review their own practice, demonstrating a certain level of competence in coloproctology. In addition, it aims to guarantee the presence and/or acquisition of new skills, as well as a certain amount of development over time, by also highlighting the deficiencies of the unit. This evaluation process is also evaluated by an external tutor not affiliated with the unit who is assigned by the AECP. Among the different facets evaluated by the program are the colorectal cancer treatment standards. There has been extensive experience in evaluating rectal cancer treatment through a program that evaluates and audits surgical treatment, known as the Viking project. After its start in Norway in 2006, the program has spread throughout Europe, including Spain, with enormous success. However, unlike in other countries, it has not been possible for the health authorities or the companies involved to provide enough support for this program to actually behave like a true healthcare audit.9
Variability in clinical practice continues to be a very common characteristic in surgical processes worldwide. However, in scheduled colorectal surgery, there are common factors that enable systematization of the intervention, using steps for homogeneity. This causes a positive impact on the quality of surgery and facilitates learning of professionals in training.7
There are many studies in which various variables have been proposed as quality indicators.10–16 In this article, we have analyzed the indicators related with the treatment of colorectal cancer. We analyzed a significant number of patients operated on with different approaches and, unlike other studies, the colon and rectum surgery results were evaluated independently. Anastomotic leakage is one of the most feared complications that increases perioperative morbidity, mortality and hospital stay. In our study, the overall anastomotic leak rate was 7.8%, and we independently observed a dehiscence rate of 6.6% in colon surgeries and 10.6% in rectal. Several authors17–19 have reported rates similar to ours with figures between 6.4% and 8.7%. Other studies, such as those by Nikolian et al.,20 Hyman et al.21 and Park et al.,22 present anastomotic leak rates of less than 3%, and the reported risk factors included male sex, immunosuppression, obesity and ileo-rectal anastomosis. However, the systematic review by Cong et al.23 defined a rate of 8.6% in rectal surgery. This variability in the percentage of anastomotic leaks may be due to the heterogeneity in the definition, variability of the inclusion criteria for each study, as well as the different experience in colorectal surgery among hospitals.24
Specifically, the dehiscence rates in rectal cancer are slightly higher compared with the 8.5% of the Viking Project, especially at the expense of open surgery. Taking into account the threshold proposed by the AECP certification manual (<10% in colon and <15% in lower rectum), these results are acceptable, although we believe that they can be improved.
During the first 30 postoperative days, 71 patients died (2.3%). This mortality rate is slightly lower than that of other publications. Thus, for example, Van der Sluis et al.25 presented a mortality rate of 9.1%, which dropped to 4.6% in patients with scheduled surgery, such as those included in our study. In our series, significantly higher mortality was observed in patients undergoing open surgery (3.8% open versus 1.3% laparoscopic/robotic surgery). It is likely that this is the result of a bias, since it is common for the group of patients with open surgery to include cases with more advanced tumors, comorbidities and conversions, whose mortality is higher.25,26 Likewise, the mortality rate in our study secondary to anastomotic dehiscence was 10.2% (colon: 12.2% vs rectum: 7.4%, P=.325); this was slightly lower than reports in the literature, which varied from 11% to 17%.27–29
Postoperative infection rates, both in the colon (11.4%) and rectal (14.8%) cancer groups, were higher than those proposed by the clinical pathway of the Spanish Association of Surgeons (10%). However, we observed a significant decrease in postoperative infections in patients treated laparoscopically and robotically (11.2%) compared to open surgery (14.48%),26 which has already been described by multiple previous studies. From the point of view of the surgical approach, we agree with other published studies, where our findings confirm that minimally invasive surgery reduces the rate of postoperative infection and days of hospitalization, but does not show differences with open surgery in the rate of anastomotic dehiscence in colon surgery. However, in our study we did observe important, though not significant, differences in the dehiscence rate of rectal surgery when comparing the open versus laparoscopic approaches.
This study has allowed us to demonstrate the functioning of a specific part of the unit certification program and the capacity for analysis that the program itself provides. The analysis of the indicators shows us that audit and improvement are key elements in the treatment of colorectal cancer in units committed to this new way of working. Undoubtedly there are certification program parameters that should be analyzed more thoroughly in future publications, such as comorbidities, surgical risk or related complications.
In conclusion, the evaluation of these quality indicators in certified hospital units has provided us a photograph of healthcare practices in our country related to the treatment of colorectal cancer. The data presented here are highly important for establishing realistic references for the healthcare practice of “units of excellence” in the treatment of colorectal processes.
AuthorshipF. de la Portilla was responsible for the concept and design of this study, as well as the data interpretation, design and composition of the article.
Sergio Builes and Alejandra Garcóa-Novoa were responsible for the analysis and interpretation of the results and the composition of the article. The remaining authors participated in the critical analysis of the article.
Conflict of InterestsThe authors have no conflicts of interests to declare.
Please cite this article as: de la Portilla F, Builes S, García-Novoa A, Espín E, Kreisler E, Enríquez-Navascues JM, et al. Análisis de los indicadores de calidad en la cirugía de cáncer colorectal de unidades acreditadas por la Asociación Española de Coloproctología. Cir Esp. 2018;96:226–233.