Fluorescence image-guided surgery (FIGS) is an optical-based imaging technique, which allows for the visualization of unapparent structures at the naked eye, and for the evaluation of dynamic metabolic activities, such as organ perfusion. Fluorescence imaging is obtained through the injection of a fluorescent dye, which can emit a fluorescent signal after being excited by ad hoc laser sources. The fluorescent signal can either be visualized directly on the operative field, in open surgical procedures, or can be captured by specific cameras and displayed on screen, in the minimally invasive setting. FIGS is adapted to the needs of surgical navigation, since it does not require bulky equipment and provides real-time images, without disrupting the surgical workflow.
There is a growing interest in the potential impact of FIGS molecular navigation on surgical outcomes. It is witnessed by the fact that there is a steep increase in the number of publications and an increasing number of manufacturers, producing imaging systems which enable FIGS. FIGS has been successfully attempted in a variety of clinical conditions pertinent to the digestive system. The most popular current clinical applications worldwide include fluorescence cholangiography,1 sentinel node navigation, and anastomotic perfusion assessment.2 However, the most groundbreaking application, which is still at an embryonic state, is the real-time fluorescence-based identification of tumor tissue, thanks to cancer-specific fluorescent probes.3
An extensive intelligence and networking activity, including major opinion leaders in the field, has allowed to identify major directions for future researches gravitating around FIGS, including: (1) the integration of computer-assisted interpretation of the fluorescent signal through dedicated software; (2) the development of targeted fluorescent probes, which precisely recognize biological targets or tumor cells and allow for image-guided cancer removal; (3) the development of miniature flexible endoscopic platforms making it possible to precisely diagnose and treat gastrointestinal neoplasia.
FIGS might improve outcomes relative to digestive surgical therapies, acting at different levels, which will be shortly described.
FIGS Enables Real-Time Bowel Perfusion EvaluationBowel perfusion is a crucial requirement to ensure optimal anastomotic healing. The rationale of Fluorescence Angiography to evaluate perfusion is based on the assumption that the diffusion of a systemically injected fluorophore staining the bowel surface is proof that the vascular supply is preserved. The most up-to-date review of the literature (2016) on clinical studies assessing fluorescence-based angiography included 10 trials of colorectal and 4 trials of esophageal resections,2 for a total of approximately 1000 and 200 patients respectively. The only possible conclusions were that fluorescence evaluation is a promising technique, but, in the absence of well-designed controlled studies, the potential impact on reducing the anastomotic leak rate remains to be demonstrated more accurately. However, in all of these studies, perfusion was evaluated on the basis of relative fluorescence intensity, without considering the diffusion of fluorophores over time. In fact, the dye can reach the boundaries of ischemic areas through capillary flow diffusion with time and may provide overestimation of the perfused zone.
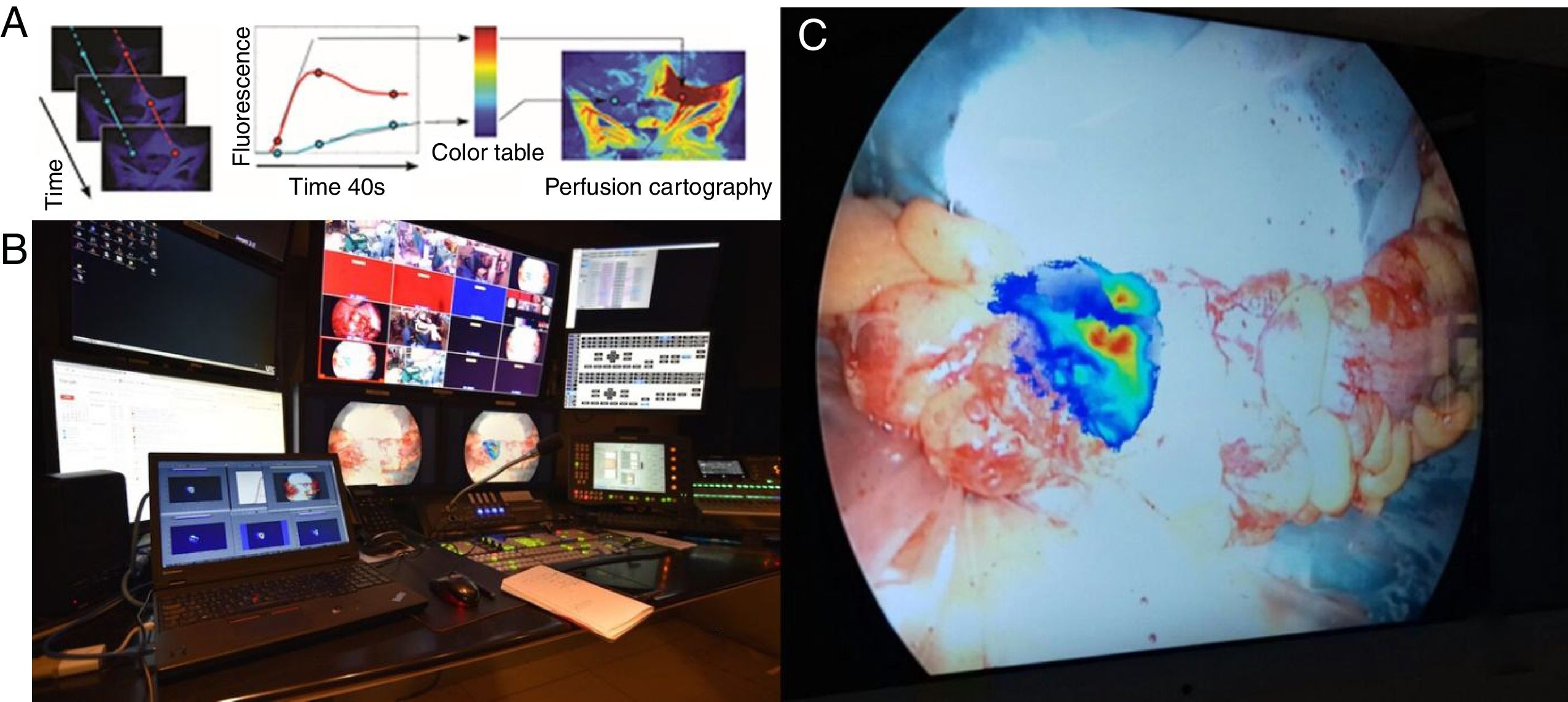
Our research and development department has developed an accurate image analyzer software (ER-PERFUSION, IRCAD; France) to obtain virtual perfusion cartography which can be overlaid onto the screen, providing an enhanced reality view of the perfusion level.4 This technology provides a dynamic, quantitative, and reproducible estimation of organ perfusion and makes it possible to precisely estimate perfusion levels and achieve a real-time visualization of resection lines directly on the laparoscopic screen. We are currently running a clinical trial (PERFECT trial: Perfusion Evaluation by Real-time Fluorescence-based EnhanCed reality; NCT02626091) with encouraging preliminary results (Fig. 1).
(A) Description of the concept of FLER (Fluorescence-based Enhanced Reality): upon injection of the fluorophore, the fluorescent signal is analyzed during 40s by ad hoc software (VR-PERFUSION, IRCAD, France). The fluorescence time-to-peak is computed pixel-by-pixel and the values of the slope are converted into a color code to generate a virtual perfusion cartography. The resultant cartography is overlapped using a video-mixer (B=broadcasting site of the IRCAD) and is overlapped in real-time on the bowel (C=proximal resection site during a sigmoid resection).
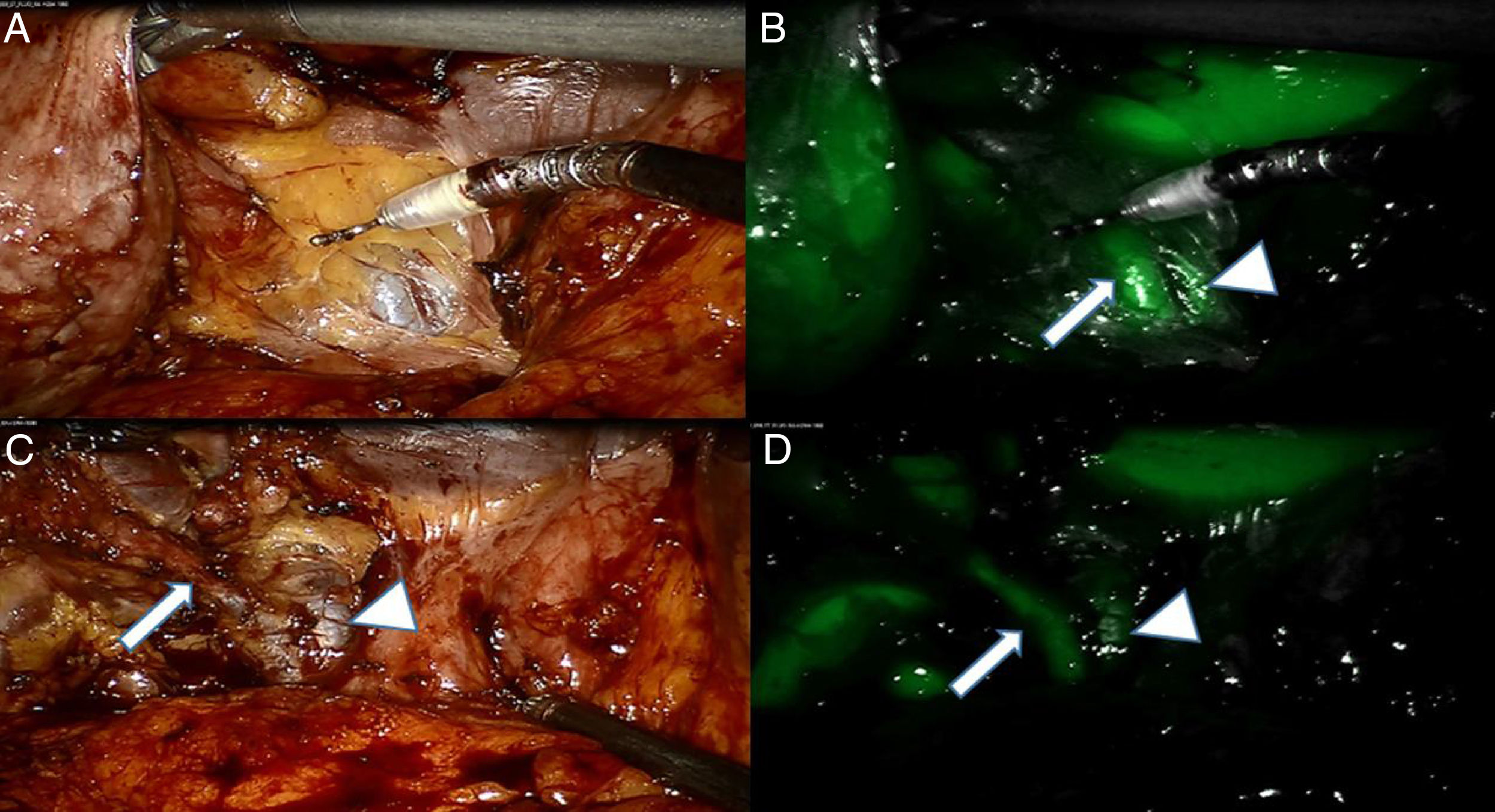
Fluorescence enhancement might help to prevent inadvertent lesions during the surgical procedures to critical anatomical structures including biliary tree, nerves, ureters, etc. As an example, near-infrared fluorescence cholangiography seems to be an accurate method to identify biliary structures and possibly to prevent bile duct injuries.1 Fluorescence cholangiography is based on the injection of a bile-excreted fluorophore (Indocyanine Green), which becomes fluorescent upon excitation by means of a near-infrared light. Such an imaging modality has the advantages of being user-friendly, real-time, low-cost, and radiation-free. A drawback with this technique lies in the high background liver fluorescence, which is disturbing. There are some strategies to reduce the fluorescence noise coming from the liver. The first strategy is to optimize the dosing and interval timing from fluorophore injection to visualization. The reported doses range from 2.5mg in a single IV administration to 0.5mg/kg (Fig. 2).1 In a study, the best biliary ducts-to-liver fluorescence ratio was obtained with 0.25mg/kg of ICG, administered at least 45min before images were acquired.5 A prolonged time interval up to 24h leads to a washout of the fluorophore with a clear view of the biliary tree and no background fluorescence from the liver.6 An alternative strategy is to inject ICG directly into the gallbladder.7 This fluorescence cholecystocholangiography provides a clear delineation of the gallbladder contour and highlights the biliary tree brightly. We have recently successfully introduced this technique into the clinical setting, and preliminary results are pending publication. Another strategy relies on software manipulation allowing for a selective erasing of liver fluorescence. Despite some limitations, it can be expected that, with some refinements, fluorescence cholangiography will be one of the more largely adopted clinical applications of FIGS.
(A) White light imaging of Calot's triangle area after an initial opening of the peri-gallbladder adipose tissue. A tubular structure can be identified. (B) At near-infrared (NIR), a second structure is identified adjacent to the previous one, allowing to identify them as the Cystic Duct (CD; arrow) and the Common Bile Duct (CBD) respectively; see arrow head. (C) White light view after some dissection allowing to dissect the CD. (D) NIR imaging confirms the accurate interpretation of the anatomy.
Another critical structure which is often at risk of iatrogenic lesions is the ureter, particularly in patients with previous abdominal surgeries. Additionally, the emergence of new minimally invasive approaches, such as “bottom-up” transanal total mesorectal excision (TME), with a radically new anatomical view, might increase the risks of ureteral injuries.8
Enhanced visualization of the ureteral course is an obvious strategy to prevent or minimize the risks of iatrogenic lesions. FIGS can provide a non-invasive and effective visualization of the ureters. However, the current clinical use of fluorescence guidance to localize ureters, is limited, since Indocyanine Green is not excreted in the urinary tract.
Promising experimental works are ongoing with modified fluorophores which can be excreted by the urinary system, such as the IRDye800CW-CA.9 This is a carboxylic acid derivative of the IRDye800CW, which is currently evaluated in several clinical trials (NCT01987375, NCT01508572, NCT02113202, NCT02129933).
FIGS has also the potential to visualize nerves, with the use of nerve-specific tracers, making it possible to potentially reduce the risk of nerve injuries, particularly during pelvic surgery, which can result in urogenital, pelvic floor, and/or anal sphincter dysfunctions. Currently, nerve visualization represents an area of active research in molecular fluorescent imaging.
FIGS Enables Real-time Intraoperative StagingThe precise identification and analysis of the sentinel lymph node (SLN) are critical in the surgical decision-making process, particularly in organ-sparing, localized procedures, such as endoscopic submucosal dissections (ESD) or limited full-thickness resections, which can be considered oncologically appropriate only if lymph nodes are not involved.
Indocyanine Green (ICG) near-infrared fluorescence-guided SLN navigation is a relatively novel and effective technique which has been successfully used in various kinds of tumors, including GI cancers, showing high detection and sensitivity rates. However, ICG is not a good candidate for SLN navigation, for at least 2 reasons: (1) it has a low quantum yield (low fluorescence brightness), and (2) it has a low retention in lymph nodes, being a way too small molecule which quickly disperses to multiple lymph nodes.10
For this reason, ICG-based techniques of SLN are limited, as clearly outlined in a recent paper of the Japan Clinical Oncology Group,11 which documented an unexpectedly high false-negative rate, leading to the suspension of the trial after enrolling 440 cases of gastric cancers.
Improved performance of SLN navigation can be achieved, acting at various levels. First, it can be improved by engineering more adapted, smart fluorescent probes to counteract ICG deficiencies, including increased retention rate and increased quantum yield and selectivity to the target tumor tissue. As an example, the retention rate can be improved by engineered fluorescent molecules combined with large molecules, such as human albumin or nano-colloids, which demonstrate less dispersion and longer retention in the nodes as compared to ICG alone.12 Secondly, it can be improved by enhancing injection methods and dosing (reduced concentration and larger volume), which still requires to be properly titrated in prospective studies. Thirdly, it can be improved by using a combined dual-mode 3D imaging lymphography and optical fluorescence-based navigation.
FIGS Enables Precise Evaluation of Radical Cancer Tissue Removal, Evaluation of Tumor Margins, and Evaluation of Response to TreatmentRadical removal of cancer cells is of paramount importance to reduce the rate of tumor recurrences and to increase tumor-free survival. The administration of a tumor-specific antibody, which fluoresces in the near-infrared ranges and which could be univocally recognized at a tumor cellular level, could provide a rapid and accurate evaluation of radical tumor removal. In the context of precision surgery, the development of tumor-specific fluorescent probes has made remarkable advances over recent years, allowing for the enhanced identification of tumor residuals and metastatic lymph nodes. In a pioneer paper published in 2011, the first human case of tumor-specific fluorescence-guided surgery was reported.13 Authors could effectively remove 34 peritoneal implants of ovarian cancer metastasis, which were invisible at the naked eye. This impressive proof-of-the-concept clearly highlights the potential impact of intraoperative tumor-specific fluorescence imaging at least in two moments of the tumor's natural history: first, tumor screening, which could become personalized and much more accurate, and secondly in the achievement of radical oncological outcomes. There is an increasing number of targeted probes which are being developed to visualize cancer cells, allowing for early-stage cancer detection and precise tumor resection. Of particular interest is the strategy of coupling a fluorescent dye (IRDye800CW) with the humanized monoclonal antibodies, currently used in anticancer therapy (e.g., Bevacizumab, which targets Vascular Endothelial Growth Factor=VEGF or Cetuximab, which targets Epithelial Growth Factor Receptor=EGFR) with the IRDye800. Ongoing clinical trials in GI applications are targeting Barrett's esophagus (NCT02129933) and colon cancer (NCT01972373).
To conclude, there is a vibrant excitement around FIGS in the recent years, which promises to be the next cutting edge toward precision therapies, providing an improved diagnostic, real-time surgical strategy decision-making and efficacy assessment and theranostics. Extensive research is ongoing at various levels, including hardware, software, and fluorophores to improve current performances.
Michele Diana is the recipient of a research grant (project ELIOS) from the Foundation ARC (Association for Cancer Research) to develop fluorescence-guided surgery.
Please cite this article as: Diana M. Cirugía guiada por fluorescencia aplicada al aparato digestivo: el ojo cibernético permite ver lo invisible. Cir Esp. 2018;96:65–68.