Radiotherapy techniques associated with breast-conserving surgery have evolved in early breast cancer thanks to a better knowledge of tumor radiobiology, highlighting intraoperative radiotherapy (IORT). However, complications have been documented with this procedure, mainly fibrosis. Transforming growth factor beta (TGF-β) is a cytokine with an active role in radiation-induced fibrosis, which could be used as an early biomarker for the development of fibrosis.
MethodsMulticentric prospective analysis of 60 patients with breast cancer who underwent breast-conserving surgery, 30 of whom had received additional IORT. TGF-β values were evaluated in serum pre-surgery and in serum collected 24 h after surgery. In addition, we evaluated surgical wound fluids collected 6 h and 24 h following surgery.
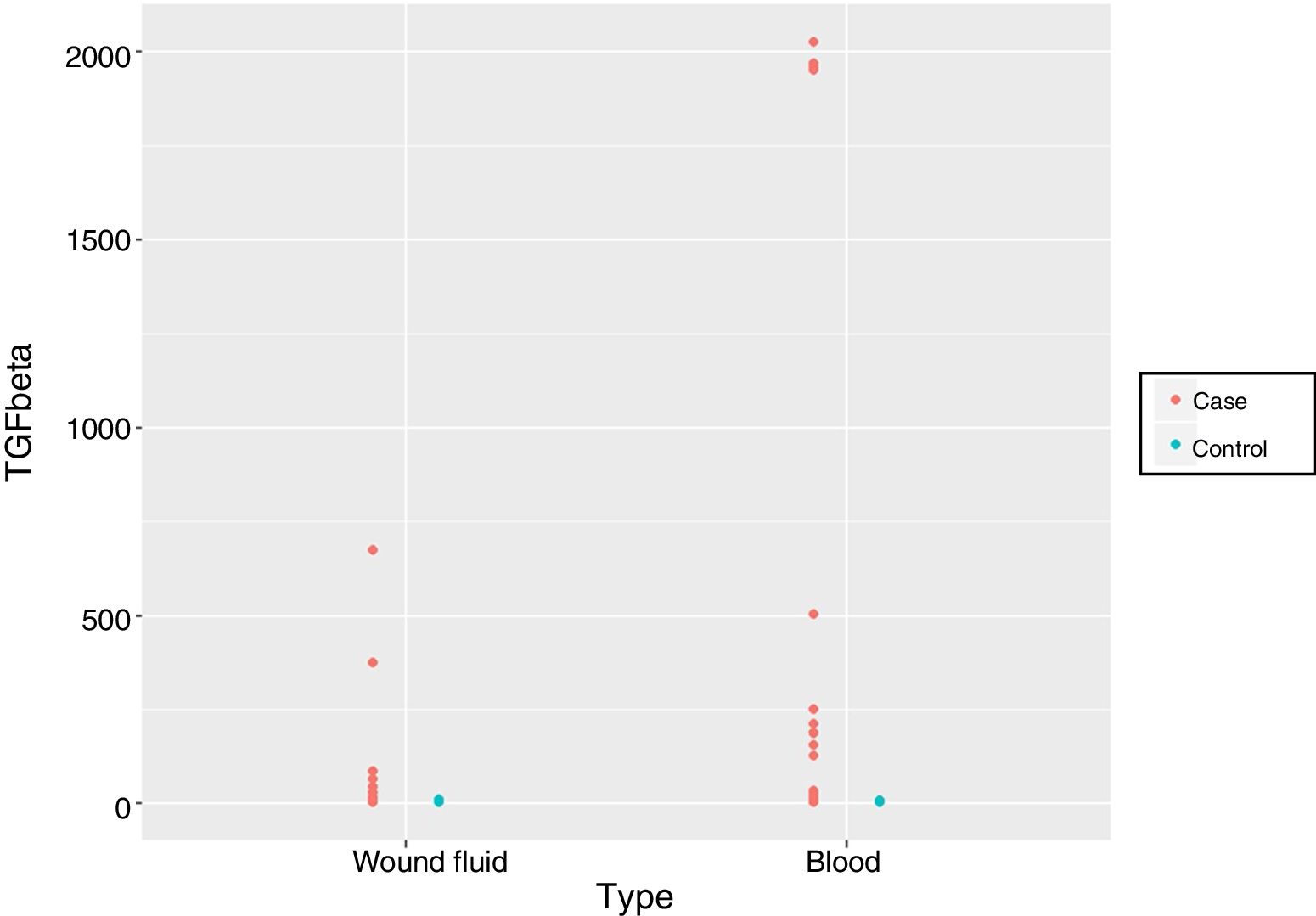
ResultsSerum and surgical wound fluids TGF-β values collected over 24 h following surgery were significantly higher in patients who received additional IORT (P < .0001). Notably, 8 of these patients showed values above 1,000 pg/ml. There were no differences between the samples (serum or surgical wound fluids) (P = .5881).
ConclusionsAlthough further investigation is needed, higher TGF-β values in IORT during breast-conserving surgery can be used as an early biomarker for the development of fibrosis.
Las técnicas de radioterapia asociadas a la cirugía conservadora del cáncer de mama precoz han evolucionado gracias a un mayor conocimiento de la radiobiología tumoral, destacando entre ellas la radioterapia intraoperatoria (RIO). Sin embargo, se han documentado complicaciones con dicha técnica, principalmente la fibrosis. El factor de crecimiento transformante beta (TGF-β) es una citocina relacionada con la fibrosis inducida después de la radiación que podría servir como marcador temprano del riesgo de desarrollo de la misma.
MétodosEstudio prospectivo multicéntrico de 60 pacientes a las que se les ha sometido a cirugía conservadora por cáncer de mama, asociada a RIO en 30 de ellas. Se evalúan los valores de TGF-β en muestras de suero preoperatorio y a las 24 h desde la cirugía, y de muestras de drenaje a las 6 y 24 h desde la cirugía.
ResultadosLos valores de TGF-β objetivados en el suero y en el débito de drenaje a las 24 h desde la cirugía de las pacientes que recibieron RIO fueron significativamente mayores que los de aquellas que no la recibieron (p < 0,0001). De entre ellas, 8 pacientes presentaron valores superiores a 1.000 pg/ml. Estas diferencias entre los grupos no se modifican por el tipo de muestra utilizada, bien sea suero, bien débito de drenaje (p = 0,5881).
ConclusionesAunque deben realizarse más estudios, valores elevados de TGF-β en las pacientes con cáncer de mama a las que se les realiza cirugía conservadora asociada a RIO pueden predecir el riesgo de fibrosis.
The treatment of early breast cancer based on breast-conserving surgery and radiotherapy is currently the treatment of choice in most patients.1–3
External radiotherapy is the standard treatment and is a fundamental component that affects both local control and survival. However, thanks to a greater understanding of tumor radiobiology, a select group of patients can benefit from accelerated partial radiation techniques by treating the tumor bed in fewer sessions at higher doses.1,4–6
Intraoperative radiotherapy (IORT) associated with breast-conserving surgery for the treatment of breast cancer is an indicator of the significant advancement of these techniques. With this method, we are able to apply higher doses in a single radiation dose directly to the tumor bed, providing potential benefits in recurrence rates and the toxicity of organs at risk.1,4–10
Veronesi et al.11 found that 6% of their patients presented complications after the application of IORT, the most frequent of which were fibrosis and fat necrosis. However, a study by Key et al. observed that, after IORT, only 2.4% presented grade II fibrosis or higher; meanwhile, when associated with external radiotherapy, this figure rose to 43.3%.12 According to the published literature, the most severe complications have been associated with higher doses of 24 Gy, with an incidence of around 30%, which drops to 25% with doses of 20–21 Gy.7,11,13–15
Fibrosis is the formation of excess connective tissue characterized by increased production of extracellular matrix proteins and the accumulation of activated fibroblasts during the healing process.16,17 Although this complication can be detected years after radiation exposure, transforming growth factor beta (TGF-β) is the main regulatory component of fibrosis from its initial stages,16,18–20 and its relationship with induced fibrosis after radiation has been demonstrated in patients.16,21
TGF-β is a cytokine involved in different processes such as radiation-related fibrosis, increasing the production of extracellular matrix proteins and the accumulation of differentiated fibroblasts.16,17
Previous studies have linked it to the development of fibrosis after breast cancer treatment, so it may be able to be used as an early marker for the development of these complications.16,22,23 It is therefore necessary to determine the effect of IORT on this determining factor, as the inhibition of its activity in selected patients could reduce these adverse effects.
The objective of our study, therefore, is to analyze the effect of IORT on TGF-β values in serum and drained wound fluid samples from our patients, as it could be useful as an early marker of the development of fibrosis.
MethodsStudy designWe designed a multicenter prospective study, divided into 2 comparative groups with consecutive cases to assess the relationship of the IORT with TGF-β values observed in the surgical wound fluid and serum samples from patients who had undergone breast-conserving surgery for breast cancer, either with or without associated IORT.
Serum samples were collected from all patients preoperatively and 24 h after surgery, and wound fluid samples were taken 6 and 24 h after surgery.
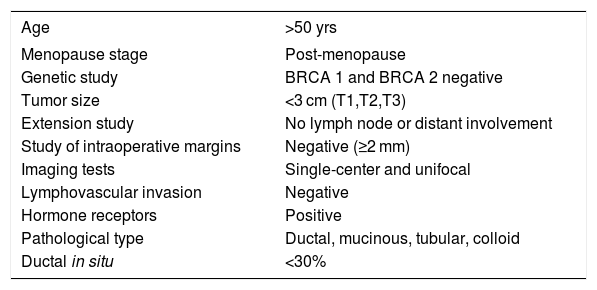
The inclusion criteria for the group of patients who had been administered IORT (case group) met the IORT selection criteria at our hospital, which are shown in Table 1.
IORT selection criteria.
| Age | >50 yrs |
|---|---|
| Menopause stage | Post-menopause |
| Genetic study | BRCA 1 and BRCA 2 negative |
| Tumor size | <3 cm (T1,T2,T3) |
| Extension study | No lymph node or distant involvement |
| Study of intraoperative margins | Negative (≥2 mm) |
| Imaging tests | Single-center and unifocal |
| Lymphovascular invasion | Negative |
| Hormone receptors | Positive |
| Pathological type | Ductal, mucinous, tubular, colloid |
| Ductal in situ | <30% |
The control group included patients with breast cancer who had undergone breast-conserving surgery without IORT, meeting the same inclusion criteria as the case group.
Patients with benign systemic diseases with known alteration of the molecular pathways of TGF-β were excluded from this study, as were patients with multiple primary tumors.
RadiotherapyIORT was administered using a Model S700 Xoft Axxent™ X-ray Source that works with 50-KV voltage, provides spherical isodoses and imparts 20 Gy on the surface of the balloon-type applicator. For dose calculation, the manufacturer provides all the characterization values of the x-ray source necessary to model the planning system (in our case, BrachyVision™ by Varian. The tissue thickness between the balloon and the skin was always greater than 1 cm, as verified by ultrasound.1
Immunodetection of TGF-βSerum and surgical drainage samples were centrifuged, sterilized and stored at −80 °C at the time of collection to keep them intact.
Subsequently, the TGF-β analysis was carried out in each of the samples collected by ELISA kits specific for human proteins (Invitrogen, Thermo Fisher Scientific) using positive and negative controls according to the instructions of the manufacturer. All determinations were made in duplicate to be considered specific.
Sample size calculationThe calculation of the sample size was based on the calculation for comparison of means of 2 independent groups, assuming that the variable corresponding to TGF-β follows a normal distribution and the Student’s t-test is used.
We assume that the variance of this variable is similar in both groups yet unknown. To quantify the magnitude of the difference between the groups, we have used the so-called standardized difference (¿) of means used by Machin et al.,24 following the recommendations of Cohen25 and assuming that the effect is large with ¿ = .8.
For a 95% confidence level and a minimum power of 80%, 25 individuals were necessary per group. Assuming a 5% of possible losses, the estimated sample necessary was 27 patients per group.
Compliance with ethical standardsAll patients included in the study signed the required specific informed consent form. The study was approved by the Research Ethics Committee of our community.
Statistical analysisThe qualitative variables are reported by absolute and relative frequencies; quantitative variables are described with mean and standard deviation if they were parametric, and with median, first quartile and third quartile when non-parametric.
Subsequently, a bivariate analysis was carried out. The statistical association between qualitative variables was studied using the Chi-squared or Fisher’s tests, and between quantitative variables using the Student’s t or Mann Whitney U test for parametric or non-parametric variables, respectively.
For all research, R 3.5.1 statistical software was used, establishing the level of statistical significance at p < 0.05.
ResultsA total of 60 patients were recruited for the study, 30 of whom received IORT after breast-conserving cancer surgery.
Mean patient age was 65.53 years (SD 9.44); in control group, it was 66.47 years (SD 7.49), while in the case group it was slightly lower at 64.60 years (SD 11.10), with no statistically significant differences.
All patients included met the inclusion criteria, and no statistical differences were observed between groups.
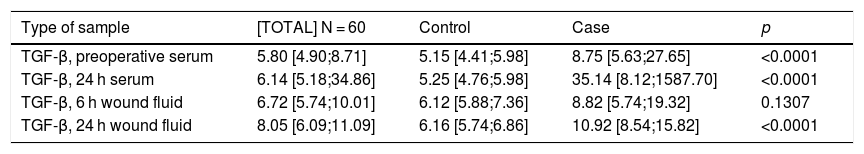
First, we compared the TGF-β values observed in the different sample types among patients who had undergone breast-conserving surgery followed by IORT (cases) or not (controls), and found that there were statistically significant differences in all cases except for the values obtained from the drained would fluid sample 6 h after surgery (Table 2).
Univariate comparison between cases and controls.
| Type of sample | [TOTAL] N = 60 | Control | Case | p |
|---|---|---|---|---|
| TGF-β, preoperative serum | 5.80 [4.90;8.71] | 5.15 [4.41;5.98] | 8.75 [5.63;27.65] | <0.0001 |
| TGF-β, 24 h serum | 6.14 [5.18;34.86] | 5.25 [4.76;5.98] | 35.14 [8.12;1587.70] | <0.0001 |
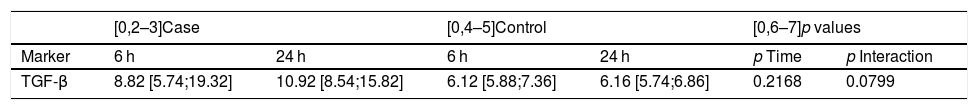
| TGF-β, 6 h wound fluid | 6.72 [5.74;10.01] | 6.12 [5.88;7.36] | 8.82 [5.74;19.32] | 0.1307 |
| TGF-β, 24 h wound fluid | 8.05 [6.09;11.09] | 6.16 [5.74;6.86] | 10.92 [8.54;15.82] | <0.0001 |
Subsequently, we analyzed the values obtained through models that contemplated whether patients had received IORT, as well as the type of sample (serum or wound fluid) and time after surgery.
When we measured TGF-β in serum comparing its preoperative values and 24 h after surgery, we observed statistically significant differences between the groups, as well as in their evolution over time and behavior (p < 0.001; 0.009 and 0.0409, respectively). In the group of patients who did not receive IORT, no variation in time was observed (medians of 5.15 and 5.25 preoperative and 24 h after surgery, respectively). However, in the group of patients who did receive IORT, the medians increased after 24 h compared to preoperative values (8.75 and 35.14, respectively). It should be noted that the values observed in the serum samples of IORT patients were higher than in the samples of patients who underwent conservative surgery only, and this group presented a series of patients with figures greater than 1000 pg/mL, which is consistent with the bibliography (Table 3).
When we compared the values obtained in the drained wound fluid samples 6 and 24 h after surgery, we observed statistically significant differences between the patient groups, with higher values found in patients who had received IORT (p = 0.0001). However, no differences were found in terms of time transpired since surgery or the behavior in the two groups (Table 4).
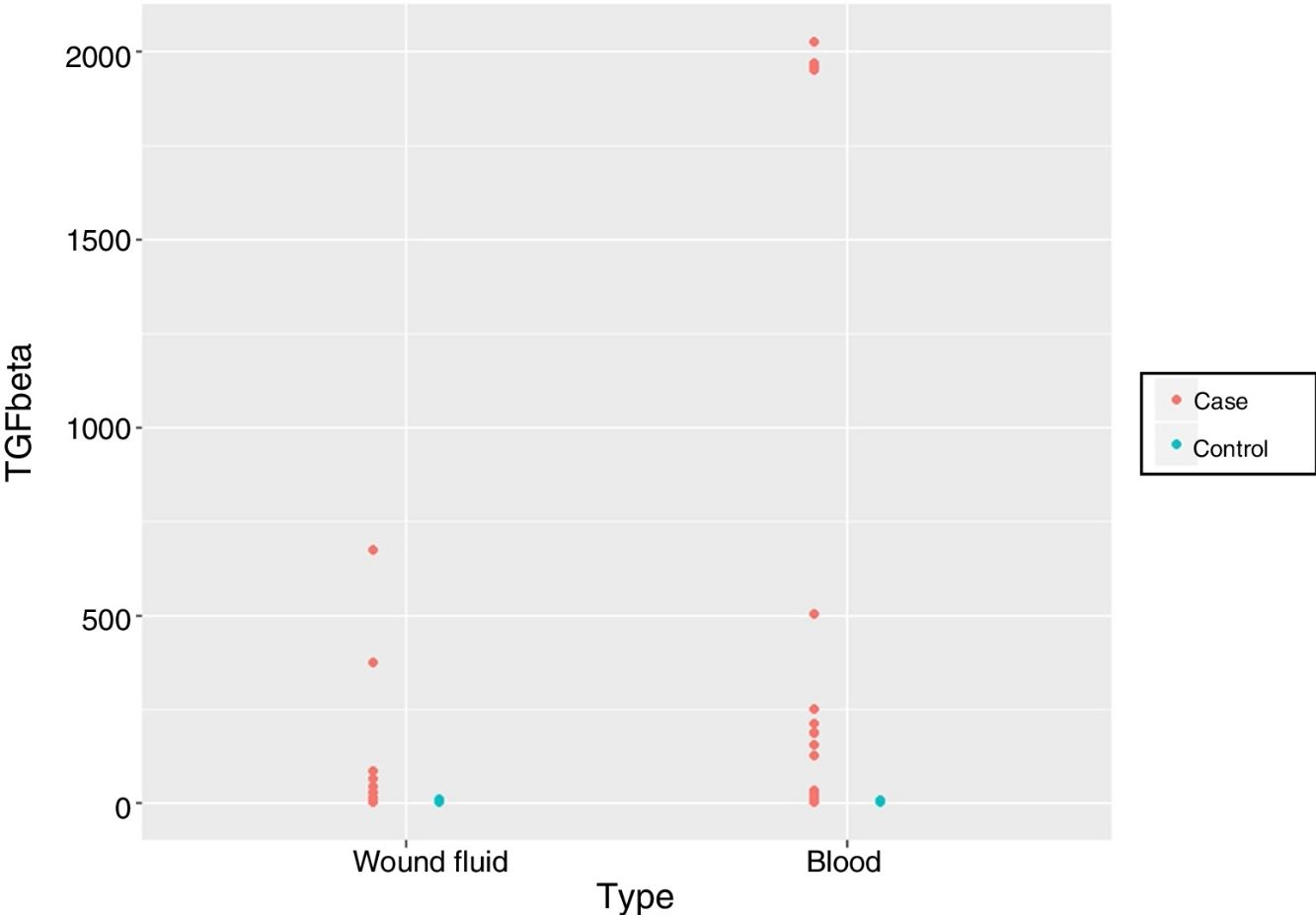
Finally, we compared the values obtained from the serum and wound discharge samples collected 24 h after surgery in both groups. We observed significant differences in magnitude that caused longitudinal changes according to whether the samples belonged to the patient group who had received IORT or not. However, it is important to note that no statistically significant differences were found regarding the type of sample used, be it either serum or wound fluid (p = 0.5881) (Fig. 1, Table 5).
DiscussionIn recent years, we have witnessed new developments in the techniques used for the treatment of breast cancer, and new therapies have appeared, such as IORT associated with breast-conserving surgery.9,10 Most studies agree that the most frequent complication after this treatment is fibrosis, so it is essential to identify predictive markers.1,7,26
Li et al.2,7 observed in their study that serum TGF-β levels were significantly higher in patients who subsequently developed moderate or severe fibrosis, further stating that serum values higher than 96pg/mL after surgery, prior to radiotherapy had a sensitivity of 76%, a specificity of 74% for the development of this complication. In addition, they concluded that, because the patients under study presented early-stage breast cancer with small tumor sizes, the altered TGF-β levels found can be, attributed to the individual’s own genetic variability, not be, affected by the tumor cells themselves. Boothe et al.2,3 demonstrated that serum TGF-β values are an early marker to predict fibrosis after surgery, prior to radiotherapy, afterwards as, well. Likewise, the authors concluded that the values found were not altered by the application of radiotherapy.
In our study, however, we have observed that patients who received IORT had serum TGF-β values that were significantly higher than patients who only underwent breast-conserving surgery for cancer, considering that this alteration is a consequence of the IORT (p < 0.0001). We should also mention that in 8 of the patients, the specific values were greater than 1.000 pg/mL. This finding is in line with the literature. Our patients presented initial stages of breast cancer with small tumors, so we can attribute this alteration to the genetic variability of the individual,27 which would make this group of patients especially susceptible to being chosen for planning subsequent treatment in order to prevent fibrosis.
Similarly, it should be noted that the TGF-β values found in preoperative serum can also be attributed to the genetic variability of the individual, since no statistically significant differences have been seen between the groups. Although further studies should be conducted, we believe that the differences in magnitude seen in the TGF-β values obtained 24 h after surgery are considerable enough to not be altered by these initial values.
Other authors have researched the altered TGF-β values after radiotherapy found in the drained surgical wound fluid. Scherer et al.22 collected wound fluid for 24 h, comparing patients who had received IORT versus those who had not, and observed that IORT did not have a significant effect on TGF-β levels in the drained discharge. However, the values they found were higher than those correlating with fibrosis and likewise demonstrated that the TGF-β found was active in the surgical drain fluid.
The present study, which concurs with the published literature, has found no statistically significant differences in TGF-β values in the surgical wound fluid after having administered IORT. However, similar to Scherer et al.,22 although the elevated levels found do not show a difference between patients with or without IORT 6 h after surgery, a significant difference was found 24 h after surgery associated with IORT (p < 0.0001).
To our knowledge of the published literature, this is the first study that compares TGF-β values obtained from patients who had undergone breast-conserving cancer surgery either with or without the application of IORT in serum samples and drained fluid samples. Differences were found between the groups without these findings being altered by the type of sample 24 h after surgery (p = 0.5881).
In conclusion, although further studies should be conducted, elevated TGF-β values in patients with breast cancer who undergo breast-conserving surgery in association with IORT can predict the risk of fibrosis.
Therefore, these patients should be chosen for treatments that aim to counteract the pro-fibrotic effect of this cytokine in order to avoid adverse effects without compromising tumor control. Numerous strategies are currently being studied, mainly using the following: antisense oligonucleotides that reduce TGF-β expression, neutralizing antibodies that prevent interaction with the TGF-β ligand receptor, chimeric recombinant proteins to sequester TGF-β and inhibitory molecules of the TGF-β kinase receptors.22–28 Local inhibition of TGF-β after surgery may be a viable option, as already demonstrated in glaucoma surgery using the lerdelimumab antibody in subconjunctival injection after surgery.29
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Bernad C et al. Cirugía conservadora de cáncer de mama y radioterapia intraoperatoria. ¿Podemos predecir la fibrosis? Cir Esp. 2019;97:517–522.