In surgical procedures of the supraclavicular and lateral cervical regions, as well as in cardiac and mediastinal surgeries, diaphragm function can be compromised by the risk of injury to the phrenic nerve and/or the C4 root. There are few publications that treat the intraoperative stimulation of these nerve structures to evaluate their functionality and, to our knowledge, until now it has not been hypothesized about whether it is possible to reduce the injury rates, which reach 26% in some cardiac surgery studies.
We describe the technique used for the neurophysiological monitoring of the phrenic nerve. Also, its usefulness and advantages over other techniques are discussed.
We conclude that, with the increasing incorporation in recent years of intraoperative neurophysiological monitoring, its application to the phrenic nerve is possible in procedures with a risk of injury and, thus, the reduction of iatrogenic injury rates may be feasible.
En procedimientos quirúrgicos de las regiones supraclaviculares y laterales cervicales, así como en cirugías cardíacas y mediastínicas, la función diafragmática puede comprometerse desde la base del riesgo de lesión del nervio frénico y/o la raíz C4. Son escasas las publicaciones que tratan la estimulación intraoperatoria de estas estructuras nerviosas para evaluar su funcionalidad y, en nuestro conocimiento, hasta ahora no se ha hipotetizado acerca de si es posible reducir las tasas de lesión situadas en hasta el 26% en algunos estudios de cirugía cardíaca.
Describimos la técnica empleada para la monitorización neurofisiológica del nervio frénico. Asimismo, se discute su utilidad y ventajas respecto a otras técnicas.
Concluimos que con la incorporación creciente de la monitorización neurofisiológica intraoperatoria en los últimos años, es posible su aplicación al nervio frénico en los procedimientos en los que se considere que existe riesgo de lesión del mismo y, con ella, puede ser factible la reducción de las tasas de lesión iatrógena.
Diaphragm function can be compromised during surgical procedures in the vicinity of the phrenic nerve (PN) and/or the C4 nerve root, which may risk their injury, often inadvertently. These structures are frequently difficult to locate, and their direct visualization, while of great help when attempting to preserve them, does not guarantee their continuing function. Different mechanisms of traction, clamping, contusion, compression, thermal damage or even vacuum pressure can cause injury, even when neuronal integrity seems to have been preserved macroscopically (grades I–IV of the Sunderland peripheral nerve injury classification1).
Few publications have studied intraoperative stimulation of the PN to evaluate the continuity of the nerve pathway. In addition, certain proposed methods for testing diaphragmatic contraction are subjective, such as palpation of the subcostal region in the ipsilateral hypochondrium,2 or indirect, using artifacts recorded on capnography3,4 and pressure-time curves of the ventilator.4
Intraoperative neurophysiological monitoring (IONM) has grown exponentially in the last 20 years. It has become a useful and objective tool, whose main goal is the prevention of neurological damage. This technique can be applied in any nervous structure, central or peripheral, that may be at risk during a surgical procedure, and the PN is no exception. Below, we describe the technique used for neurophysiological monitoring of this nerve.
TechniqueIONM of the PN can be performed in surgical procedures where the surgeon deems there is risk of injury, including cardiac, mediastinal, supraclavicular, as well as cervical surgeries, especially in cases of lateral lymph node dissection. In these latter anatomical regions, IONM can be extended to the C4 root.
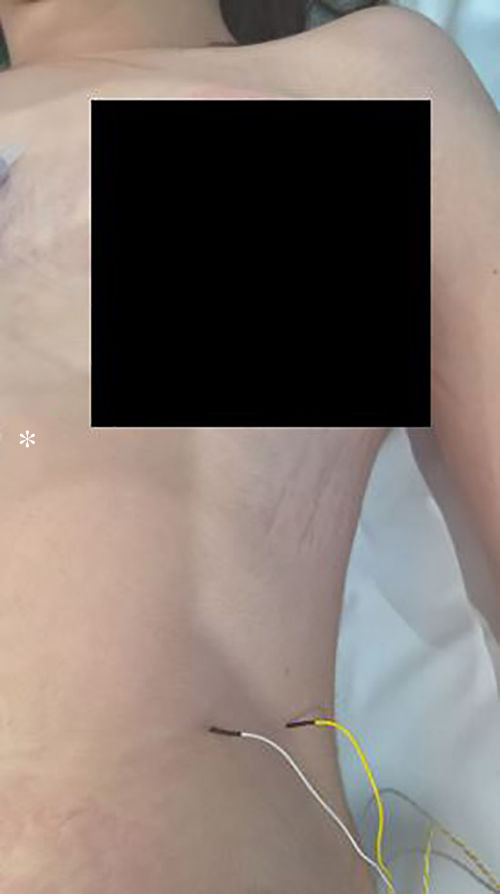
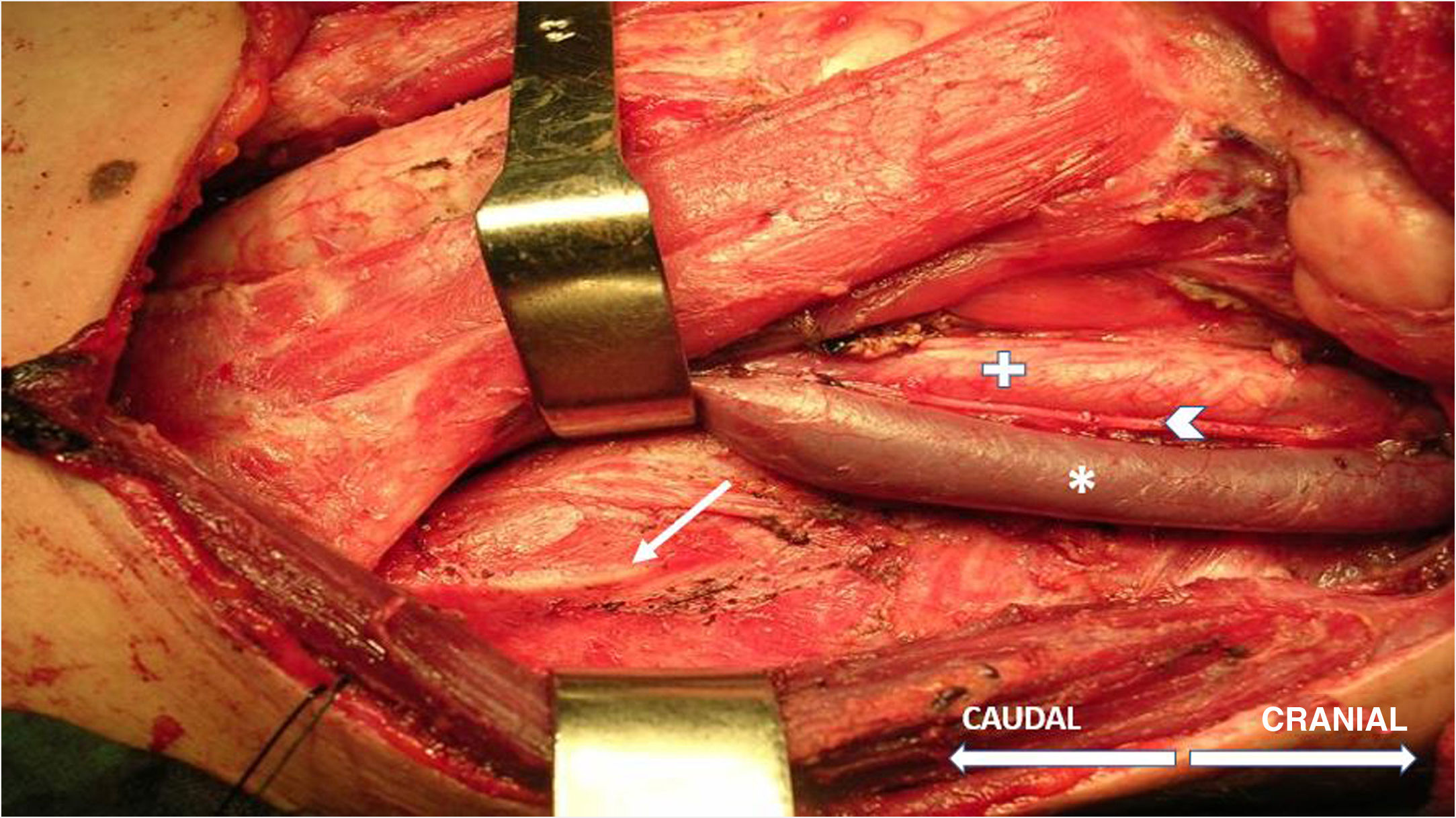
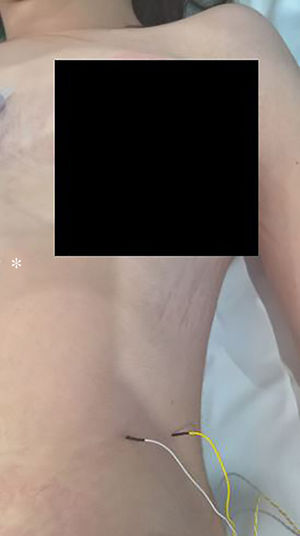
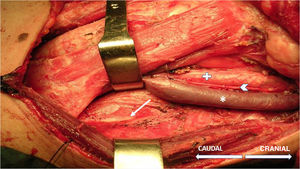
The technique begins after the induction of anesthesia, with the palpation of the 7th or 8th intercostal spaces, located some 16cm along the costal margin from the tip of the xiphoid process.5 At that point, the needle electrodes are inserted: the active electrode, in the midclavicular line, adjacent to the costochondral joint; and the reference needle, laterally about 2cm, on the anterior axillary line (Fig. 1); another grounding electrode is placed in the xiphoid process, and the impedances are checked to not exceed 5kΩ. Stimulation of the PN and/or root of the C4 is done intermittently with a monopolar probe handled by the surgeon at intensities of usually 2–3mA (3pulses/s; duration of the stimulus: 200ms) for mapping by zones (Fig. 2) and 1–1.5mA, once located, to evaluate functionality, when considered appropriate. In cardiac and mediastinal surgeries, it is also possible to place additional stimulation needle electrodes in the supraclavicular fossa (posterior edge of the sternocleidomastoid with the cathode 3cm from the clavicle and the anode 2cm above the former5), which provides more continuous stimulation of the PN (1.5 pulses/s; duration of the stimulus: 50ms; supramaximal intensity with adjustment from about 10mA). This is done according to the criteria of the neurophysiologist, especially after risk maneuvers in the proximity, which makes it possible to detect an injury as soon as it may happen.
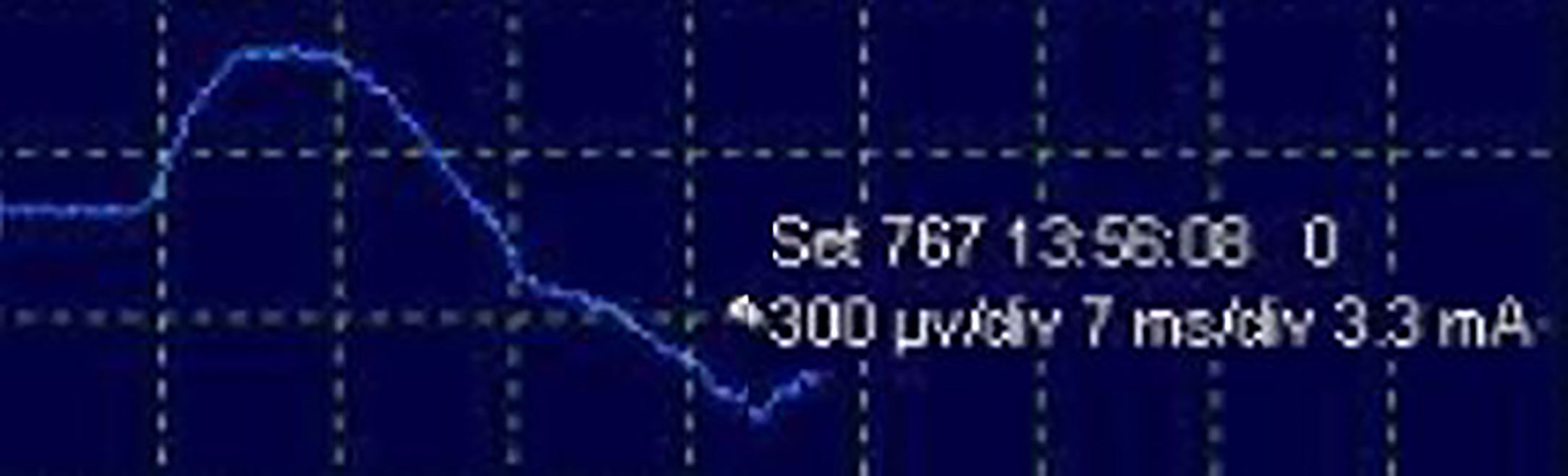
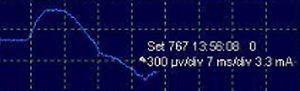
After stimulation of the nerve path at any point (PN to C4 root), either intermittent (filters: low frequency, 20Hz; high, 3kHz) or continuous (filters: low frequency, 10Hz; high, 1kHz), the compound muscle action potential (CMAP) of the PN is obtained (Fig. 3) with a mean amplitude generally greater than 300μV (the latency is variable depending on where the nerve path is stimulated; around 6.5ms from more proximal areas in the neck5). The work methodology during IONM involves detecting significant neurophysiological changes (decrease >50% in amplitude and/or increase >10% in latency of the baseline CMAP), correlating these with the surgical risk maneuvers that may have caused them, and alerting the surgeon, who will take the appropriate precautions in an attempt to reverse the changes and thereby minimize the possibility of postoperative neurological deficit. If an interruption is detected in the transmission of the stimulus along the nerve pathway, achieving a CMAP with a more distal stimulus (indicative of segmental lesion), it would be possible to map, from distal to proximal levels, the exact point of the lesion in order to try to repair it.
To perform the technique, several points must be considered. Some are common to any other IONM procedure, such as close collaboration and adequate communication with the surgical team and the anesthesiologist. The anesthesiologist must also use a regimen of total intravenous anesthesia, avoiding the administration of muscle relaxants as they interfere in CMAP registration, as well as other agents, such as halogenated gases.6 A particularly difficult technical factor arises in patients who are overweight or obese, with voluminous abdomens, which make it difficult to properly connect the registration electrodes to the diaphragm, once inserted. This can be resolved, at least partially, with the use of longer needle electrodes and/or by increasing the sensitivity of the CMAP acquisition. This problem does not exist in cardiac surgeries, in which the surgeon inserts the electrodes and even sutures them directly to the diaphragm.
DiscussionMost publications about PN stimulation include patients treated with central alveolar hypoventilation (for example, after high cervical spinal cord injury) as an alternative to mechanical ventilation; basically, it involves the surgical implantation of an electrode in the PN, in either the neck or thorax, which causes diaphragmatic contraction with an electrical stimulus.7,8 However, there are few study groups that have attempted intraoperative electrical stimulation of the PN and/or the C4 root in order to assess its functional integrity during the course of different surgical procedures,2–4 and only one used a methodology similar to what we propose.9 None of the studies hypothesize about whether it is possible to reduce PN injury rates, which reach 26% in cardiac surgery studies.9,10
Bilateral PN injuries generate a significant restrictive respiratory disorder that usually requires non-invasive mechanical ventilation,11 whereas unilateral injuries generally tend to be well tolerated,2 especially in the absence of comorbidity. However, the loss of muscle tone in one hemidiaphragm is sufficient to alter the differential pressure between the abdominal and thoracic compartments, which results in a reduction in the volume of the latter that may be further worsened by the upwards migration of abdominal contents, especially in supine decubitus.12 Thus, orthopnea and gastroesophageal reflux associated with varying degrees of dyspnea and respiratory sleep disorder can coexist in a situation of restrictive deficit.13 It should be noted that, in the case of iatrogenic injury, these symptoms will be more relevant as they will begin in the immediate postoperative period of a major surgical procedure, with observed higher associated rates of atelectasis, pneumonias, pulmonary effusions, as well as difficulties in weaning from mechanical ventilation and longer ICU stays.10
In the evaluation of the function of a nerve pathway susceptible to injury during a specific surgical procedure, IONM is undoubtedly the method of choice. The parameters it provides are objective, reproducible and generally have established standard values, and any significant deviation defines abnormality. In addition, in the specific case of the C4 root and/or the PN, the registration of CMAP gives the technique greater precision compared to others, in which the methods of checking the diaphragmatic contraction are subjective (palpation of the subcostal region in the ipsilateral hypochondrium2) or indirect (through registered artifacts on capnography3,4 and ventilator pressure-time curves4), with presumably higher rates of false negatives and positives, respectively.
On the other hand, since the acquisition and interpretation of signals takes place in real time during IONM, if neurophysiological changes are detected (after ruling out that they are not related to the administration of drugs and verifying proper functioning of the system), many are reversible, and a correlation can be established with surgical maneuvers involving risk, with high sensitivity and specificity.6 This makes it possible to make decisions in order to avoid postoperative neurological deficit. Despite all efforts, if this main objective is not achieved, the IONM also allows for an initial pathophysiological approach (probably demyelinating/axonal or mixed injury; complete/incomplete). This must ultimately be confirmed in a second surgery by means of electroneurography and electromyography study of the PN and diaphragm, respectively.10,13 These studies are essential for a prognosis of the deficit (degree, transitory/permanent) and to complement the documentation of the IONM in case of litigation, which is becoming increasingly popular. Other theoretical advantages are the help in the localization and dissection of the PN, which is especially important in cases of reoperation, cancer lesions that distort the normal anatomy, or previous radiotherapy. Also, in these instances, it can facilitate greater radicality of the intervention, when necessary.
Probably, the main limiting factor in the application of IONM of the PN and/or C4 root is the accessibility to adequate monitoring equipment and, above all, the presence of a clinical neurophysiologist in the operating room with experience in IONM, which is not always available. However, surgical procedures where this technique may be used usually take place in third-level hospitals, which have the aforementioned resources. As for technical limitations, the most important is related to the need to avoid the use of muscle relaxants (at least during the course of the procedure, after intubation); if this is not possible with an adequate regimen of total intravenous anesthesia, and specific increments in its depth if the patient moves or the muscle plane dissection is difficult, the muscle relaxants used should have the shortest half-life. In this case, as long as the effect lasts, it will not be possible to register an optimal CMAP of the PN.
We conclude that, with the increasing use in recent years of IONM, currently not only as a tool to prevent neurological damage in traumatology and neurosurgery procedures exclusively, IONM of the PN is possible in procedures with a risk of injury. Likewise, it may be feasible to reduce iatrogenic injury rates. However, this statement needs to be demonstrated, and in that case quantified, in future prospective studies. To this end, we are currently designing a study in cardiac surgeries, which are procedures with high PN injury rates, to establish stronger conclusions in this regard.
FundingThe study conducted for this manuscript received no funding.
Conflict of InterestThe authors have no conflicts of interest to declare associated with this publication.
Please cite this article as: Grande-Martín A, Martínez-Moreno A, Sánchez-Honrubia RM, Pardal-Fernández JM. Monitorización neurofisiológica intraoperatoria del nervio frénico: utilidad y descripción de la técnica. Cir Esp. 2019;97:103–107.