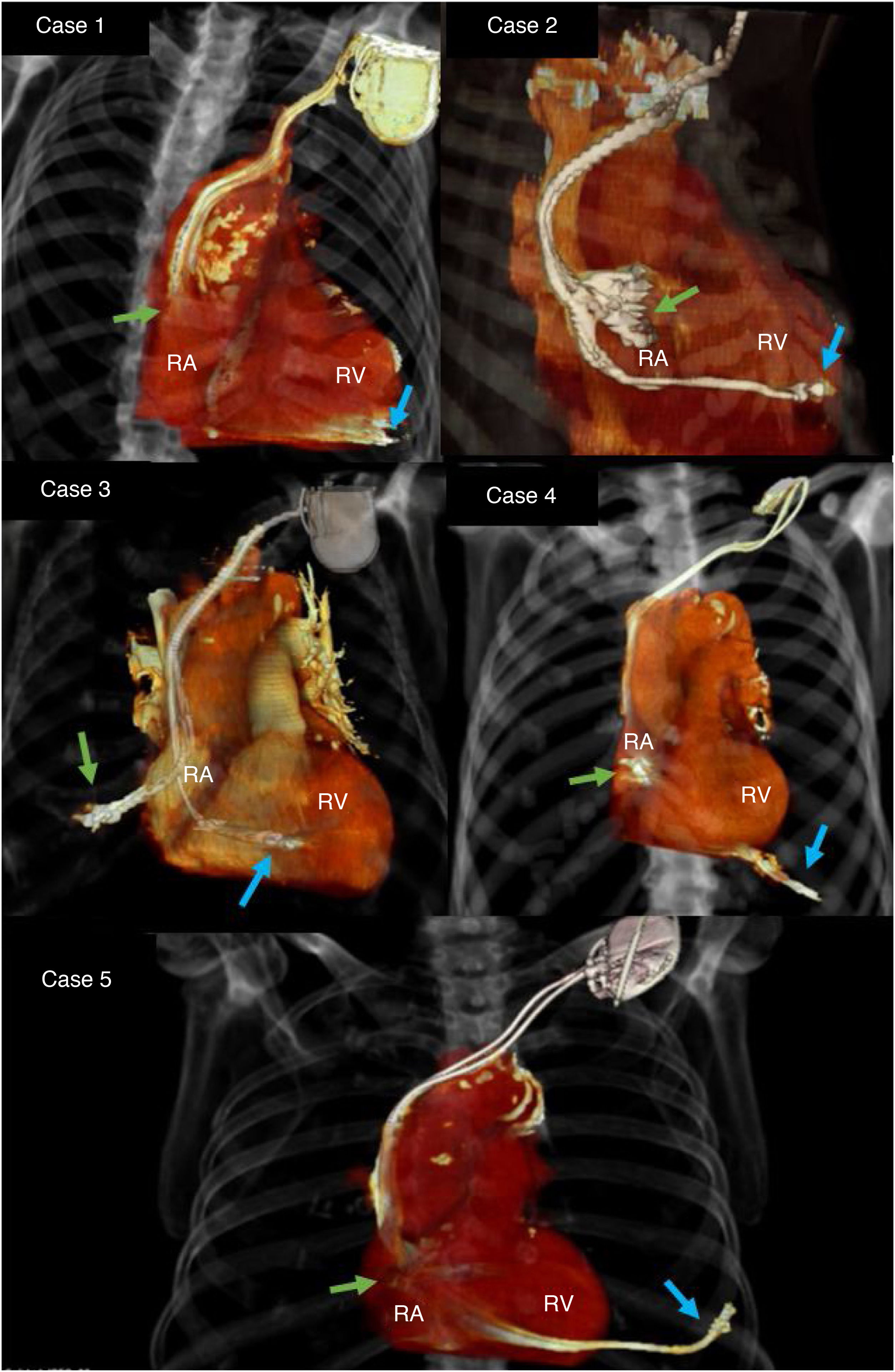
Delayed myocardial perforation (DMP) secondary to an implantable cardiac electronic device (ICED) lead is uncommon1,5. Its management is not standardised in the current literature. The aim of the present is to present our experience in five patients.
MethodsAll patients from January 2018 to October 2020 with a diagnosis of DMP secondary to ICED were recruited. DMP was considered when the event occurred 15 days after implantation of the device, after confirming migration of the electrode from its initial location by imaging tests.
ResultsWe present a series of 5 patients with a mean age of 79 years (SD 7), 3 males, with a median of 47 days to perforation. The patients’ baseline characteristics, device, indication for implantation and clinical features are shown in Table 1.
Characteristics of the patients, devices, and clinical presentation.
| Case | Age | Sex | Indication for implant | Device | Fixation type | Stimulation and perforation site | Time to diagnosis (days) | Principal sign or symptom |
|---|---|---|---|---|---|---|---|---|
| 1 | 72 | Male | CHD with LVEF < 30% | ICD-CRT | Active | RV apex | 30 | Cardiac tamponade |
| 2 | 88 | Male | A-V block | MP bicameral | Active | RV apex | 56 | Cardiac tamponade |
| 3 | 77 | Male | HNOC with NSVT | DAI bicameral | Active | RA orifice | 47 | Right haemothorax (dyspnoea) |
| 4 | 74 | Female | Full A-V block | Dual-chamber P | Active | RV apex | 395 | Syncope |
| 5 | 86 | Female | 2:1 AV block | Dual-chamber P | Active | RV apex | 28 | Absence of P capture on ECG |
A-V: Atrioventricular; CHD: Coronary Heart Disease; ECG: Electrocardiogram; ICD-CRT: Implantable Cardioverter Defibrillator - Cardiac Resynchronisation Therapy; HNOC: Hypertrophic non-obstructive Cardiomyopathy; LVEF: Left Ventricular Ejection Fraction; NSVT: Nonsustained Ventricular Tachycardia; P: Pacemaker; RA: Right Atrium; RV: Right Ventricle.
Three of the patients had a dual-chamber pacemaker, one an ICD-CRT and an ICD. In four cases the perforation was of the right ventricle (RV), three were secondary to the ventricular lead of the pacemaker, and one by the ventricular lead of the ICD-CRT. In the ICD patient the perforation was of the right atrial free wall.
All the patients underwent chest radiography to confirm the displacement of the leads from their original position. A CT scan was requested to identify the exact location of the lead and to rule out concomitant complications (Fig. 1). After the implantation, the lead thresholds were checked to confirm normal electrode function and rule out inadvertent acute perforation.
Management was similar in all cases, with some peculiarities in preoperative management depending on the clinical presentation. Case 1 underwent ultrasound guided pericardiocentesis with a pigtail catheter to relieve cardiac tamponade. In case 2 a pericardial window via subxiphoid approach was performed during the surgical procedure. In case 3 the right haemothorax was drained with a chest tube.
Surgical procedureUnder general anaesthesia. The anterior chest was prepared for potential sternotomy. Both groins were prepared for femoral vessel access. The femoral artery and vein were cannulated, leaving a 4 Fr and 6 Fr introducer, respectively, for percutaneous cannulation should extracorporeal circulation be required due to a major complication.
The lead was removed by simple traction up to the superior vena cava. Once active bleeding was ruled out by invasive monitoring and transoesophageal echocardiography (absence of pericardial or pleural effusion), the leads were repositioned. The ventricular leads of the pacemaker were repositioned at the RV outflow tract, the ICD-CRT was reimplanted in the apex and the atrial lead was reimplanted in the RA appendage.
All the patients were extubated and did not require vasoactive and/or inotropic support. No intraoperative complications were reported.
In the cases of haemothorax and pericardial effusion, the drains were removed at 24 h and they were discharged after 48 h. The other two cases were discharged after 24 h. Chest X-ray and transthoracic echocardiography were performed to rule out complications. All the patients were discharged home in good condition. Clinical follow-up was completed at 1 month and 6 months, with no morbidity or mortality.
DiscussionThe purpose of this study was to analyse all the patients operated in our centre with a diagnosis of DMP secondary to ICED and to describe the clinical characteristics, the presentation of symptoms and to report our centre’s protocolised management.
DMP is an unusual complication with a reported incidence of .1%–.8%, and our incidence is .1%2–4. DMP has been arbitrarily classified as acute (first 24 h), subacute (between 24 h and one month), and delayed (after one month)2,5. Other authors propose a classification of acute (first 15 days) and delayed (more than 15 days)6. We have opted for the latter, as we consider that the therapeutic approach does not differ between subacute and delayed.
Some risk factors for perforation are implantation in the RA free wall or RV apex, female sex, obesity, age over 75 years, anticoagulants, implantation of active fixation leads1. Some of these factors coincide in our series.
The clinical presentation varies, ranging from pacemaker dysfunction to patients with cardiac tamponade. Signs suggestive of DMP are increased thresholds, capture failure and/or electrode censoring. On chest X-ray, lead displacement, which may be as obvious as migration to other chambers. On echocardiogram, pericardial effusion and even the presence of the lead in the pericardial sac. CT provides detailed information on the path of the lead and confirms diagnosis in borderline cases.
The management of DMP varies considerably: removal of the lead by surgery (thoracotomy, sternotomy, or videothoracoscopy with concomitant wall repair), simple traction, or even watchful waiting, refraining from lead removal. All with good results7.
Our approach is conservative, removing the electrode, with preparation for emergent surgery, avoiding increased risk and the comorbidity inherent to the surgical approach.
ConclusionsIn cases of DMP by ICED lead, mobilisation, and repositioning of the lead in an appropriate surgical setting offers good results.
Although we had no complications of active bleeding or myocardial rupture, the main limitation of our series is the small number of cases due to the unusual nature of this complication, therefore we do not recommend moving leads in a non-surgical area.
The management of DMP should be properly protocolised, adjusted to the availability of equipment and personnel in each centre.