The diagnosis of thyroid nodules is challenging without surgical intervention. In routine clinical practice, it is usually established with ultrasound and fine-needle aspiration (FNA). If the cytology shows follicular neoplasm with indeterminate cytology or follicular proliferation (Bethesda III or IV)1, surgery is indicated for diagnosis.
A preoperative prediction model has recently been published for follicular thyroid carcinoma (FTC) calculated from the data of 3649 patients treated with thyroidectomy,2 which has obtained a sensitivity of 90.2%, specificity 87.7%, negative predictive value 99.8% and positive predictive value 90.7%.
The objective of this study is to verify the usefulness and clinical applicability of this model in our setting.
We designed an observational, descriptive and prospective study on the population of patients operated on for nodular thyroid pathology with FNA. Surgery was indicated in all cases of follicular proliferation (Bethesda T3 and T4). Radiological reports were taken into consideration. FNA was performed following the ACR-TIRADS classification, based on TR3, applying the clinical protocol.3 Preoperative serum thyroglobulin and antithyroglobulin antibodies (TgAb) were analyzed. A modification by our group was applied to the described formula (2) so that the cut-off point was 0:
- calcification: 1 yes; 0 no
- nodule component: 1 solid, 0 cystic or solid-cystic
- blood flow: 1 yes; 0 no
Y values were compared with the definitive result of the biopsy after surgery. Y values >0 were considered an effective test for diagnosis.
From February 2020 to February 2021, 23 cases were included. The sex distribution was 16 women and 7 men. Mean age was 58 years. The indication for thyroidectomy was by FNA T3 in 15 cases and by FNA T4 in the remaining 8. In 4 cases, total thyroidectomy was performed due to contralateral nodules, while in the remainder hemithyroidectomy was done with isthmectomy.
The result of the formula was Y > 0 in 13 cases (56%) and less than 0 in the remaining 10 (44%). There were 10 FTC, including 4 microcarcinomas. The prevalence in this series was 43%.
The agreement of the formula with the results is shown by means of a 2 × 2 contingency table (Table 1) following the Standards for Reporting of Diagnostic Accuracy Studies (STARD) recommendations.
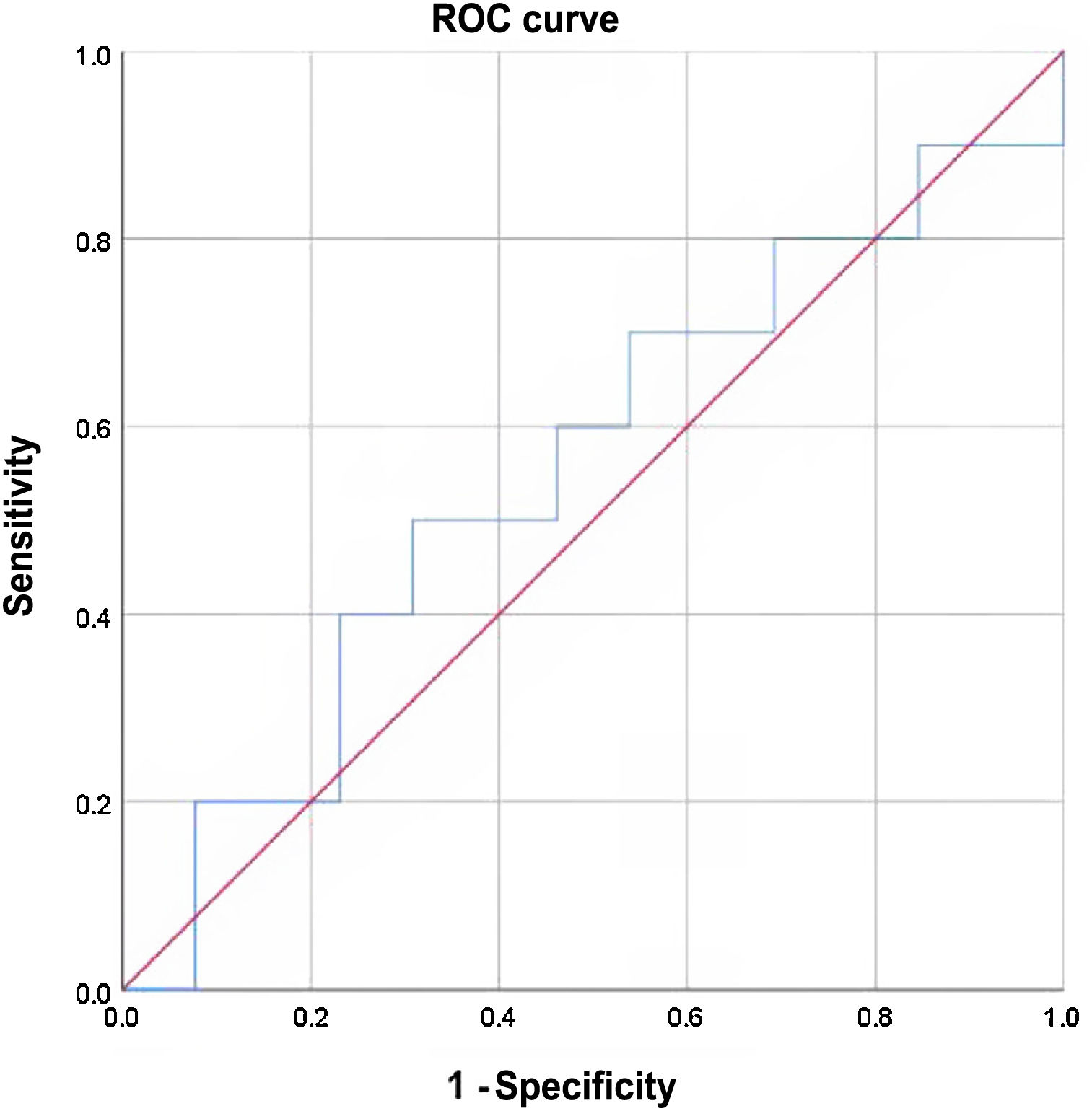
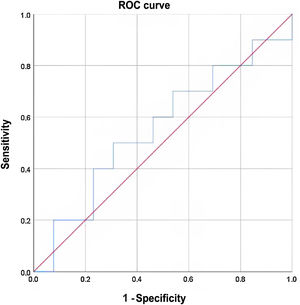
Sensitivity was 60% and specificity 46%. The positive predictive value was 46% and the negative predictive value 60%. Expressed in an ROC curve as a continuous numerical variable according to thyroglobulin levels, the results for various cut-off points were no better (Fig. 1).
The area under the curve (AUC) was 0.55, with a standard deviation of 0.125 (with a 95% confidence interval of 0.3 to 0.79).
When we compared patients whose TgAb was not elevated (less than 115 IU), the results were similar: sensitivity 55% and specificity 50%.
The diagnostic challenge is due to the fact that the only certainty is the capsular and/or vascular invasion of the follicular cells in the complete piece of the nodule. In addition, the ultrasound characteristics associated with a higher probability of malignancy are more evident in papillary carcinoma, but not so much in FTC.4
The recruitment of cases was affected by the COVID pandemic, similar to what has happened in other regions.5
In trying to reproduce the prediction model for FTC designed by Yu et al, our results were not comparable to the authors’ results.2
This discrepancy may be due to the individual variability of the radiological interpretation,6 exclusion of patients with previous FNA,2 and/or differences in the incidence of FTC (1.7%2 vs 43% of the population in this series). Patients with Bethesda III or IV FNA have a risk of carcinoma from 10% to 40%,4 which is consistent with our findings.
Thyroglobulin is influenced by TgAb3 and by previous FNA.7 In the series presented, the FNA was performed at least 3 weeks before; therefore, its determination should not be influenced.8
The sample size is not close to what is necessary for statistical power.
In conclusion, in our setting and with certain limitations, the positive predictive value results for the determination of FTC were not reproduced when we applied the Yu et al formula based on thyroglobulin levels and ultrasound parameters. Therefore, our results do not support the systematic use of this formula without defining certain factors, such as the target population, methodology, population prevalence, and other factors that must be analyzed by more groups. Further studies are necessary to compile a foundation on which to base critical clinical decisions when applying this formula.
Please cite this article as: Díaz-Roldán J, Martín-Jiménez MC, Recio Moyano G, Rubio-Sánchez T, Román-Rando A. Estudio de aplicabilidad de un modelo de predicción preoperatoria en carcinoma folicular de tiroides. Cir Esp. 2022;100:312.