A borderline resectable group (APBR) has recently been defined in adenocarcinoma of the pancreas. The objective of the study is to evaluate the results in the surgical treatment after neoadjuvancy of the APBR.
MethodBetween 2010 and 2014, we included patients with APBR in a neoadjuvant and surgery protocol, staged by multidetector computed tomography (MDCT). Treatment with chemotherapy was based on gemcitabine and oxaliplatin. Subsequently, MDCT was performed to rule out progression, and 5-FU infusion and concomitant radiotherapy were given. MDCT and resection were performed in absence of progression. A descriptive statistical study was performed, dividing the series into: surgery group (GR group) and progression group (PROG group).
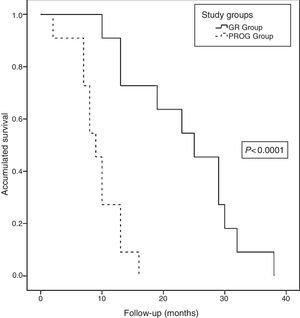
ResultsWe indicated neoadjuvant treatment to 22 patients, 11 of them were operated, 9 pancreatoduodenectomies, and 2 distal pancreatectomies. Of the 11 patients, 7 required some type of vascular resection; 5 venous resections, one arterial and one both. No postoperative mortality was recorded, 7 (63%) had any complications, and 4 were reoperated. The median postoperative stay was 17 (7–75) days. The pathological study showed complete response (ypT0) in 27%, and free microscopic margins (R0) in 63%. At study clossure, all patients had died, with a median actuarial survival of 13 months (9.6–16.3). The median actuarial survival of the GR group was higher than the PROG group (25 vs 9 months; P<.0001).
ConclusionThe neoadjuvant treatment of APBR allows us to select a group of patients in whom resection achieves a longer survival to the group in which progression is observed. Post-adjuvant pancreatic resection requires vascular resection in most cases.
Se ha definido un grupo de resecabilidad borderline resectable (APBR) en el adenocarcinoma de páncreas. El objetivo del estudio es evaluar los resultados en el tratamiento quirúrgico tras neoadyuvancia del APBR.
MétodoEntre 2010 y 2014 incluimos pacientes afectos de APBR en un protocolo de neoadyuvancia y cirugía, estadificados mediante tomografía computarizada multidetector (TCMD). El tratamiento con quimioterapia se basó en gemcitabina y oxaliplatino (GEMOX). Posteriormente, se realizó TCMD para descartar progresión, y se administró 5-FU en infusión y radioterapia concomitante. Se practicó TCMD y resección en ausencia de progresión. Se realizó un estudio estadístico descriptivo, dividiendo la serie en grupo resección (grupo GR) y grupo progresión (grupo PROG). El seguimiento finalizó en febrero de 2016.
ResultadosIndicamos tratamiento neoadyuvante a 22 pacientes, 11 de ellos fueron finalmente intervenidos. Se realizaron 9 duodenopancreatectomías cefálicas, una duodenopancreatectomía total y una pancreatectomía corporocaudal. De los 11 pacientes, 7 requirieron algún tipo de resección vascular; 5 resecciones venosas, uno arterial y otro ambas. No hubo mortalidad postoperatoria, 7 (63%) tuvieron alguna complicación y 4 fueron reintervenidos. La estancia hospitalaria postoperatoria mediana fue 17 días (7-75). El estudio patológico evidenció márgenes microscópicos libres (R0) en el 63% de los pacientes y ausencia de afectación adenopática en 10 pacientes (ypN0). Al cierre del estudio, todos los pacientes habían fallecido, con una supervivencia actuarial mediana de 13 meses (9,6-16,3). La supervivencia actuarial mediana del grupo GR fue superior al grupo PROG (25 vs 9 meses; p<0,0001).
ConclusiónEl tratamiento neoadyuvante del APBR permite seleccionar un grupo de pacientes en el que la resección consigue una supervivencia superior al grupo en el que se observa progresión. La resección pancreática posneoadyuvancia requiere resecciones vasculares en la mayoría de los casos.
The term “borderline-resectable pancreatic cancer” (BRPC) describes a borderline resectability concept first used by Maurer in 1999.1 This concept was introduced to classify tumors that are between resectable and unresectable tumors.2 The definition is based on the findings of multidetector computed tomography (MDCT). In 2006, the MD Anderson3 group published a classification that included BRPC. Three groups were defined according to vascular involvement: resectable tumors, borderline-resectable tumors, and unresectable tumors. The borderline-resectable group included patients with borderline resectability, whose tumors could be resected after neoadjuvant therapy. The authors have published good post-resection results in BRPC patients after neoadjuvant treatment, especially considering that in the past they were considered unresectable tumors. Recently, this classification has been adopted in an international consensus, with minimal changes.4 The aim of this study is to review the short- and medium-term results obtained at our hospital for the surgical treatment of BRPC after neoadjuvant therapy, and to analyze morbidity and mortality after post-neoadjuvant therapy surgery.
Material and MethodsWe have collected data from the experience in the surgical treatment of BRPC after neoadjuvant therapy from July 2010 to November 2014 at the Hospital Universitari de Bellvitge and the Servei d’Oncologia Mèdica at Institut Català d’Oncologia in L’Hospitalet, Spain. We prospectively registered data for patient demographics, neoadjuvant regimen, surgery performed, anatomic pathology results and the follow-up period of all patients. The patient follow-up was finalized in February 2016.
Staging StudyA 64-MDCT was used for diagnosis and staging. Patients were classified according to criteria published by the MD Anderson Group3 as having resectable, borderline-resectable or unresectable tumors. BRPC was defined as tumors of the head of the pancreas in contact with the superior mesenteric artery (SMA) of less than 180°, obliteration of the portal vein/superior mesenteric vein (PV/SMV) with the possibility of reconstruction, and/or contact with the hepatic artery at its union with the gastroduodenal artery. We have also included lesions in the neck/body of the pancreas encompassing the celiac artery (CA), as long as they presented a tumor-free distance from the aortic root to the tumor.5 Once included in the BRPC protocol, patients with jaundice underwent biliary drainage using a coated metallic stent and cytological confirmation by ultrasound-guided endoscopy.6
Neoadjuvant TherapyNeoadjuvant therapy was based on the GEMOX regimen (1000mg/m2 of gemcitabine during 100min of infusion on day 1, followed by 100mg/m2 of oxaliplatin in infusion during 2h on days 2, every 2 weeks) for 6 cycles. During the period analyzed, 6 patients participated in a clinical trial (GEMOX+erlotinib 100mg/day) for 6 cycles. After re-staging patients with MDCT, the patients without progression of the disease received 5 weeks of chemotherapy (CTX) with infusion of 5-FU at 250mg/m2/day, concomitant to radiotherapy (50.4Gy). The patients who were treated within this clinical trial received during radiotherapy 40mg/m2 of gemcitabine 2 times per weeks and 100mg/day of erlotinib. After re-staging with MDCT, between 4 and 6 weeks after the end of concomitant chemoradiotherapy (CRTX), surgical resection was indicated in cases with no progression. Progression of the disease was determined by greater local tumor involvement or distant disease. Thus, surgery was indicated in cases in which the radiological study did not detect changes in staging prior to treatment or evidence of tumor regression.
Surgical Technique and Pathology StudyThe surgical techniques utilized included pancreaticoduodenectomy (PD), distal pancreatectomy with splenectomy or total pancreaticoduodenectomy, depending on the tumor location, with regional lymphadenectomy.7 During surgery, the resection margins were remitted for analysis, then the margins were extended in case of involvement. Vascular resection was planned individually. As for the pathology study, the TNM classification by the Union for International Cancer Control (UICC)8 was used; the study of surgical resection margins was based on the Royal College study.9 Finally, the presence of post-neoadjuvant therapy changes were recorded according to the tumor regression grade (TRG) by the College of American Pathologists (2009).10 Depending on the pathological response to neoadjuvant treatment, cases with no viable tumor cells were considered TRG0 (complete response); if tumor cells were isolated or in small groups, TRG1; if there was residual tumor but the percentage of fibrosis was greater than the tumor percentage, TRG2; and if there was extensive residual tumor (minimal or no response), TRG3.
Statistical StudyWe completed a descriptive analysis according to measures of central tendency (mean, median) and dispersion (standard deviation and interquartile range) according to normality criteria (Kolmogorov–Smirnov test). Subsequently, the series was divided into the resection group (RG) and progression group (PROG), depending on the patient progress recorded after neoadjuvant treatment. Next, a comparative study was performed between qualitative variables using the chi-squared or Fisher's tests and between quantitative variables according to the Mann–Whitney U. Radiological involvement was classified according to contact with the SMA, PV/SMV occlusion, contact with the CA and hepatic artery (HA). The period of time elapsed between diagnosis and the onset of neoadjuvant therapy was referred to as T1, between the beginning and the end of neoadjuvant therapy as T2, and between the end of neoadjuvant therapy and surgery as T3. Actuarial survival and disease-free time of the global series were calculated with a Kaplan–Meier analysis. The survival time was defined as the time transpired from the date of CTX initiation to the date of death. Finally, actuarial survival was compared between the RG and PROG groups using the log-rank test (P<.05). Among the patient of the RG group, disease-free time was defined as the time transpired from surgery until the appearance of recurrence. In patients in the PROG group, time to progression was defined as the time from the onset of CTX until progression. The SPSS 18.0® statistical package was used, and a P value <.05 was considered statistically significant in all cases.
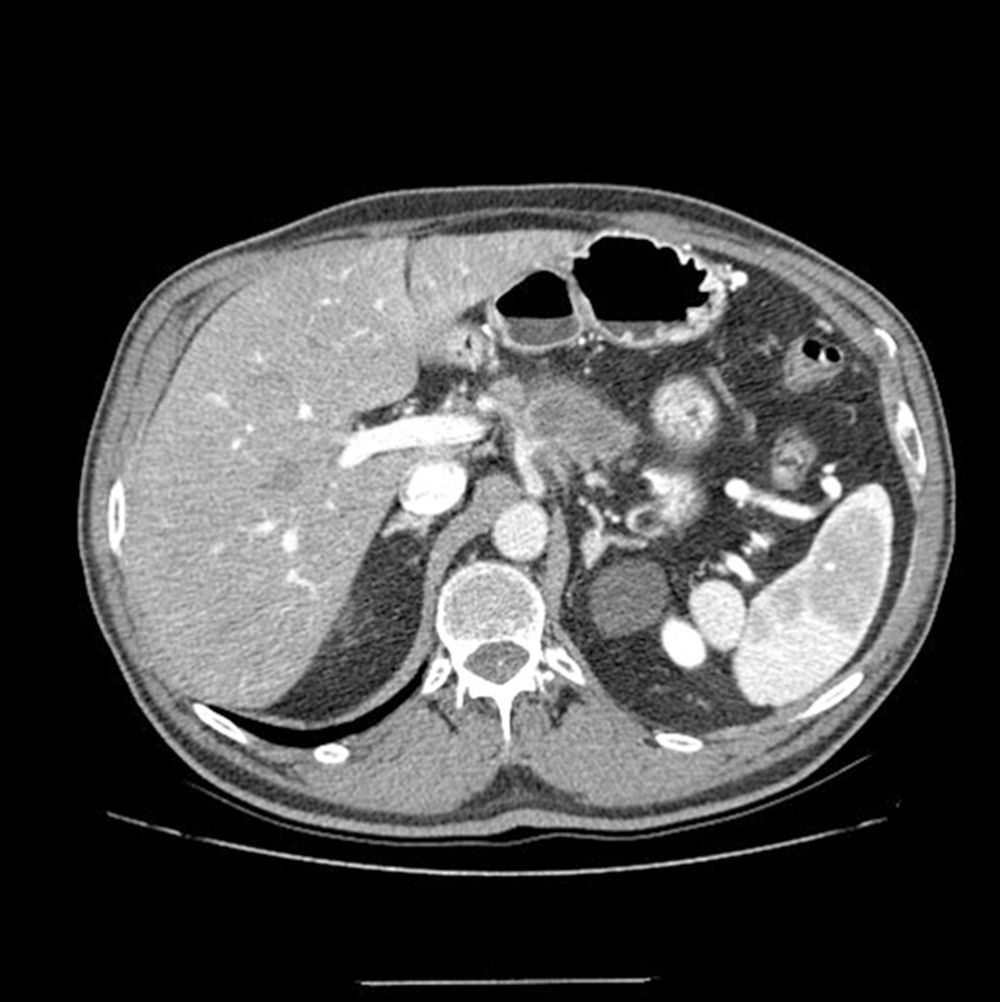
ResultsInitial Assessment and StagingFrom 2010 to 2014, 22 patients were included in the treatment protocol with neoadjuvant therapy for BRPC: 13 men and 9 women, with a mean age of 62.7 (±10.3) (range: 41–78). The staging MDCT demonstrated that the patients studied had lesions in the head and neck/body of the pancreas (19 and 3 cases, respectively). Likewise, evidence was seen of contact of the tumor with the superior mesenteric artery (SMA) in 13, occlusion of the PV/SMV in 9, the tumor encompassed the CA in 4 (Fig. 1) and contact of the tumor with the HA in 5 (Table 1).
Multidetector computed tomography scan: tumor mass is evident in the neck/body of the pancreas, encompassing the splenic artery and in contact with the hepatic and celiac arteries. At the time of diagnosis, a second tumor mass was identified in the head of the pancreas; therefore, the two-stage surgical plan included total pancreaticoduodenectomy, four/fifths gastrectomy, splenectomy, celiac trunk resection and end-to-end arterial anastomosis after arterial embolization.
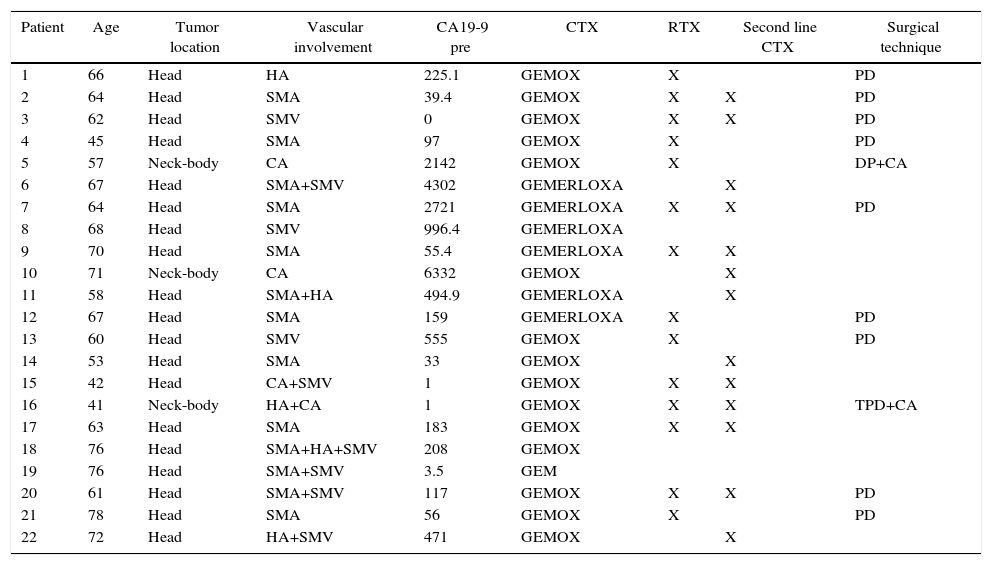
Vascular Involvement and Therapeutic Regimen of Patients Treated With Neoadjuvant Therapy for Borderline-Resectable Pancreatic Adenocarcinoma.
| Patient | Age | Tumor location | Vascular involvement | CA19-9 pre | CTX | RTX | Second line CTX | Surgical technique |
|---|---|---|---|---|---|---|---|---|
| 1 | 66 | Head | HA | 225.1 | GEMOX | X | PD | |
| 2 | 64 | Head | SMA | 39.4 | GEMOX | X | X | PD |
| 3 | 62 | Head | SMV | 0 | GEMOX | X | X | PD |
| 4 | 45 | Head | SMA | 97 | GEMOX | X | PD | |
| 5 | 57 | Neck-body | CA | 2142 | GEMOX | X | DP+CA | |
| 6 | 67 | Head | SMA+SMV | 4302 | GEMERLOXA | X | ||
| 7 | 64 | Head | SMA | 2721 | GEMERLOXA | X | X | PD |
| 8 | 68 | Head | SMV | 996.4 | GEMERLOXA | |||
| 9 | 70 | Head | SMA | 55.4 | GEMERLOXA | X | X | |
| 10 | 71 | Neck-body | CA | 6332 | GEMOX | X | ||
| 11 | 58 | Head | SMA+HA | 494.9 | GEMERLOXA | X | ||
| 12 | 67 | Head | SMA | 159 | GEMERLOXA | X | PD | |
| 13 | 60 | Head | SMV | 555 | GEMOX | X | PD | |
| 14 | 53 | Head | SMA | 33 | GEMOX | X | ||
| 15 | 42 | Head | CA+SMV | 1 | GEMOX | X | X | |
| 16 | 41 | Neck-body | HA+CA | 1 | GEMOX | X | X | TPD+CA |
| 17 | 63 | Head | SMA | 183 | GEMOX | X | X | |
| 18 | 76 | Head | SMA+HA+SMV | 208 | GEMOX | |||
| 19 | 76 | Head | SMA+SMV | 3.5 | GEM | |||
| 20 | 61 | Head | SMA+SMV | 117 | GEMOX | X | X | PD |
| 21 | 78 | Head | SMA | 56 | GEMOX | X | PD | |
| 22 | 72 | Head | HA+SMV | 471 | GEMOX | X |
HA: hepatic artery; SMA: superior mesenteric artery; CA19-9PRE: carbohydrate antigen 19-9 prior to treatment; PD: pancreaticoduodenectomy; TPD: total pancreaticoduodenectomy with splenectomy; GEM: gemcitabine: GEMERLOXA: gemcitabine oxaliplatin erlotinib; GEMOX: gemcitabine oxaliplatin; DP: distal pancreatectomy with splenectomy; CTX: chemotherapy; RTX: radiotherapy; CA: celiac artery; SMV: superior mesenteric vein.
Source: Hospital Universitari de Bellvitge (2010–2014).
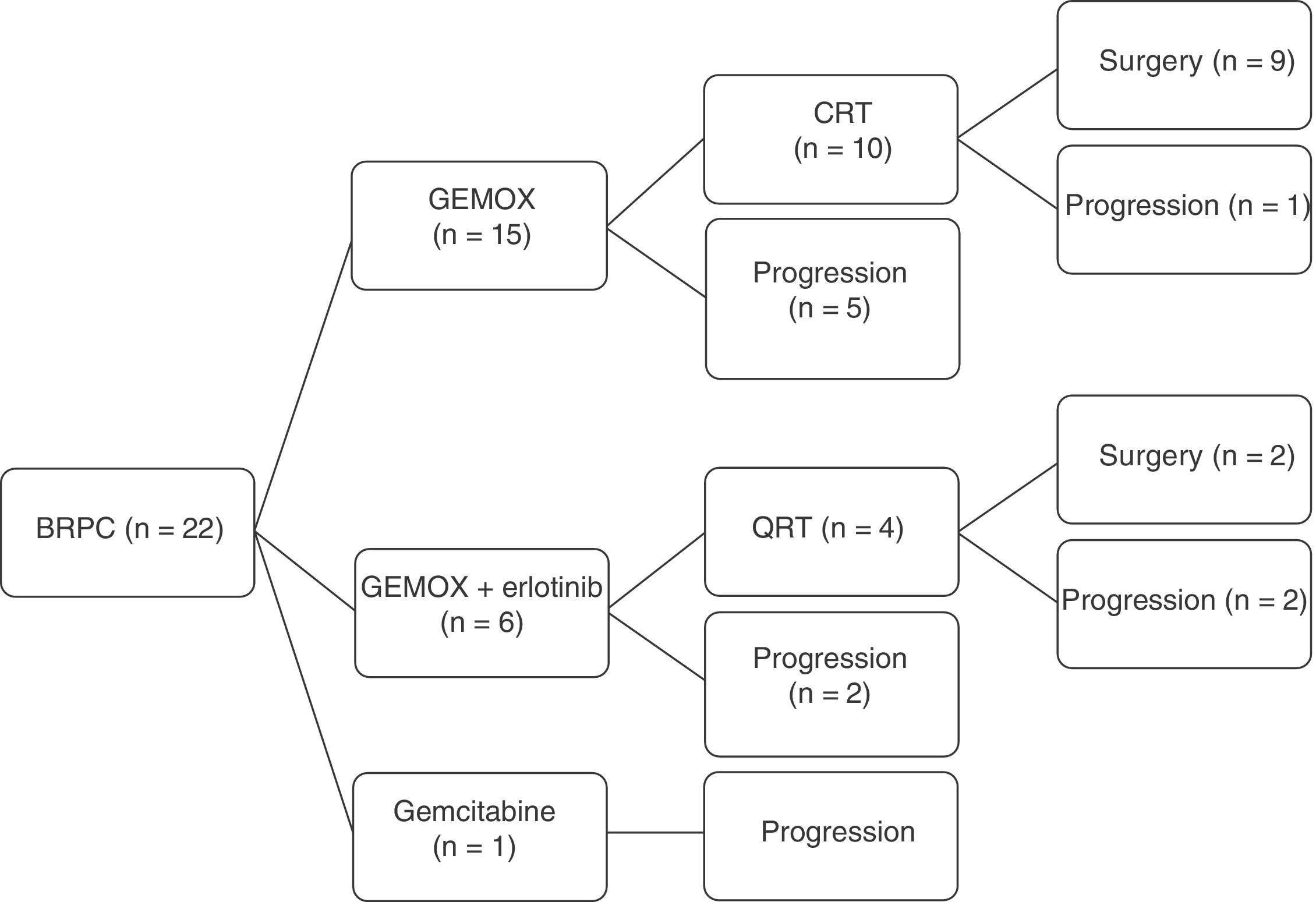
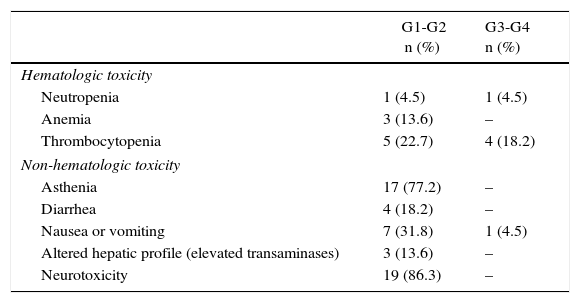
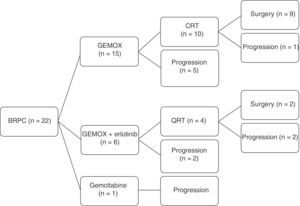
A total of 22 patients were included for neoadjuvant treatment, which involved induction CTX for 3 months. Fifteen patients received the GEMOX regimen for 6 cycles, 6 patients participated in a clinical trial (GEMOX+erlotinib [100mg day]) for 6 cycles, and one patient received gemcitabine monotherapy for 3 cycles (Fig. 2). As for the toxicity profile, 5 patients had hematologic toxicity grades 3 or 4 (neutropenia and thrombocytopenia) and one patient grade 3 emesis syndrome. The most frequent grade 1–2 toxicities were asthenia and neurotoxicity (Table 2). A total of 8 patients did not receive treatment with CRTX due to progression of the disease. At the end of the neoadjuvant treatment, 11 patients did not present evidence of tumor progression and underwent surgical resection (RG group), and in 11 cases disease progression was detected (PROG group). The median time elapsed between diagnosis and start of treatment (T1) was 58 days (range: 40–66), the time from the beginning of treatment to the end (T2) was 83 days (range: 45–204) and the time from end of treatment to surgery (RG group, T3) was 141 days (range: 63–250). When the comparative study was carried out between the 2 groups, we showed that the PROG group presented a CA19-9 level prior to initiation of the neoadjuvant treatment superior to the RG group, without reaching statistical significance (P=.06, Table 3).
Toxicity Profile After Induction Chemotherapy; Neoadjuvant Therapy for Borderline Resectable Pancreatic Adenocarcinoma.
| G1-G2 n (%) | G3-G4 n (%) | |
|---|---|---|
| Hematologic toxicity | ||
| Neutropenia | 1 (4.5) | 1 (4.5) |
| Anemia | 3 (13.6) | – |
| Thrombocytopenia | 5 (22.7) | 4 (18.2) |
| Non-hematologic toxicity | ||
| Asthenia | 17 (77.2) | – |
| Diarrhea | 4 (18.2) | – |
| Nausea or vomiting | 7 (31.8) | 1 (4.5) |
| Altered hepatic profile (elevated transaminases) | 3 (13.6) | – |
| Neurotoxicity | 19 (86.3) | – |
Source: Hospital Universitari de Bellvitge (2010–2014).
Comparative Analysis Between the RG Group (Resection Group) and PROG Group (Progression Group); Neoadjuvant Therapy for Borderline-Resectable Pancreatic Adenocarcinoma.
| RG Group | PROG Group | P | |
|---|---|---|---|
| Age mean (yrs) | 60.4 (±10.1) | 65 (±10.4) | NS |
| Serum albumin (g/dL) | 43 (±3.9) | 39 (±3.3) | .01 |
| Lymphocytes (mm3) | 2330 (±1095) | 2336 (±975) | NS |
| Leukocytes (mm3) | 7418 (±2177) | 7518 (±2028) | NS |
| Platelets (mm3) | 286000 (±78571) | 275000 (±68511) | NS |
| Mean hemoglobin (g/dL) | 12.9 (±41) | 12.2 (±9.7) | .01 |
| Weight, baseline visit (kg) | 65 (±9.5) | 68 (±12.6) | NS |
| Usual weight (kg) | 71 (±9.1) | 79 (±17.3) | NS |
| CA19-9 | 555 (±199) | 1189 (±1565) | .06 |
| CEA | 5.8 (±6.5) | 25 (±10.3) | NS |
| T1 (days between diagnosis and start of medical treatment) | 53 (±19) | 51 (±53) | NS |
| T2 (days between start and end of medical treatment) | 111 (±55) | 83 (±13) | NS |
CA19-9: carbohydrate antigen 19-9 prior to start of treatment; CEA: carcinoembryonic antigen prior to start of medical treatment; RG group: resection group; PROG: progression group; NS: not significant.
In bold, statistically significant results.
Source: Hospital Universitari de Bellvitge (2010–2014).
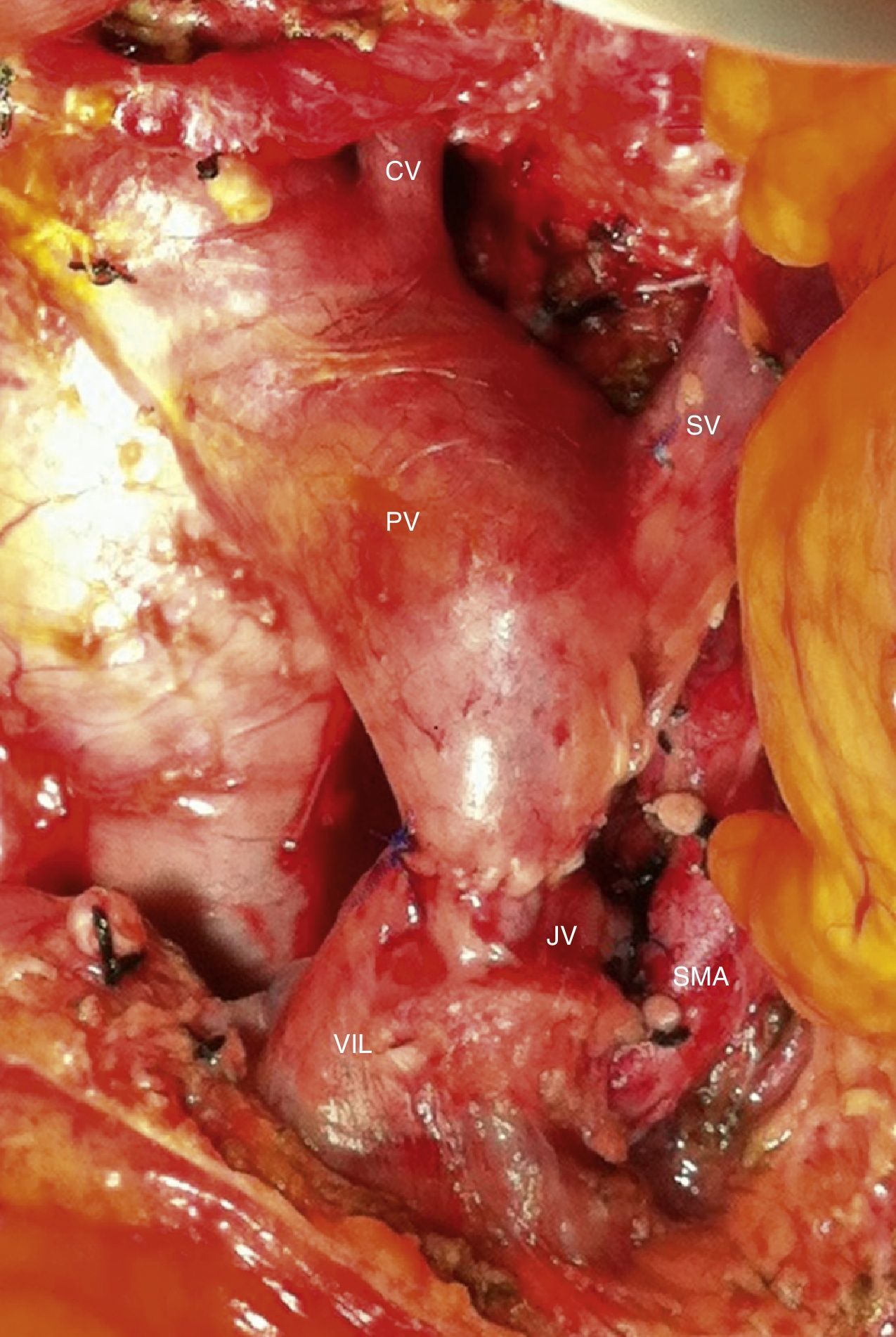
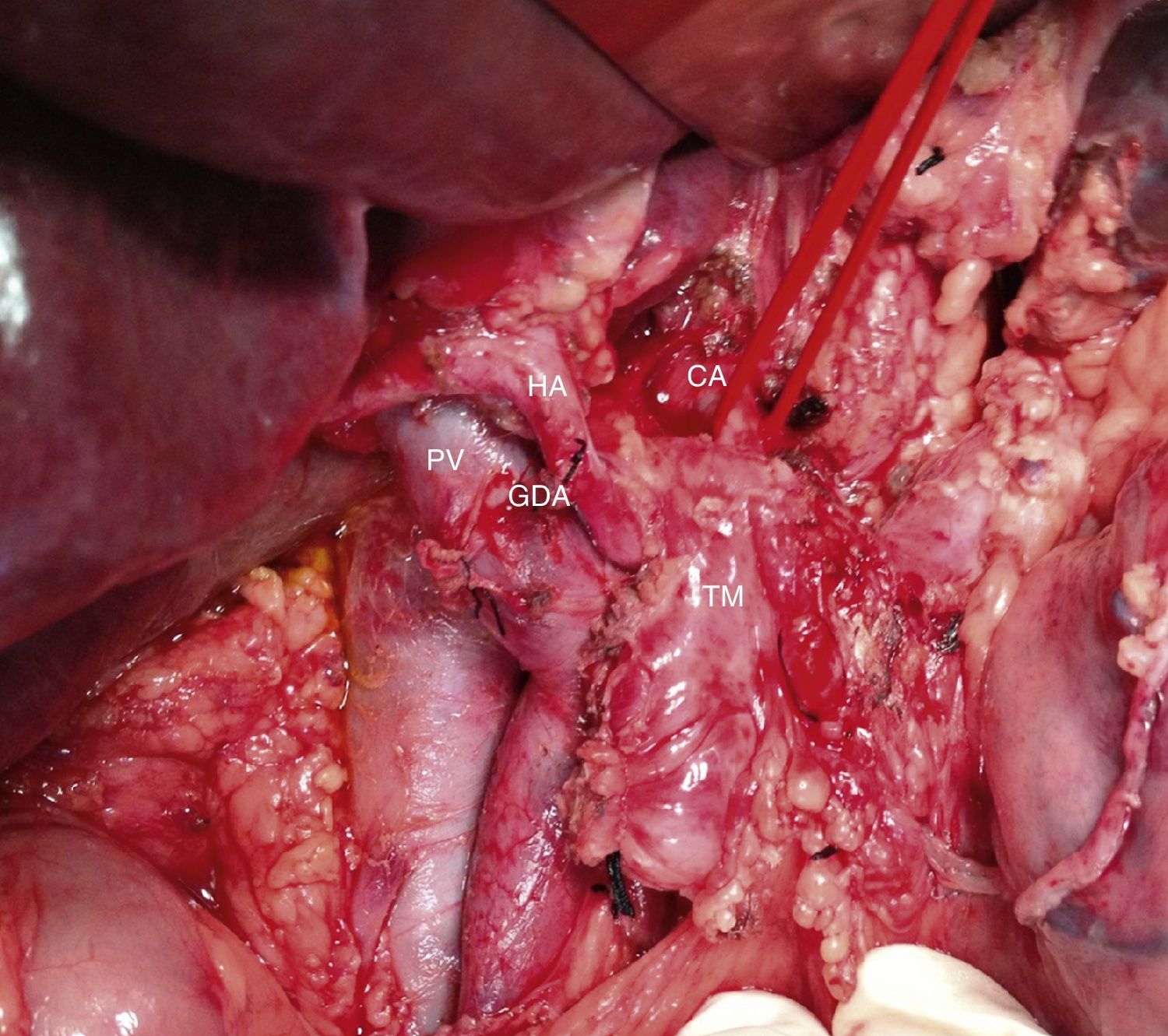
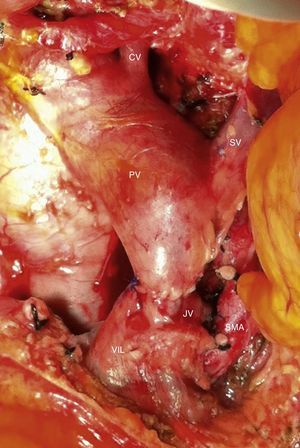
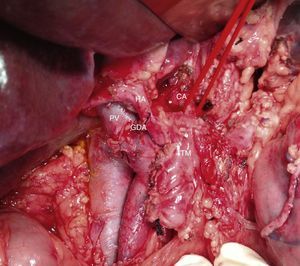
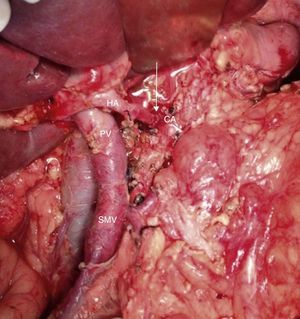
Among the 11 patients who underwent surgical resection, 6 presented tumor contact with the SMA, in 2 the tumors encompassed the CA, in 2 they caused PV/SMV obliteration, and had contact with the hepatic artery in one. In 9 cases, these were lesions in the head of the pancreas: we performed 9 PD, 5 of them with venous resection and end-to-end vascular anastomosis (Fig. 3). We recorded 2 cases with lesions in the neck/body of the pancreas. In one case, distal pancreatectomy was performed with splenectomy with venous and CA resection (Appleby's procedure).12 Since the lesion encompassed the CA, arteriography and preoperative embolization of the common HA were performed with the intention of increasing the hepatic blood flow through the gastroduodenal artery.5 Finally, we treated a patient with double neoplastic lesions in the head and neck/body of the pancreas with double preoperative embolization. Initially, the right gastric artery, splenic artery and left gastric artery were embolized. In a second procedure, the gastroepiploic artery was embolized, leaving the gastric blood flow dependent on the phrenic arteries in order to preserve the well-supplied gastric stump. Finally, a pancreaticoduodenectomy was performed with a four-fifths gastrectomy, splenectomy, resection of the CA and end-to-end arterial anastomosis (Figs. 4 and 5).
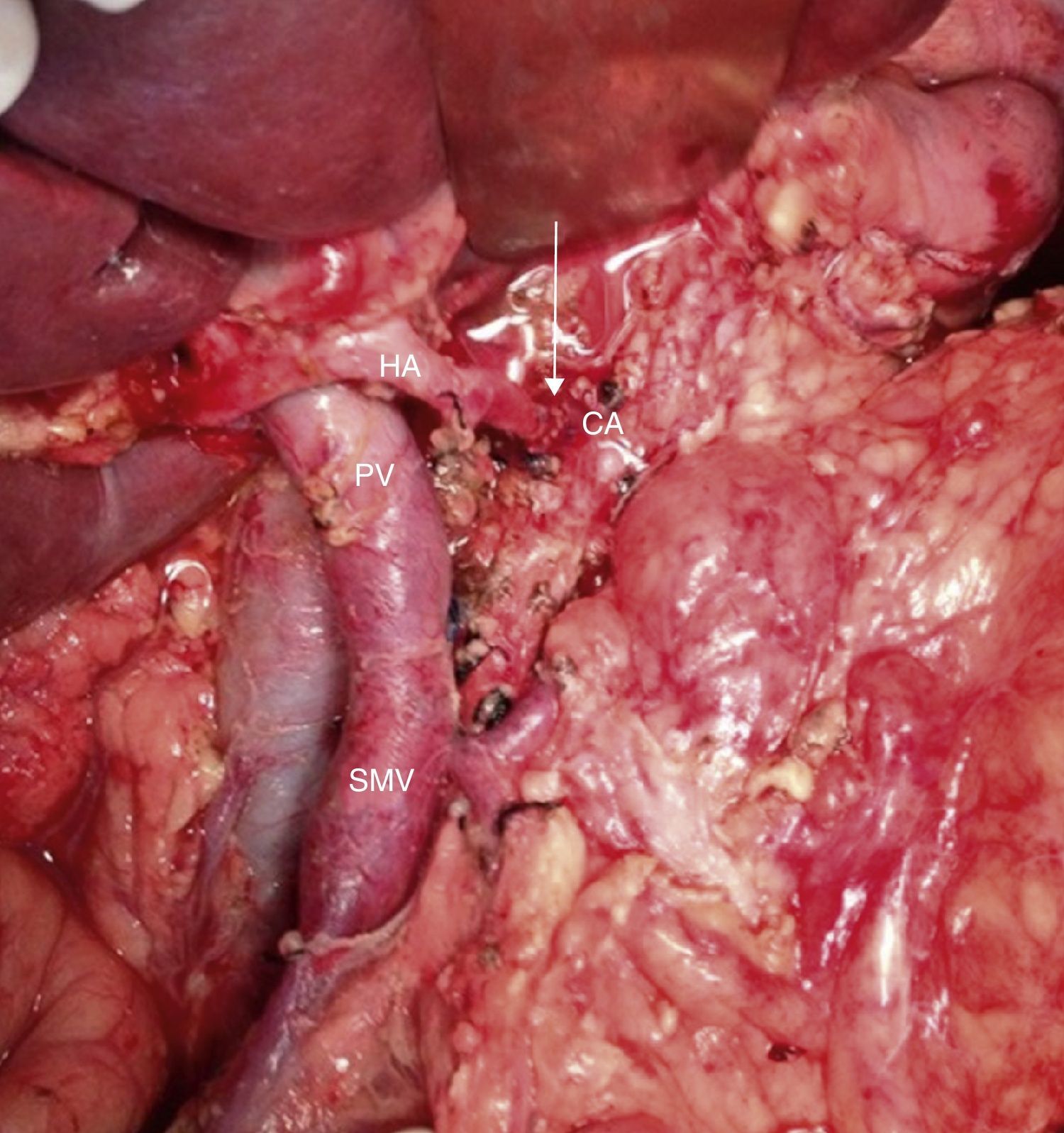
Intraoperative image after resection of borderline-resectable pancreatic adenocarcinoma with obliteration of the superior mesenteric vein and PV. The image shows the end-to-end venous anastomosis between the SMV and the confluence of the ileocolic vein and the JV. SMA: superior mesenteric artery; CV: coronary vein; SV: splenic vein; PV: portal vein; JV: jejunal vein.
Intraoperative image (patient referred to in Fig. 1) after resection of the head of the pancreas due to periampullary neoplasm and prior to resection of the distal pancreas: borderline-resectable pancreatic adenocarcinoma is observed with involvement of the CHA. GDA: gastroduodenal artery; HA: hepatic artery; CHA: common hepatic artery; CA: celiac artery; TM: pancreatic body tumor; PV: portal vein
Intraoperative image (patient referred to in Figs. 1–3) after arterial embolization in 2-stages and subsequent total pancreaticoduodenectomy, four-fifths gastrectomy, splenectomy, CT resection and end-to-end arterial anastomosis. In the photo, the arterial anastomosis is seen between the CA and common HA, marked with an arrow. HA: hepatic artery; CA: celiac trunk; SMV: superior mesenteric vein; PV: portal vein.
The mean operative time was 488min (360–650), and transfusion of blood products was necessary in 4 cases (36%). Postoperative morbidity was recorded in 7 patients (63%). Upon analyzing all the complications, we observed 2 cases of slow gastric emptying (type A), 2 pancreatic fistulas (type A and B), 2 hepatic abscesses, 2 gastrointestinal fistulas, one case of upper gastrointestinal bleeding, one hemoperitoneum due to late-onset arterial lesion, one gastric ischemia, one respiratory distress, one wound infection, one bacteremia and one ascites. Four patients required re-operation: in two cases to achieve hemostasis, one case with late arterial injury (on the 12th day post-op) and one case with hemorrhage of the gastroenteric suture. One patient was re-operated due to gastric ischemia, which underwent total gastrectomy; another patient was re-operated due to respiratory distress and ascites, with suspected gastric ischemia, although there were no pathological findings in the re-operation. The median postoperative hospital stay was 17 days (range: 7–75), and there was no postoperative mortality (Table 4).
Vascular Involvement and Therapeutic Regimen of Patients Treated With Neoadjuvant Therapy for Borderline-Resectable Pancreatic Adenocarcinoma.
| Patient | CTX | Response | Surgical technique | Vascular resection | Morbidity | Morbidity, clavien | Margins | TRG | ypT | ypN | Recurrence | DFS | TUP | OS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | GEMOX | PR | PD | PV/SMV | Hemoperitoneum, HA pseudoaneurysm | IVA | R0 | 3 | ypT3 | ypN1 | No | 15 | ||
| 2 | GEMOX | SD | PD | – | VGL | I | R0 | 0 | ypT0 | ypN0 | Locoregional | 10 | 40 | |
| 3 | GEMOX | PR | PD | SMV | No | – | R1 | 3 | ypT3 | ypN0 | Distant | 7 | 21 | |
| 4 | GEMOX | SD | PD | – | HDA, intestinal fistula, hepatic abscess | IVA | R0 | 2 | ypT3 | ypN0 | No | 13 | ||
| 5 | GEMOX | SD | DP+CA | PV+CA | Gastric necrosis, hepatic abscess | IVA | R0 | 2 | ypT3 | ypN0 | No | 31 | ||
| 6 | GEMERLOXA | PROG | Distant | 2 | 10 | |||||||||
| 7 | GEMEROLOXA | PR | PD | PV | No | – | R1 | 2 | ypT3 | ypN0 | Distant | 7 | 34 | |
| 8 | GEMERLOXA | SD | Locoregional | 6 | 10 | |||||||||
| 9 | GEMERLOXA | SD | Locoregional | 8 | 14 | |||||||||
| 10 | GEMOX | PROG | Distant | 3 | 9 | |||||||||
| 11 | GEMERLOXA | PROG | Locoregional | 2 | 8 | |||||||||
| 12 | GEMERLOXA | SD | PD | – | No | – | R0 | 1 | ypT0 | ypN0 | Distant | 13 | 25 | |
| 13 | GEMOX | PR | PD | SMV | Ascites | II | R1 | 3 | ypT1 | ypN0 | Locoregional | 19 | 31 | |
| 14 | GEMOX | PROG | Locoregional | 3 | 20 | |||||||||
| 15 | GEMOX | SD | Distant | 5 | 17 | |||||||||
| 16 | GEMOX | PR | TPD+CA | HA+CA | Respiratory distress | IVA | R0 | 1 | ypT0 | ypN0 | Distant | 9 | 31 | |
| 17 | GEMOX | PR | Distant | 6 | 11 | |||||||||
| 18 | GEMOX | SD | Distant | 9 | 11 | |||||||||
| 19 | GEM | PROG | Distant | 2 | 3 | |||||||||
| 20 | GEMOX | PROG | PD | PV/SMV | No | – | R1 | 2 | ypT3 | ypN0 | Locoregional | 6 | 26 | |
| 21 | GEMOX | SD | PD | – | Pancreatic fistula | I | R0 | 2 | ypT3 | ypN0 | Distant | 3 | 14 | |
| 22 | GEMOX | SD | Distant | 6 | 12 |
HA: hepatic artery; PD: pancreaticoduodenectomy; TPD: total pancreaticoduodenectomy with splenectomy; SD: stable disease; GEM: gemcitabine; GEMERLOXA: gemcitabine oxaliplatin erlotinib; GEMOX: gemcitabine oxaliplatin; TRG: tumor regression grade; Morbidity Clavien: according to Clavien-Dindo Classification criteria (Dindo: Ann Surg 2004); OS: overall survival (time from start of chemotherapy until death, in months); DP: distal pancreatectomy with splenectomy; PROG: disease progression; CTX: chemotherapy regimen; PR: partial response; DFS: disease-free survival (time from surgery to recurrence, in months); CA: celiac artery; TUP: time until progression (time from start of chemotherapy until progression: months); SMV: superior mesenteric vein; PV: portal vein.
Source: Hospital Universitari de Bellvitge (2010–2014).
The histological study of the resection specimens revealed a TRG of 0 in one case, and a TRG of 1 in 2 cases; likewise, one case was also classified as ypT1 and 7 cases ypT3. Finally, 10 patients presented lymph node involvement. The study of the surgical margins showed microscopic involvement (R1) in 4 cases, whereas in 7 cases (63%) there was no tumor (R0) (Table 4).
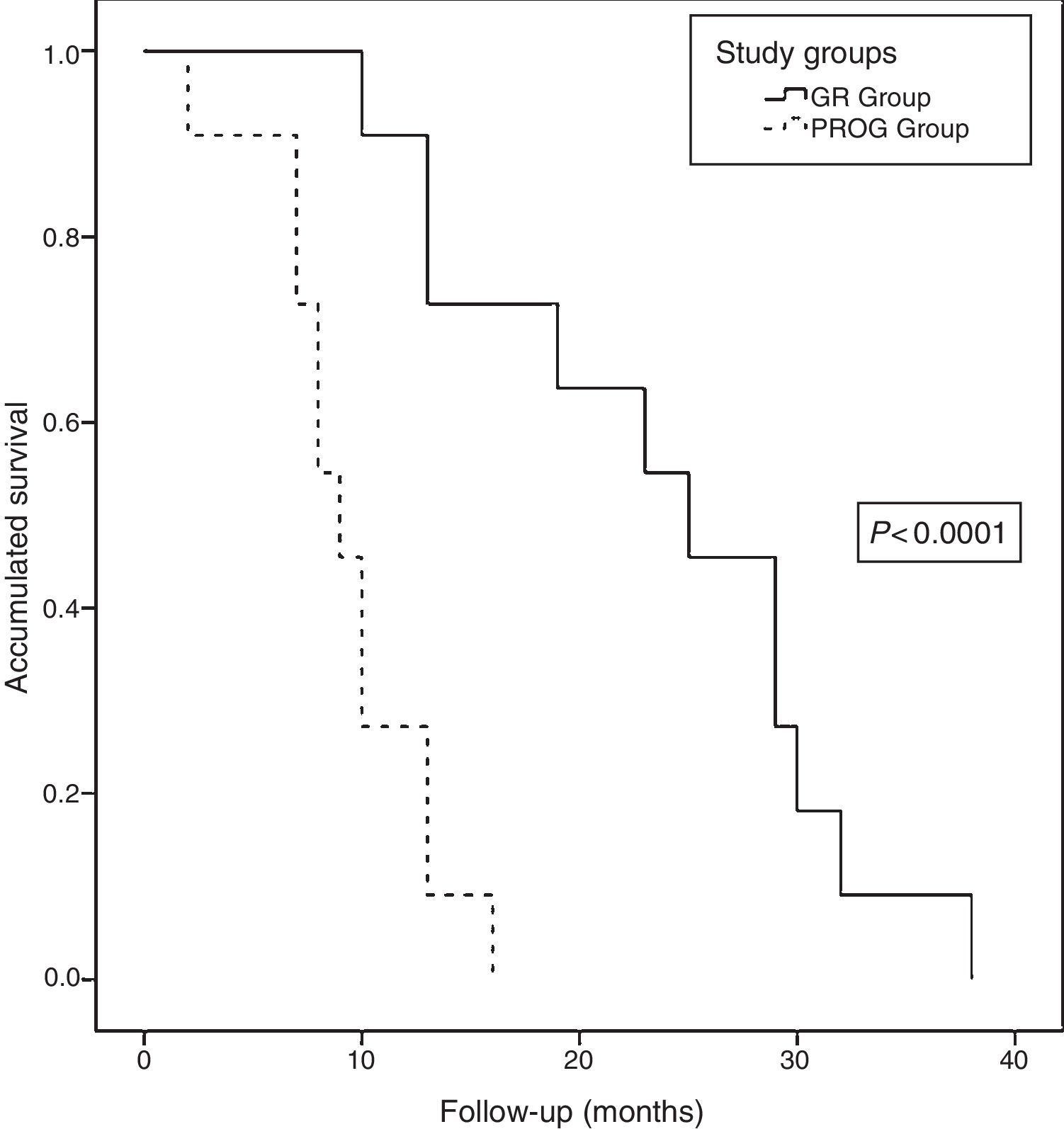
Long-term Follow-upAt the close of the study, all patients (22 patients) had died, with a median actuarial survival of 13 months (range: 9.6–16.3). The median actuarial survival of the RG group was statistically higher than that of the PROG group (25 [16.9–33] vs 9 [7–10.9] months, P<.0001) (Fig. 6). The median disease-free time was 8 months (range: 3–19) and the median time to progression was 5 months (range: 2–8). Regarding the progression or recurrence of the disease, all the patients in the PROG group presented progression and 8 of the patients in the RG group presented recurrence. The progression or recurrence of the disease was diagnosed locally in 7 and at a distance in 12. Among patients who progressed or relapsed, 13 received CTX: 6 based on fluoropyrimidines, 4 gemcitabine and 3 received new treatments based on clinical trials.
Actuarial survival analysis after neoadjuvant treatment and subsequent resection for borderline-resectable pancreatic adenocarcinoma at the Hospital Universitari de Bellvitge (2010–2014); comparison of the actuarial survival curves (Kaplan–Meier curves) of the study groups: RG group (resection group, 11 patients) and PROG group (progression group, 11 patients), using the log-rank test <0.0001.
Adjuvant treatment is standard management after surgery for pancreatic cancer. However, this approach has some drawbacks. First of all, the high morbidity of pancreatic surgery implies that approximately only 60% of resected patients will receive adjuvant treatment after surgery.13 Second, the high relapse rate during the first postoperative year would suggest that the selection of patients for surgery should be improved. Finally, surgical resection is often performed with microscopic involvement of the margins (R1),14 which would justify some type of preoperative treatment to reduce this percentage and thus improve survival. In contrast, neoadjuvant treatment can treat almost all patients staged from the beginning and select patients with the worst tumor biology; in addition, it could improve the rate of surgery with microscopically free margins (R0).
Some international groups have defended its use for years, such as MD Anderson Cancer Center of Texas (United States),15 even though neoadjuvant therapy can also lead to problems, such as biliary drainage-related morbidity, delayed surgery, or progression during treatment. In the 1990s, some studies indicated that neoadjuvant CRTX may improve resectability and reduce recurrence after surgery.16,17 In 2001, Mehta18 published the first series with 15 patients with “marginally” resectable lesions seen on the preoperative CT scan, with a median survival of 30 months in the 9 resected patients. Nearly 10 years later, in 2010, Landry19 published the first randomized phase II multicenter study comparing different treatment regimens in BRPC based on 2 regimens with gemcitabine. In spite of being a promising study, it was ended prematurely due to the low incorporation of patients. In the end, 23 patients were included, 5 of whom underwent resection; the median survival was 26 months, which showed adequate tolerance to the treatment regimens. A recent meta-analysis involving 959 patients with BRPC11 demonstrated that resection after neoadjuvant therapy, in this scenario, is feasible and can be performed in up to 63% of patients, with a percentage of resections with no involvement of the margins (R0) of 57.4%.
The therapeutic regimens used by most groups are based on gemcitabine, either alone or in association with other chemotherapy and radiotherapy (50.4Gy). Recently, good results have been reported with the FOLFIRINOX combination, although it appears to be associated with increased morbidity. A meta-analysis showed a median survival of 17.9 months (range: 14–21): 25.9 months (range: 21.1–30.7) for resected patients and 11.9 (range: 10.4–13.5) for unresected patients, which are results similar to those found by our group. Thus, it seems that, with preoperative treatment, some patients with advanced disease could be selected for resection, and it is possible to achieve survival rates similar to that demonstrated in resectable cases.13
Vascular Involvement in Pancreatic Cancer and the Borderline-Resectable ConceptIn the 1960s and 1970s, the first descriptions of pancreaticoduodenectomy with venous resection were published.20,21 However, it was in 1992 that Ishikawa22 laid the foundation for venous resection in pancreatic cancer. This study demonstrated that partial venous involvement was a candidate for surgical resection, but that stenosis and venous obliteration would be contraindications because survival did not improve. Since then, most groups have performed pancreatic resection with vascular involvement, reporting similar survivals between patients with and without vein resection.23,24 However, some authors have published worse survival rates among patients with venous resection.25
In 2006, the Texas group published an article defining new resectability criteria according to vascular involvement.3 They proposed that cases with venous obliteration (with the possibility of reconstruction after neoadjuvant therapy), isolated involvement of the HA (with the possibility of reconstruction after neoadjuvant therapy) and involvement of the superior mesenteric artery (less than 180°) be included in a category called borderline-resectable.3,4,26,27
Following the publication of the paper by the Texas group, the National Comprehensive Cancer Network (NCCN)28 published guidelines on the management of these patients and the BRPC concept. In 2009, an international consensus of the American Hepatopancreatobiliary Association (AHPBA) of the Society for Surgery of the Alimentary Tract (SSAT) and the Society of Surgical Oncology (SSO) redefined the BRPC concept.29 Lastly, in 2014, the MD Anderson group30 and the Society of Abdominal Radiology and the American Pancreatic Association31 reviewed the BRPC concept taking into account the contact of the tumor with the SMV or SMA (greater or less than 180°).
As we have mentioned, in our series we followed the criteria published by the MD Anderson group in 2006, ruling out neoadjuvant therapy in patients with partial involvement of the SMV or the portal vein as these situations were considered resectable. Our study also included lesions in the body of the pancreas that encompassed the CA. When we analyzed long-term survival, we found evidence that the resected patients had longer survival rates compared to non-surgical patients (25 vs 9 months). Thus, the survival of the 11 patients with borderline-resectable tumor resection seemed comparable to the survival outcome observed in patients with resectable tumors.13 These results could probably be explained by the careful selection of patients after neoadjuvant therapy and the effect of the preoperative treatment itself.
Morbidity After Post-Neoadjuvant Therapy SurgeryMorbidity outcomes after surgical treatment with radiotherapy and neoadjuvant CTX have already been published.32 In our experience, up to 63% of the patients resected had some type of complication, with a high rate of re-operations. However, we did not record postoperative mortality, despite the aggressive nature of the surgery performed, as shown by the fact that 7/11 patients required some type of vascular resection. Among the complications recorded, late-onset arterial damage and gastric ischemia (in cases of CA resection) are the most severe. The combination of preoperative treatment, lymphadenectomy with aggressive arterial dissection and the effect of the probable pancreatic fistula could be the cause of the arterial lesions.32,33 Furthermore, resection of the CA together with pancreatic resection or the Appleby technique12 may entail increased morbidity, such as gastric or hepatic ischemia. Therefore, preoperative embolization is useful for the preparation of patients, since it reduces the rate of postoperative gastric ischemia,5,34 similar to that used in gastric preconditioning prior to esophagogastric surgery.35
In conclusion, the neoadjuvant treatment of BRPC allows us to select a group of patients in which resection achieves a significantly higher survival than the group in which progression is observed during neoadjuvant therapy. Surgical resection in borderline-resectable pancreatic cancer involves a high rate of vascular resection and elevated morbidity, so it should be performed at experienced referral centers.
Authorship/CollaborationsJ. Busquets: composition of the article, critical review and approval of the final version, study coordination.
J. Fabregat: composition of the article, critical review and approval of the final version.
H. Verdaguer: composition of the article, critical review and approval of the final version.
B. Laquente: composition of the article, critical review and approval of the final version.
N. Peláez: data collection.
Ll. Secanella: data collection.
D. Leiva: data collection.
T. Serrano: analysis and interpretation of the results.
M. Cambray: analysis and interpretation of the results.
R. López-Urdiales: analysis and interpretation of the results.
E. Ramos: analysis and interpretation of the results.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Busquets J, Fabregat J, Verdaguer H, Laquente B, Pelaez N, Secanella L, et al. Experiencia inicial en el tratamiento del adenocarcinoma de páncreas borderline resectable. Cir Esp. 2017;95:447–456.