Since the beginning of the pandemic, morbidity and mortality in emergency care of surgical patients have been the subject of several studies. However, most of these have compared this variable with that of the pre-COVID period, ignoring its evolution during the pandemic itself. In order to analyze this possible change, we performed a comparative study of morbidity and mortality in emergency surgery between the first and second waves of the pandemic in our center.
MethodsRetrospective longitudinal study including all patients over the age of 18 admitted and/or operated in the emergency setting in the two maximum incidence periods (MIP) of COVID-19 infection (1st MIP: 22/03/2020-31/05/2020; 2nd MIP: 26/08/2020-30/11/2020). The incidence of SARS-CoV-2 infection, treatment received, early morbidity and mortality and possible risk factors for complications were analyzed.
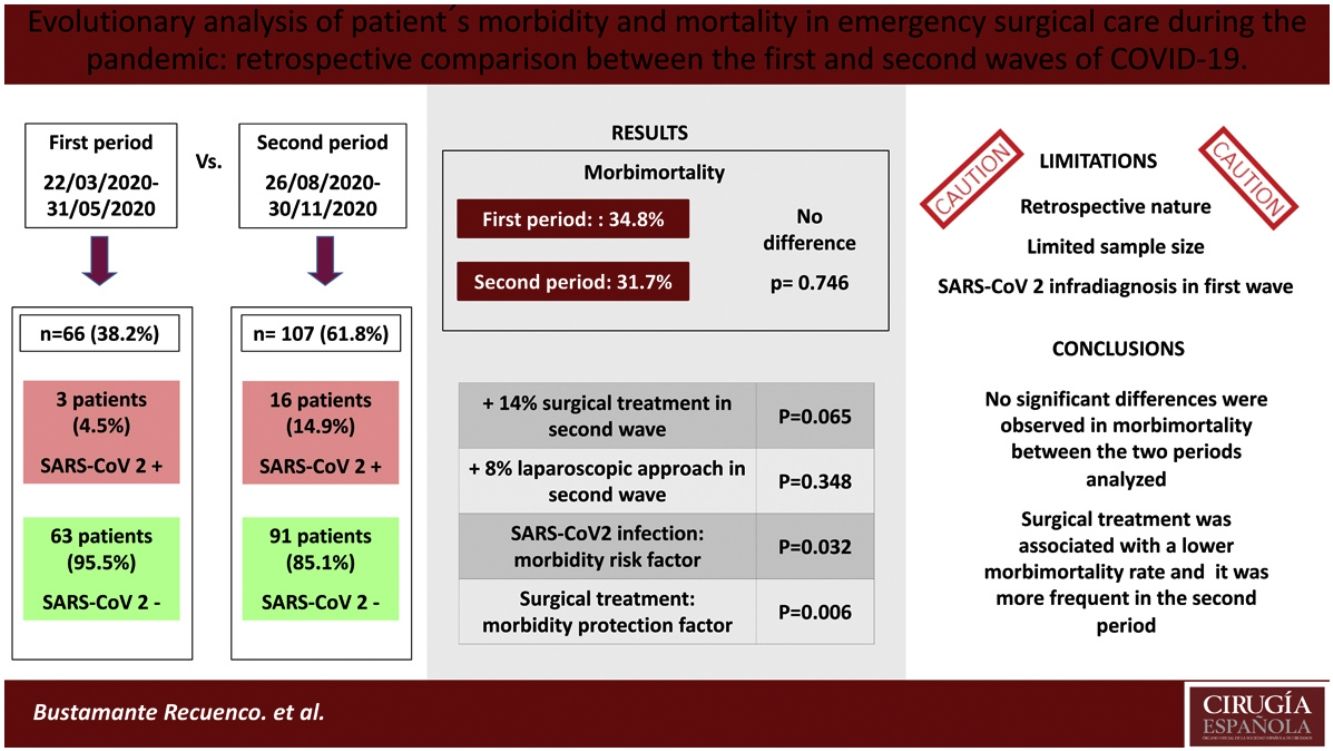
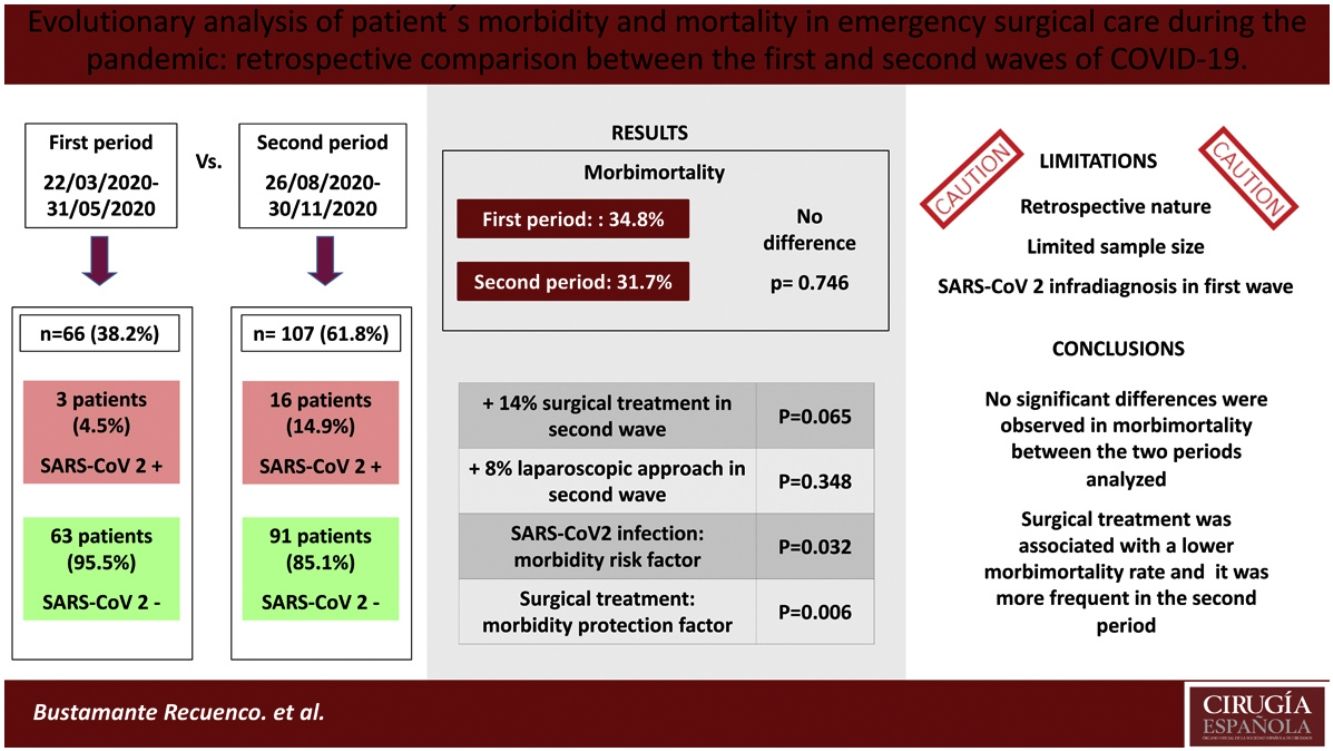
ResultsA total of 173 patients were analyzed (1st MIP: 66; 2nd MIP: 107). The incidence of COVID-19 was higher in the second period (14.95% vs. 4.54%). SARS-CoV-2 infection was associated with a higher rate of complications; however, no statistically significant differences were observed in morbimortality rate, either in the total sample (P = .746) or in patients with a positive COVID-19 test (P = .582) between both periods. Surgical treatment was found to be associated with a lower complication rate in both the first (P = .006) and second waves (P = .014), and it was more frequent in the second MIP (70.1% vs 57.6%), although statistical significance was not reached (P = .065).
ConclusionsNo significant differences were observed in morbidity and mortality of patients admitted and/or operated in the emergency setting in the two periods of maximum incidence of SARS-CoV-2 at our center. Surgical treatment was associated with lower morbidity and mortality rates, and it was more frequent in the second MIP.
Desde el comienzo de la pandemia, la morbimortalidad en la atención urgente al paciente quirúrgico ha sido objeto de estudio. Sin embargo, la mayoría de los estudios compararon dicha variable con la propia de la época pre-COVID, obviando la evolución de la misma durante la propia pandemia. Con el objetivo de analizar este posible cambio, realizamos un estudio comparativo de morbimortalidad en cirugía de urgencias entre la primera y segunda ola de la pandemia en nuestro centro.
Material y métodosEstudio retrospectivo longitudinal que incluyó a todos los pacientes mayores de 18 años ingresados y/o intervenidos quirúrgicamente de forma urgente en los dos períodos de máxima incidencia (PMI) de infección por COVID-19 (1ºPMI: 22/03/2020-31/05/2020; 2ªPMI: 26/08/2020-30/11/2020). Se analizó la incidencia de infección por SARS-CoV2, el tratamiento recibido, la morbimortalidad precoz y los posibles factores de riesgo de complicaciones.
ResultadosSe analizaron 173 pacientes (1ºPMI: 66; 2ºPMI: 107). La incidencia de COVID-19 fue mayor en el segundo periodo (14,95% vs. 4,54%). La infección por SARS-CoV-2 se asoció a una mayor tasa de complicaciones, sin embargo, no se observaron diferencias estadísticamente significativas en la morbimortalidad general (p = 0,746) ni en la de los pacientes COVID positivos (p = 0,582) entre ambos períodos. El tratamiento quirúrgico se asoció con una menor tasa de complicaciones tanto en la primera (p = 0,006) como en la segunda ola (p = 0,014). Dicho tratamiento quirúrgico fue más frecuente en el segundo PMI (70,1% vs 57,6%) aunque no se alcanzó la significación estadística al respecto de esta afirmación (p = 0,065).
ConclusionesNo se observaron diferencias significativas en la morbimortalidad de los pacientes ingresados y/o intervenidos quirúrgicamente de urgencias en los dos períodos de máxima incidencia de SARS-CoV-2 en nuestro centro. El tratamiento quirúrgico se asoció con una menor morbimortalidad, siendo este más frecuente en el segundo PMI.
SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) is an infectious agent of animal origin and is the seventh coronavirus described in our species.1 It is the cause of the disease known as COVID-19, whose symptoms are either mild or moderate in 80% of cases (fever, dry cough and asthenia). In its initial forms, it caused acute respiratory failure, metabolic acidosis, coagulopathy and multiple organ failure in 50%–60% of cases.2 Its rapid transmission through aerosols led to global expansion of the disease, and the World Health Organization (WHO) declared a pandemic in March 2020.3 Since then, we have had to make many changes to standard clinical practice. Without a doubt, healthcare systems around the globe have been negatively affected by the current pandemic.
Confronted by the propagation of COVID-19, Spanish hospitals were forced to reorganize themselves to respond to the demand for medical care, which increased dramatically at the onset of this new disease. Emergency surgery immediately underwent changes, as it was surgery that could not be delayed. The new situation led to the creation of protocols and recommendations by different organizations, such as the Spanish Association of Surgeons (Asociación Española de Cirujanos, AEC) or the World Society of Emergency Surgery (WSES),4,5 with the aim to unify and facilitate this transition. Although several articles have been published regarding the differences in morbidity and mortality in emergency surgery between the current era and the pre-COVID era,6–8 the evolution of that variable during the pandemic itself has been the subject of a much smaller study.
Therefore, we have decided to retrospectively compare morbidity and mortality rates between the 2 peak periods of maximum incidence of COVID-19 infection at our hospital, which is a level II public hospital with 330 hospitalization beds and 12 intensive care beds (ICU) that provides healthcare to a population of almost 200 000 inhabitants in a large, mainly rural, region. Our secondary objectives were to analyze the percentage of surgical procedures performed in each period and to identify possible risk factors for morbidity and mortality.
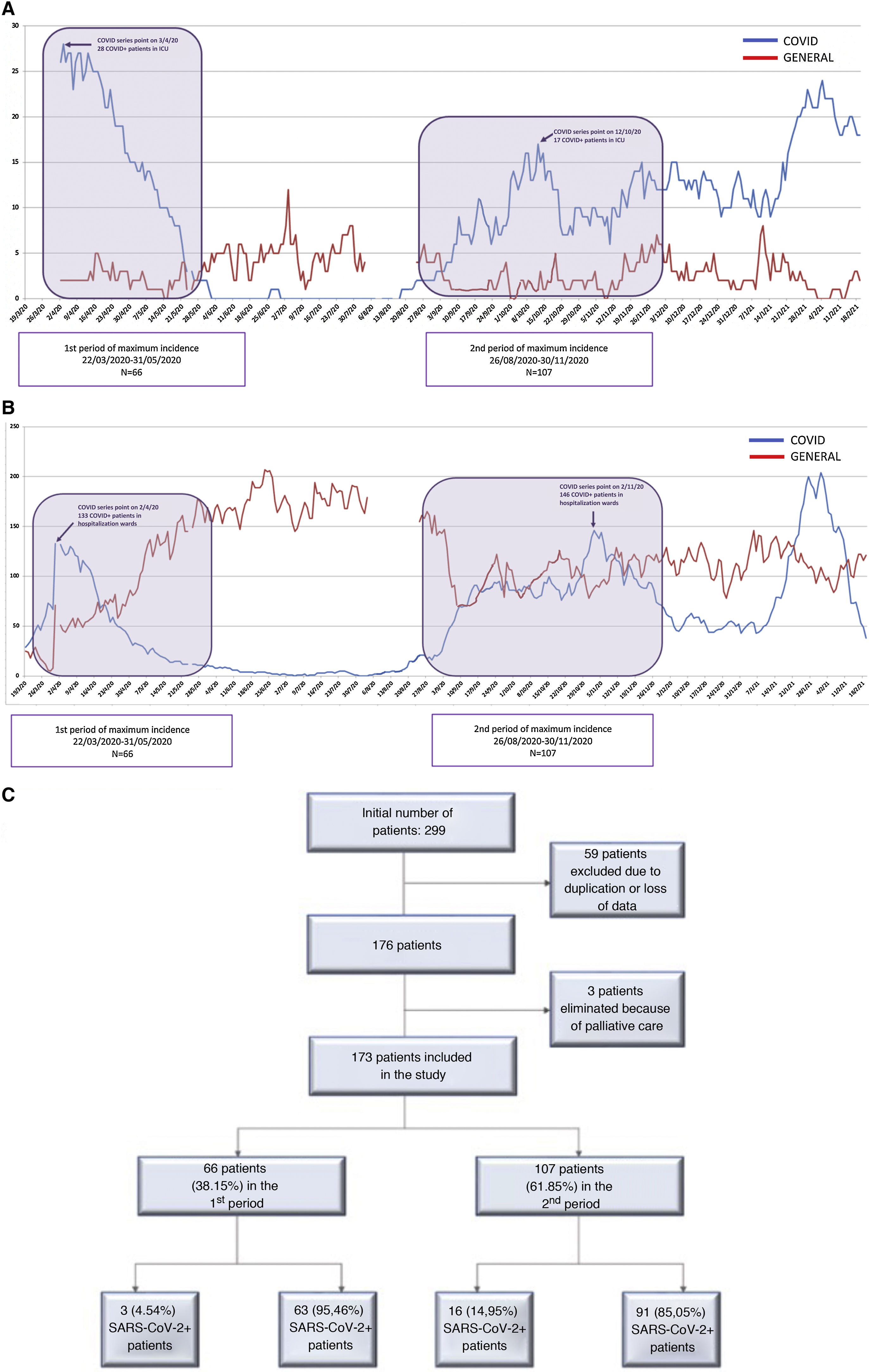
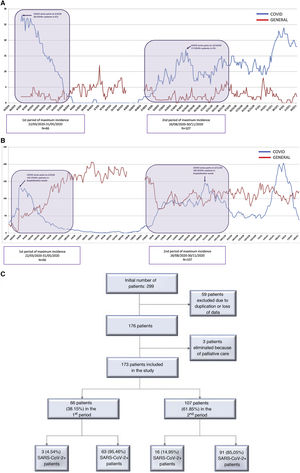
MethodsTwo maximum incidence periods (MIP) of COVID-19 cases were defined: the first period from March 22, 2020 to May 31, 2020 (70 days), and the second period from August 26, 2020 to November 30, 2020 (96 days) (Fig. 1A and B). These periods were established based on the criteria defined in the documents published by the AEC.10 These documents establish 5 pandemic phases based on the percentage of beds occupied by COVID-positive patients and the impact on medical care. We used the cut-off point in phase II to define the time intervals because surgical activity was limited after that date. This phase is also defined by a COVID occupancy of 0%–25% of hospital and ICU beds.
For these 2 MIP, we retrospectively analyzed and compared all patients older than 18 years of age who were admitted by the general surgery department, regardless of the treatment performed. Likewise, admissions from other services that subsequently required specific urgent management (surgical or conservative) were also included. Patients with the following criteria were excluded: lack of data, incomplete follow-up, or hospitalization for the dignified death protocol. The following variables were analyzed and compared: age, sex, anesthetic risk (ASA), hypertension (HTN), diabetes mellitus (DM), dyslipidemia (DLP), lung disease, cardiac disease, kidney disease, immunosuppression status, screening for SARS-CoV-2 before admission, SARS-CoV-2 infection defined as positive in the reverse transcriptase-polymerase chain reaction (RT-PCR) test or appearance of symptoms during hospitalization or within the first 20 days after hospital discharge, surgical treatment, hospital stay, and early morbidity/mortality. The Clavien-Dindo (C–D) classification was applied to determine the severity of postoperative complications.11 Patient data were extracted from an internal surgery department database.
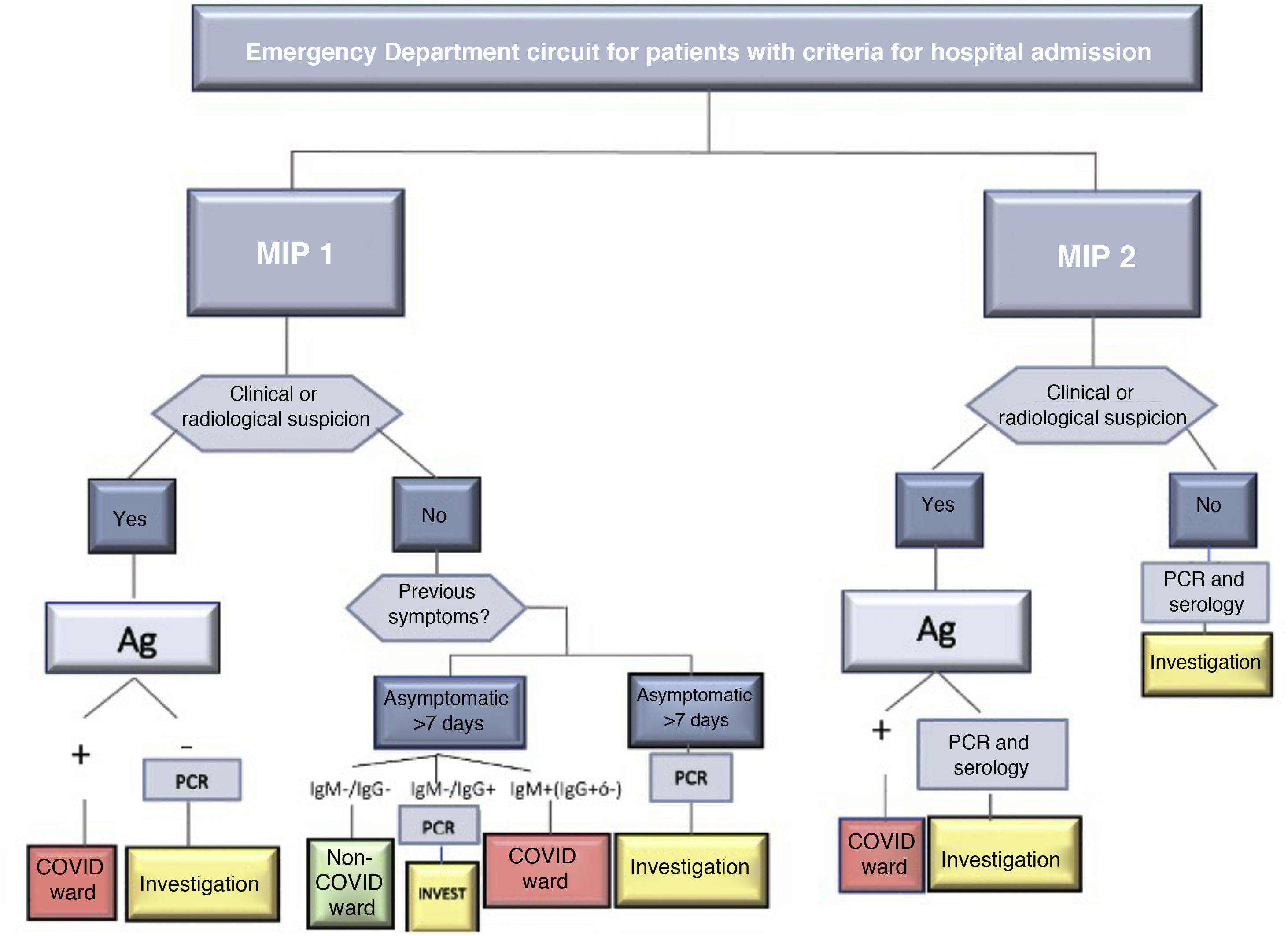
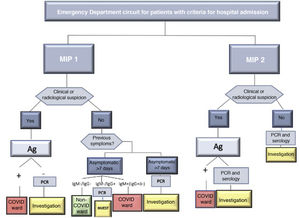
SARS-CoV-2 infection was determined by positive RT-PCR. In the absence of said test, the diagnosis was determined by the presence of symptoms or radiological signs on the computed tomography (CT) scan that were highly suggestive of infection and/or a positive IgM result in the serological tests for COVID-19. In case of discrepancy, priority was given to the result of the RT-PCR. Diagnostic and screening tests were requested based on the protocol of the center during both periods, which is shown in Fig. 2.
This study was conducted in accordance with the STROBE recommendations12 and the principles of the Declaration of Helsinki.13 Approval was obtained from the Clinical Research Ethics Committee.
Statistical analysisQuantitative variables were expressed according to the mean and standard deviation if they followed normal distribution (Shapiro–Wilk or Kolmogorov–Smirnov test) or the median and interquartile range otherwise. As a hypothesis contrast test, the chi-square test was used for qualitative variables and the Student’s t test or Mann–Whitney U for quantitative variables according to compliance with the assumption of normality. The analysis of risk factors for morbidity and mortality was performed using multiple binary logistic regression, including those variables with P < .020 in the univariate analysis or those considered possible predictors theoretically. The data analysis was performed with the SPSS version 25.0® statistical program (IBM, SPSS Statistics for Windows, Version 25.0, Amonk, NY).
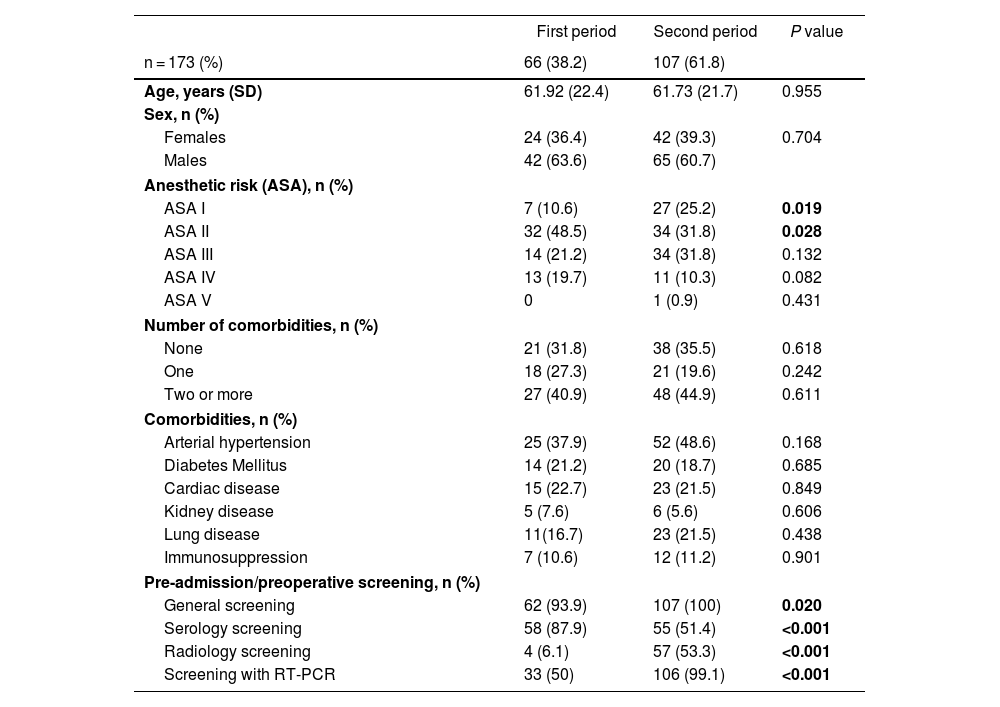
ResultsAs shown in Fig. 1C, 66 patients were included in the first peak period and 107 in the second (total = 173). No significant differences were detected in age, sex or specific comorbidities (Table 1). A higher percentage of ASA I patients (P = .019) and a lower percentage of ASA II patients (P = .028) were detected in the second MIP, while the proportion of ASA III–IV cases was similar in both groups (P = .875). In the second wave, greater screening for SARS-CoV-2 infection was performed (P = .020) compared to the first, using more radiological and RT-PCR screening (P < .001).
Demographic characteristics of the study sample.
| First period | Second period | P value | |
|---|---|---|---|
| n = 173 (%) | 66 (38.2) | 107 (61.8) | |
| Age, years (SD) | 61.92 (22.4) | 61.73 (21.7) | 0.955 |
| Sex, n (%) | |||
| Females | 24 (36.4) | 42 (39.3) | 0.704 |
| Males | 42 (63.6) | 65 (60.7) | |
| Anesthetic risk (ASA), n (%) | |||
| ASA I | 7 (10.6) | 27 (25.2) | 0.019 |
| ASA II | 32 (48.5) | 34 (31.8) | 0.028 |
| ASA III | 14 (21.2) | 34 (31.8) | 0.132 |
| ASA IV | 13 (19.7) | 11 (10.3) | 0.082 |
| ASA V | 0 | 1 (0.9) | 0.431 |
| Number of comorbidities, n (%) | |||
| None | 21 (31.8) | 38 (35.5) | 0.618 |
| One | 18 (27.3) | 21 (19.6) | 0.242 |
| Two or more | 27 (40.9) | 48 (44.9) | 0.611 |
| Comorbidities, n (%) | |||
| Arterial hypertension | 25 (37.9) | 52 (48.6) | 0.168 |
| Diabetes Mellitus | 14 (21.2) | 20 (18.7) | 0.685 |
| Cardiac disease | 15 (22.7) | 23 (21.5) | 0.849 |
| Kidney disease | 5 (7.6) | 6 (5.6) | 0.606 |
| Lung disease | 11(16.7) | 23 (21.5) | 0.438 |
| Immunosuppression | 7 (10.6) | 12 (11.2) | 0.901 |
| Pre-admission/preoperative screening, n (%) | |||
| General screening | 62 (93.9) | 107 (100) | 0.020 |
| Serology screening | 58 (87.9) | 55 (51.4) | <0.001 |
| Radiology screening | 4 (6.1) | 57 (53.3) | <0.001 |
| Screening with RT-PCR | 33 (50) | 106 (99.1) | <0.001 |
SD: Standard deviation; ASA: Classification using the American Society of Anesthesiologists anesthetic risk stratification; RT-PCR: Polymerase chain reaction test reverse transcription.
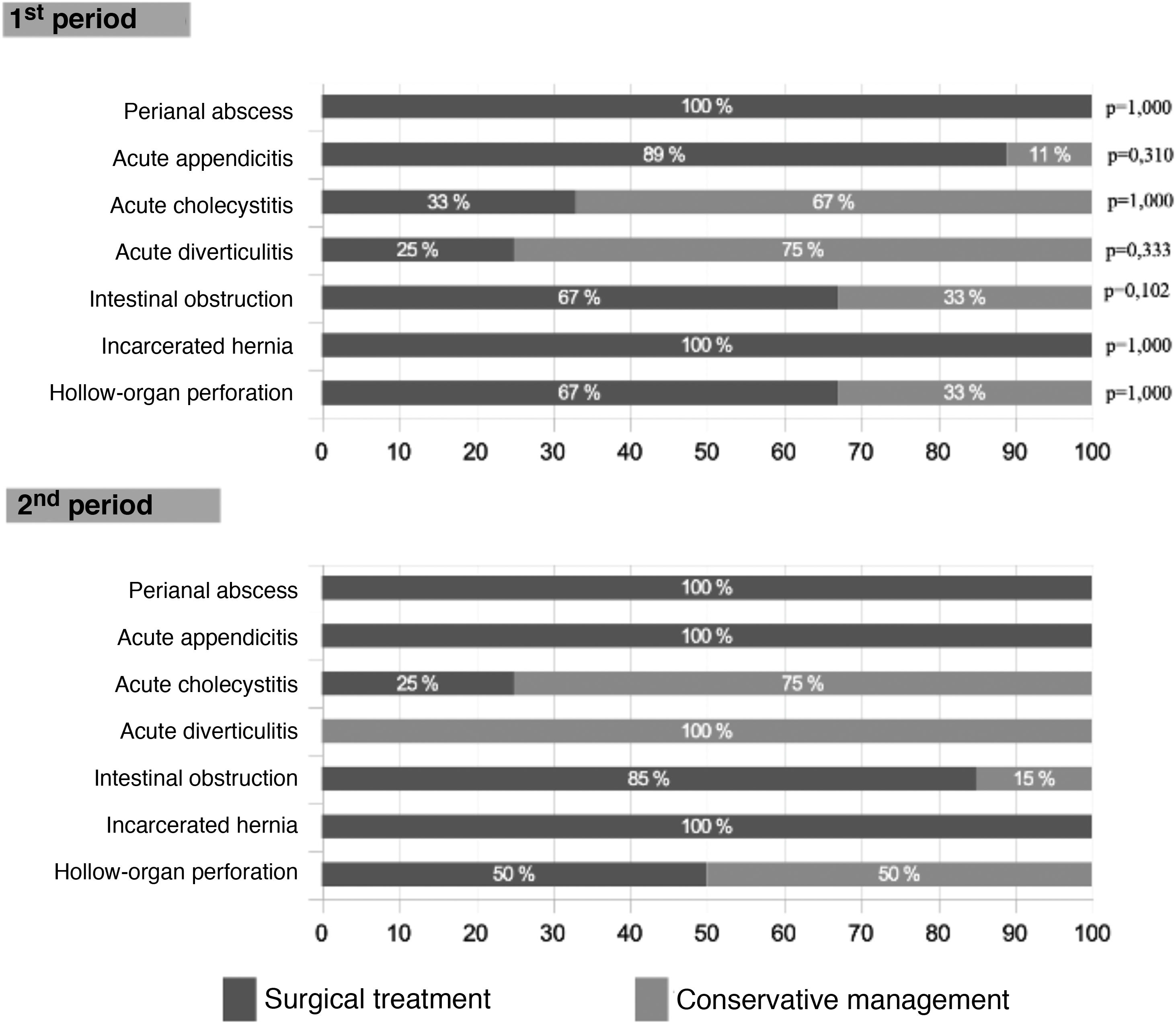
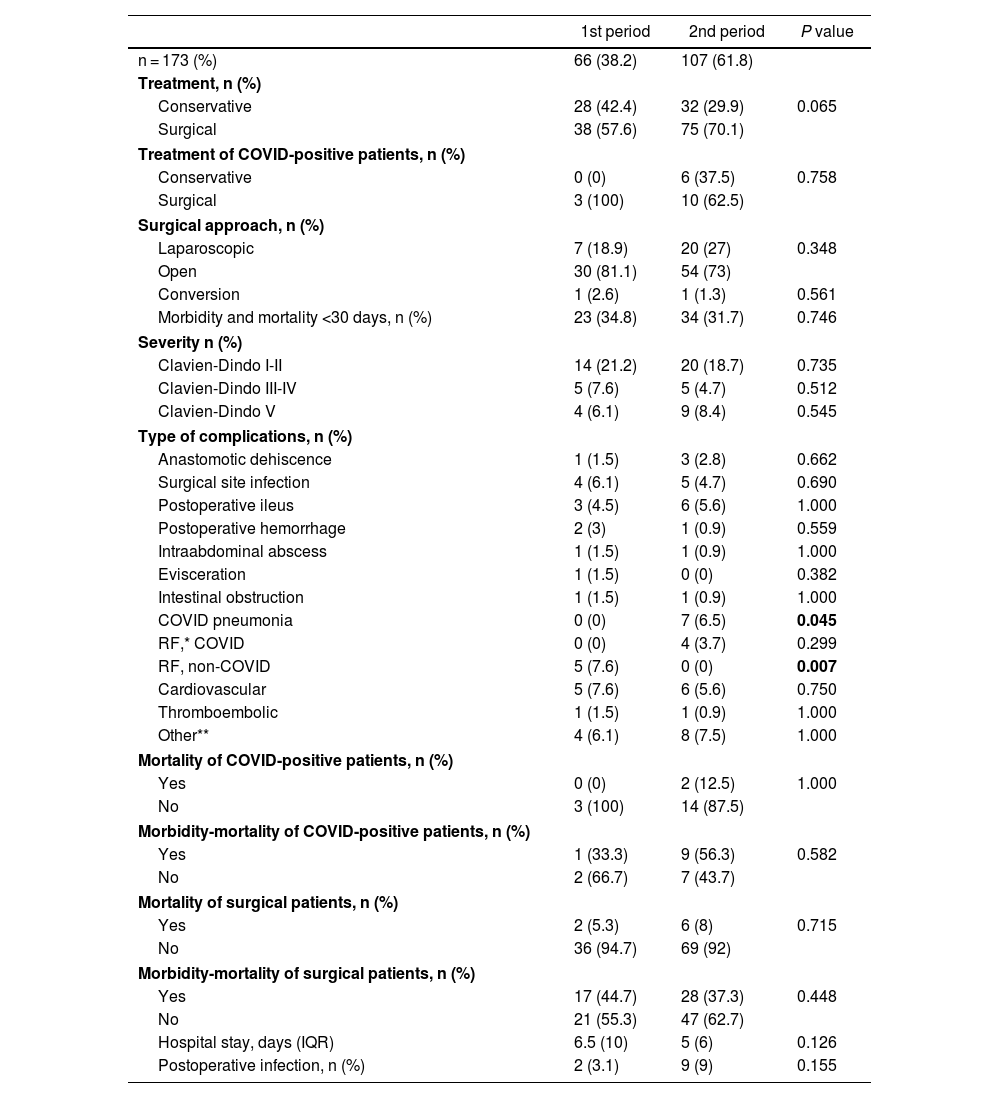
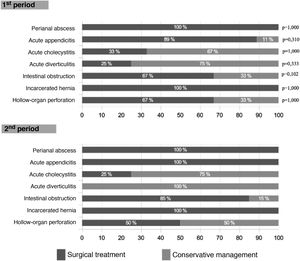
Surgical treatment was 14% lower in the first wave (Table 2), although this difference did not reach statistical significance (P = .065) and occurred due to a higher rate of interventions in cases of intestinal obstruction in the second wave (85% vs 67%; P = .102). As shown in Fig. 3, this difference was not observed in other pathologies. The laparoscopic approach was 8% higher in the second period (P = .348).
General therapeutic management, morbidity and mortality in the sample studied.
| 1st period | 2nd period | P value | |
|---|---|---|---|
| n = 173 (%) | 66 (38.2) | 107 (61.8) | |
| Treatment, n (%) | |||
| Conservative | 28 (42.4) | 32 (29.9) | 0.065 |
| Surgical | 38 (57.6) | 75 (70.1) | |
| Treatment of COVID-positive patients, n (%) | |||
| Conservative | 0 (0) | 6 (37.5) | 0.758 |
| Surgical | 3 (100) | 10 (62.5) | |
| Surgical approach, n (%) | |||
| Laparoscopic | 7 (18.9) | 20 (27) | 0.348 |
| Open | 30 (81.1) | 54 (73) | |
| Conversion | 1 (2.6) | 1 (1.3) | 0.561 |
| Morbidity and mortality <30 days, n (%) | 23 (34.8) | 34 (31.7) | 0.746 |
| Severity n (%) | |||
| Clavien-Dindo I-II | 14 (21.2) | 20 (18.7) | 0.735 |
| Clavien-Dindo III-IV | 5 (7.6) | 5 (4.7) | 0.512 |
| Clavien-Dindo V | 4 (6.1) | 9 (8.4) | 0.545 |
| Type of complications, n (%) | |||
| Anastomotic dehiscence | 1 (1.5) | 3 (2.8) | 0.662 |
| Surgical site infection | 4 (6.1) | 5 (4.7) | 0.690 |
| Postoperative ileus | 3 (4.5) | 6 (5.6) | 1.000 |
| Postoperative hemorrhage | 2 (3) | 1 (0.9) | 0.559 |
| Intraabdominal abscess | 1 (1.5) | 1 (0.9) | 1.000 |
| Evisceration | 1 (1.5) | 0 (0) | 0.382 |
| Intestinal obstruction | 1 (1.5) | 1 (0.9) | 1.000 |
| COVID pneumonia | 0 (0) | 7 (6.5) | 0.045 |
| RF,* COVID | 0 (0) | 4 (3.7) | 0.299 |
| RF, non-COVID | 5 (7.6) | 0 (0) | 0.007 |
| Cardiovascular | 5 (7.6) | 6 (5.6) | 0.750 |
| Thromboembolic | 1 (1.5) | 1 (0.9) | 1.000 |
| Other** | 4 (6.1) | 8 (7.5) | 1.000 |
| Mortality of COVID-positive patients, n (%) | |||
| Yes | 0 (0) | 2 (12.5) | 1.000 |
| No | 3 (100) | 14 (87.5) | |
| Morbidity-mortality of COVID-positive patients, n (%) | |||
| Yes | 1 (33.3) | 9 (56.3) | 0.582 |
| No | 2 (66.7) | 7 (43.7) | |
| Mortality of surgical patients, n (%) | |||
| Yes | 2 (5.3) | 6 (8) | 0.715 |
| No | 36 (94.7) | 69 (92) | |
| Morbidity-mortality of surgical patients, n (%) | |||
| Yes | 17 (44.7) | 28 (37.3) | 0.448 |
| No | 21 (55.3) | 47 (62.7) | |
| Hospital stay, days (IQR) | 6.5 (10) | 5 (6) | 0.126 |
| Postoperative infection, n (%) | 2 (3.1) | 9 (9) | 0.155 |
No correlation was found between the MIP and general morbidity and mortality (1st MIP: 34.8%, 2nd MIP: 31.7%; P = .746), and this result remained unchanged when the adverse events were classified according to the Clavien-Dindo scale. COVID-positive patients had high morbidity and mortality (52.6%), a datum that was similar in both periods. Specifically, in the second wave, a greater number of COVID-related pneumonias and respiratory failure (RF) were recorded, complications that were not detected in any cases of the first wave. In contrast, RF due to non-COVID causes was statistically significantly higher in the first period (7.6% vs 0%; P = .007). Surgical complications were similar, as was hospital stay.
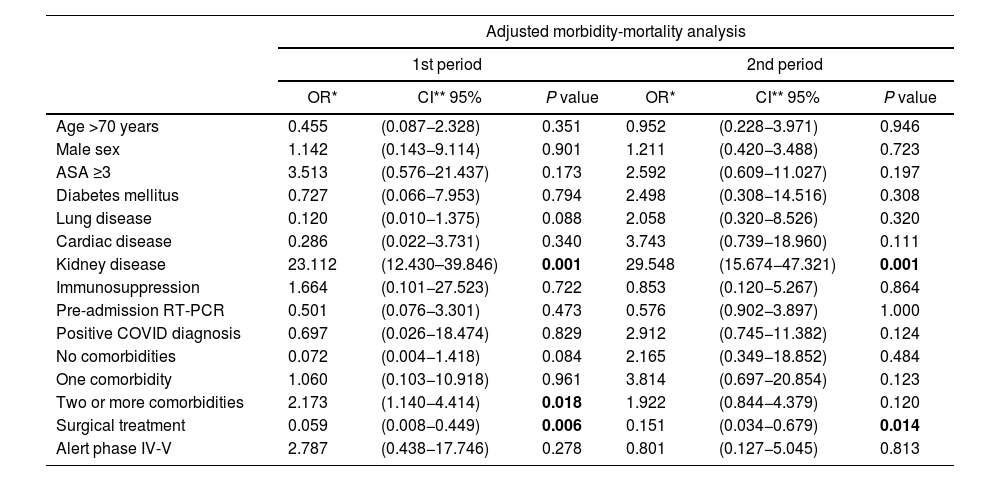
By applying the previously mentioned selection criteria, a multiple binary logistic regression model was obtained with 15 variables (Table 3). First, it was applied to the entire sample, and the MIP was included as an extra predictor variable, but no influence was detected on morbidity and mortality (OR: 0.857; 95% CI: 0.306–2.397; P = .768) or on C–D III–IV complications (OR:0.578; 95% CI:0.086−3.886; P = .573). The diagnosis of COVID was associated with a higher rate of C–D III–IV complications (OR: 7.559; 95% CI: 1.193–47.913; P = .032), yet this relationship was lost when the sample was divided by MIP. Surgical treatment was associated with lower morbidity and mortality (1st MIP: P = .006; 2nd MIP: P = .014). Kidney disease and the existence of ≥2 comorbidities were associated with a higher rate of complications; however, in the case of this latter variable, the relationship was only detected in the first wave (OR: 2.173; 95% CI: 1.140–4.414; P = .018).
General and comparative analysis of risk factors for morbidity and mortality.
| Adjusted morbidity-mortality analysis | ||||||
|---|---|---|---|---|---|---|
| 1st period | 2nd period | |||||
| OR* | CI** 95% | P value | OR* | CI** 95% | P value | |
| Age >70 years | 0.455 | (0.087−2.328) | 0.351 | 0.952 | (0.228−3.971) | 0.946 |
| Male sex | 1.142 | (0.143−9.114) | 0.901 | 1.211 | (0.420−3.488) | 0.723 |
| ASA ≥3 | 3.513 | (0.576−21.437) | 0.173 | 2.592 | (0.609−11.027) | 0.197 |
| Diabetes mellitus | 0.727 | (0.066−7.953) | 0.794 | 2.498 | (0.308−14.516) | 0.308 |
| Lung disease | 0.120 | (0.010−1.375) | 0.088 | 2.058 | (0.320−8.526) | 0.320 |
| Cardiac disease | 0.286 | (0.022−3.731) | 0.340 | 3.743 | (0.739−18.960) | 0.111 |
| Kidney disease | 23.112 | (12.430–39.846) | 0.001 | 29.548 | (15.674−47.321) | 0.001 |
| Immunosuppression | 1.664 | (0.101−27.523) | 0.722 | 0.853 | (0.120−5.267) | 0.864 |
| Pre-admission RT-PCR | 0.501 | (0.076−3.301) | 0.473 | 0.576 | (0.902−3.897) | 1.000 |
| Positive COVID diagnosis | 0.697 | (0.026−18.474) | 0.829 | 2.912 | (0.745−11.382) | 0.124 |
| No comorbidities | 0.072 | (0.004−1.418) | 0.084 | 2.165 | (0.349−18.852) | 0.484 |
| One comorbidity | 1.060 | (0.103−10.918) | 0.961 | 3.814 | (0.697−20.854) | 0.123 |
| Two or more comorbidities | 2.173 | (1.140−4.414) | 0.018 | 1.922 | (0.844−4.379) | 0.120 |
| Surgical treatment | 0.059 | (0.008−0.449) | 0.006 | 0.151 | (0.034−0.679) | 0.014 |
| Alert phase IV-V | 2.787 | (0.438−17.746) | 0.278 | 0.801 | (0.127−5.045) | 0.813 |
Several studies have compared morbidity and mortality in elective and emergency surgery during the pandemic versus the pre-COVID era. To date, the conclusions have been practically unanimous regarding higher morbidity and mortality.14–16 However, the possible change of this variable during successive waves has been the subject of less study. In this context, we believe that its analysis will allow conclusions to be drawn that, without intending to replace the current recommendations of scientific societies, could contribute to the improvement of urgent care.
The periods of our study were defined according to the AEC criteria,10 focusing on the repercussions of care on urgent surgical activity and creating 2 groups of patients treated under similar conditions, thereby reducing the periodicity bias of the target variable.
Series like Rosenthal et al.17 have reported a lower incidence of surgical emergencies during the first wave, which has been easily attributable to the fear of infection when visiting medical centers. As reported in the study by Cano-Valderrama,18 it was mainly patients with comorbidities who made fewer visits to the emergency department. In our series, a lower incidence of surgical pathology was observed in the 1st MIP compared to the 2nd MIP (0.97 admissions/day vs 1.11 admissions/day; P = .179); the lower comorbidity of the first wave was only reflected by a higher percentage of ASA-II patients, since the percentages of ASA I-II were similar (59.1% 1st MIP vs 57% 2nd MIP). The fact that it is not a comparison with the “pre-COVID” era, but instead a comparison between the first and second waves, justifies the smaller differences in these variables compared to those obtained in the aforementioned studies.
As seen in Fig. 2, the screening protocol underwent certain changes between the 1st and 2nd MIP. COVID infection was defined in such a way that said definition was applicable in both periods, with the aim of reducing detection bias. The main difference in the screening protocol was the failure to perform RT-PCR on asymptomatic patients with negative serology in the first wave. The greater availability of this latter test in this period may explain why this decision was made, despite its lower reliability compared to RT-PCR.19 This fact explains the higher use of serological screening during the 1st MIP compared to the second, when the necessary RT-PCR tests were available. Accurate screening methods for SARS-CoV-2 infection facilitated epidemiological and therapeutic management, and this may also explain the higher percentage of surgical treatment of the 2nd MIP (70.1% vs 57%), which in turn reflects a situation closer to normal. However, the higher COVID occupancy of the ICU in the 1st MIP (Fig. 1B) could have influenced the lower surgical treatment in that period; meanwhile, the ward occupancy was similar in both periods. The laparoscopic approach also increased in the 2nd MIP. The development of pneumoperitoneum evacuation methods and the reduction of the initial controversy20 regarding the possible aerosolization of viral particles may explain this difference.21
When the surgical procedures were analyzed, we found 18% more interventions for partial or total intestinal obstruction in the 2nd MIP (P = .102). This may be explained by the fact that it is a pathology that can be treated with conservative management, and such management was used more extensively during the 1st MIP due to the excess patient load. No significant differences were observed in the remaining procedures.
In our study, the rate of C–D III–IV complications (7.6%) and mortality (6.1%) obtained in the 1st MIP were lower than those reported in the national series by Pérez Rubio et al6 (12% and 11%, respectively) and Maldonado-Marcos et al9 (21.7% and 6.5%). Being a level II hospital with a smaller population, as well as having a preparation period of 10–15 days between the involvement of these centers and ours, may explain this difference. Regarding COVID-positive patients, mortality and morbidity rates of 10.5% and 52.6%, respectively, were detected; these results are comparable to international series.22,23 Specifically, an absence of COVID-related respiratory complications was detected, together with 7.6% of non-COVID RF in the first wave, results that are opposite to those of the second period, which we feel reflect an underdiagnosis of the infection by SARS-COV-2 in the 1st MIP. In the comparative analysis, no differences were detected in general morbidity and mortality between the two periods, despite the lower healthcare burden and the better epidemiological management of the second wave. In this context, the lower volume of surgical emergencies in the 1st MIP may explain this result.
The AEC recommendations for emergency care establish the need to maintain the indications for urgent surgery in the pandemic, introducing SARS-CoV-2 infection in the protocols, which are related to greater postoperative morbidity and mortality24–26 and the epidemiological scenario.5 This statement is based on the inherent benefit of surgery and COVID-positive patient series with acceptable postoperative results.27 In our series, although morbidity and mortality could be influenced by several factors, surgical treatment was associated with a lower rate of complications in both periods in the adjusted analysis, a fact that reinforces current recommendations.5 Also in line with the literature,26 the diagnosis of COVID infection led to a greater number of C–D III–IV events in our series, while the existence of 2 or more previous comorbidities was also associated with greater morbidity and mortality, but only in the 1st MIP.
Based on our results, the creation of a specific therapeutic algorithm that takes into account the healthcare scenario, as well as the diagnosis of COVID-19 and the number of patient comorbidities, is the first objective to improve urgent surgical care in the future.
Our research has the same limitations of any single-center retrospective study, such as selection bias and the lower external validity of our results. Furthermore, the limited sample size may have influenced the results of certain sub-analyses. Likewise, an information bias may have occurred due to the underdiagnosis of both the SARS-CoV-2 infection and surgical pathology due to the decrease in the number of urgent consultations17,18 in the first wave. The strengths of the study include the definition of periods using the AEC phase criteria, strict definition of the variables, analysis of the screening methods, and use of an adjusted analysis of morbidity and mortality to reduce the effect of possible confounding variables.
Based on our results, we conclude that there were no differences in the morbidity and mortality rates in emergency surgery between the first and second waves of the pandemic at our hospital. Surgical treatment was more frequent in the second wave and was statistically associated with lower morbidity and mortality, while COVID-19 infection, chronic kidney disease, and the existence of 2 or more comorbidities were associated with a higher rate of complications. In our opinion, it is necessary to plan and execute prospective studies with larger sample sizes to obtain more evidence and improve urgent surgical care.
FundingThis study has received no specific funding.
Conflict of interestsThe authors confirm the absence of any conflict of interests with funding or academic organizations regarding the content of this manuscript.