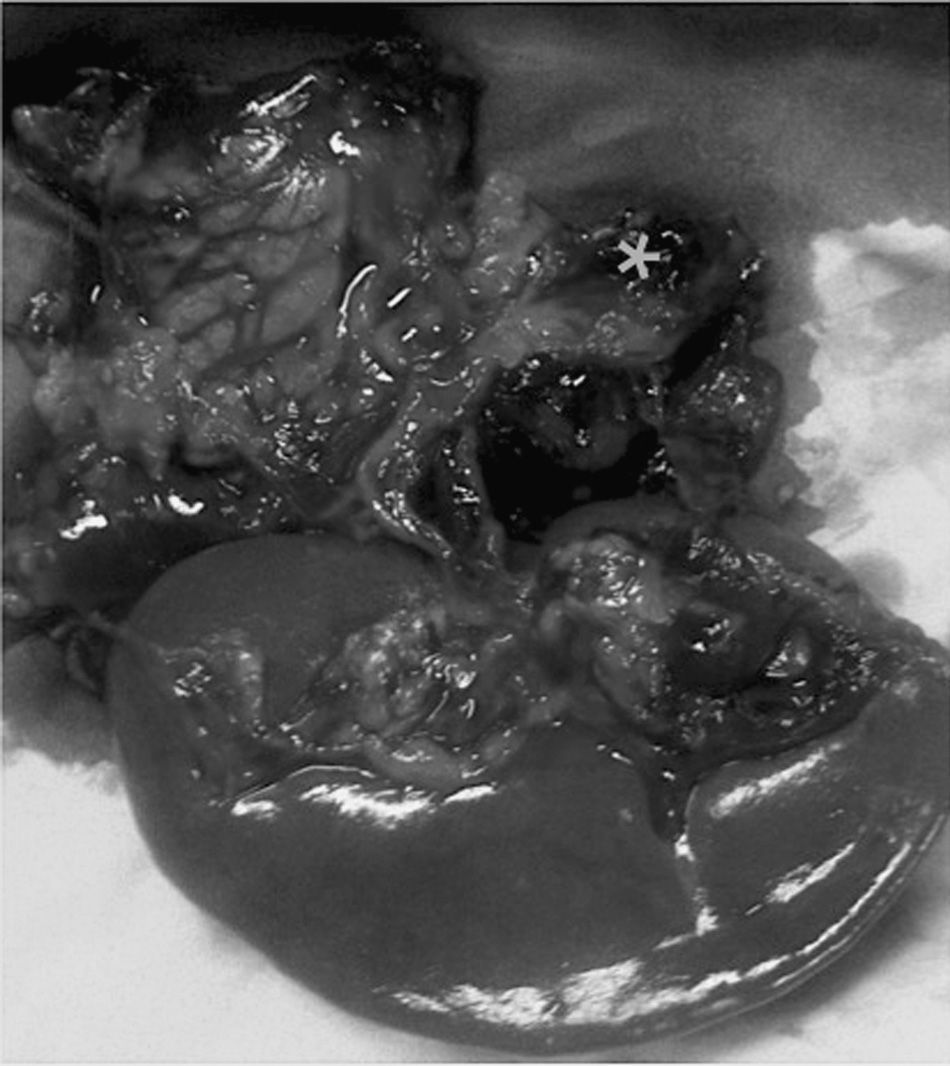
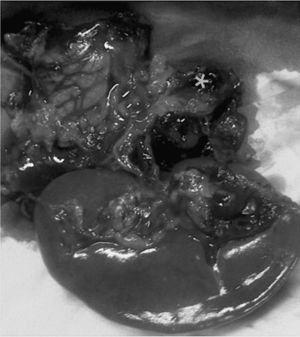
Spontaneous rupture of a splenic artery aneurysm (SAA) during pregnancy is a serious complication that has very high maternal and fetal mortality rates (70% and 90%, respectively).1 Proper early diagnosis and treatment are fundamental for improving the survival of both.
We present the case of a 41-year-old patient at 33 weeks of gestation after in vitro fertilization who consulted for pain in the left hypochondrium and epigastrium. She came to the Emergency Room 10h later with general malaise, abdominal pain in the hypogastrium and vomiting. Physical examination showed: BP 80/40mmHg, heart rate 120bpm. Abdominal pain was compatible with uterine contractions, and signs of associated fetal suffering indicated emergency cesarean section. After delivery of the fetus, a massive hemoperitoneum was observed that did not originate in the maternal uterus. The on-call surgeon was summoned. During exploratory laparotomy, a large tension hematoma was found in the left epigastrium–hypochondrium. With the suspected diagnosis of visceral aneurysm fissure, abdominal packing was used and, after the patient was hemodynamically stabilized, we initiated diagnostic–therapeutic angiography of the celiac trunk. During the procedure, the patient presented active bleeding and hemodynamic instability and was therefore transferred urgently to the operating room. With a presumed diagnosis of ruptured splenic artery aneurysm, the bleeding was initially controlled with clamping of the thoracic artery through a left thoracotomy (30min) and corporocaudal splenopancreatectomy, with the identification of a ruptured SAA (Fig. 1) after the ligation of the artery. The patient required transfusion of 8 units of packed red blood cells, plasma and support with vasoactive drugs; temporary closure of the abdomen was done with open vacuum. 48h later, the laparotomy was closed, with no evidence of further retroperitoneal hemorrhage. In the postoperative period, the patient presented a splenic hematoma that required percutaneous drainage. At the time of discharge, the mother presented no exocrine/endocrine pancreatic insufficiency, and the newborn was in good health.
SAA was described by Beaussier in 17702 and in 1869 it was reported in association with pregnancy.3 It is the third most frequent intraabdominal aneurysm, after the infrarenal aorta and iliac artery.
Pathogenic mechanisms of SAA during gestation include the hormonal and hemodynamic alterations associated with pregnancy. Other risk factors include multiple births, portal hypertension, atherosclerosis and fibrodysplasia of the vascular wall.4,5
More than 400 cases of SAA have been reported; 30% of these were during pregnancy, generally in the third trimester.5–7
The probability of aneurysm rupture is directly related to its size: the risk is higher in those larger than 2cm,5 and mortality is 10% higher.8 The symptoms of SAA rupture are non-specific: pain in the left hypochondrium or epigastrium associated with nausea and vomiting or the palpation of a pulsatile mass, with hemodynamic instability. Episodes have also been reported of upper gastrointestinal bleeding due to erosion of the posterior gastric wall, as well as the “double rupture phenomenon”, which happens in 20% of cases. This involves a limited initial hemorrhage to the omental bursa, which causes epigastric pain with hemodynamic stability and, after a variable period of latency, massive hemoperitoneum with hypovolemic shock.5,8,9 In our case, the patient presented this rupture in 2 stages, with no diagnosis in the first phase.
Indications for elective SAA surgery are symptomatic aneurysms larger than 2cm in patients with previous surgery for portal hypertension or liver transplantation and in pregnant women or those of fertile age (with aneurysms of any size).5,8 In these cases, elective treatment can include endovascular techniques (stents or selective embolization) or surgery (either open or laparoscopic in selected cases)5,8,10 in order to resect the aneurysm or ligate the splenic artery, while attempting to preserve the spleen.
For the diagnosis of ruptured splenic aneurysms, computed tomography with intravenous contrast is the best option in the emergency setting in hemodynamically stable patients. Hemorrhage in the omental bursa, retrogastric hematoma or contrast medium leak at the splenic artery should raise suspicions for the diagnosis and indicate therapeutic angiography with selective embolization.5,8
In emergency surgery due to SAA rupture, early aortic clamping (supra-celiac or thoracic) is a very useful maneuver for the initial control of exsanguinating hemorrhage. Afterwards, ligation of the splenic artery is carried out, generally with distal splenopancreatectomy.5,8–10 In our case, vascular control of the thoracic artery was necessary as it was impossible to safely access the abdominal aorta due to the large hematoma; the abdominal approach was used with a midline laparotomy, and ligation of the splenic artery was done with distal splenopancreatectomy.
SAA rupture is a rare but potentially fatal complication in pregnancy. It should be suspected in pregnant women with pain in the left hypochondrium and epigastrium and associated hemodynamic instability, especially since early diagnosis and treatment are key for the survival of both the fetus and mother.
Please cite this article as: Ledezma Peredo NV, Díaz-Tobarra M, Soria Estrems J, León Espinoza CA, Calvete Chornet J. Pinzamiento aórtico precoz en el tratamiento quirúrgico de la rotura del aneurisma de la arteria esplénica. Cir Esp. 2015;93:263–264.