Venous thromboembolism (VTE) represents a serious postoperative complication that can be prevented by adequate thromboprophylaxis. Surveys provide relevant information about clinician’s attitudes and preferences regarding VTE prophylaxis.
MethodsTransversal, descriptive study based on a survey sent to general surgeons members of the Spanish Association of Surgeons (AEC), that included 31 questions regarding postoperative VTE and its prevention, as well as three clinical scenarios.
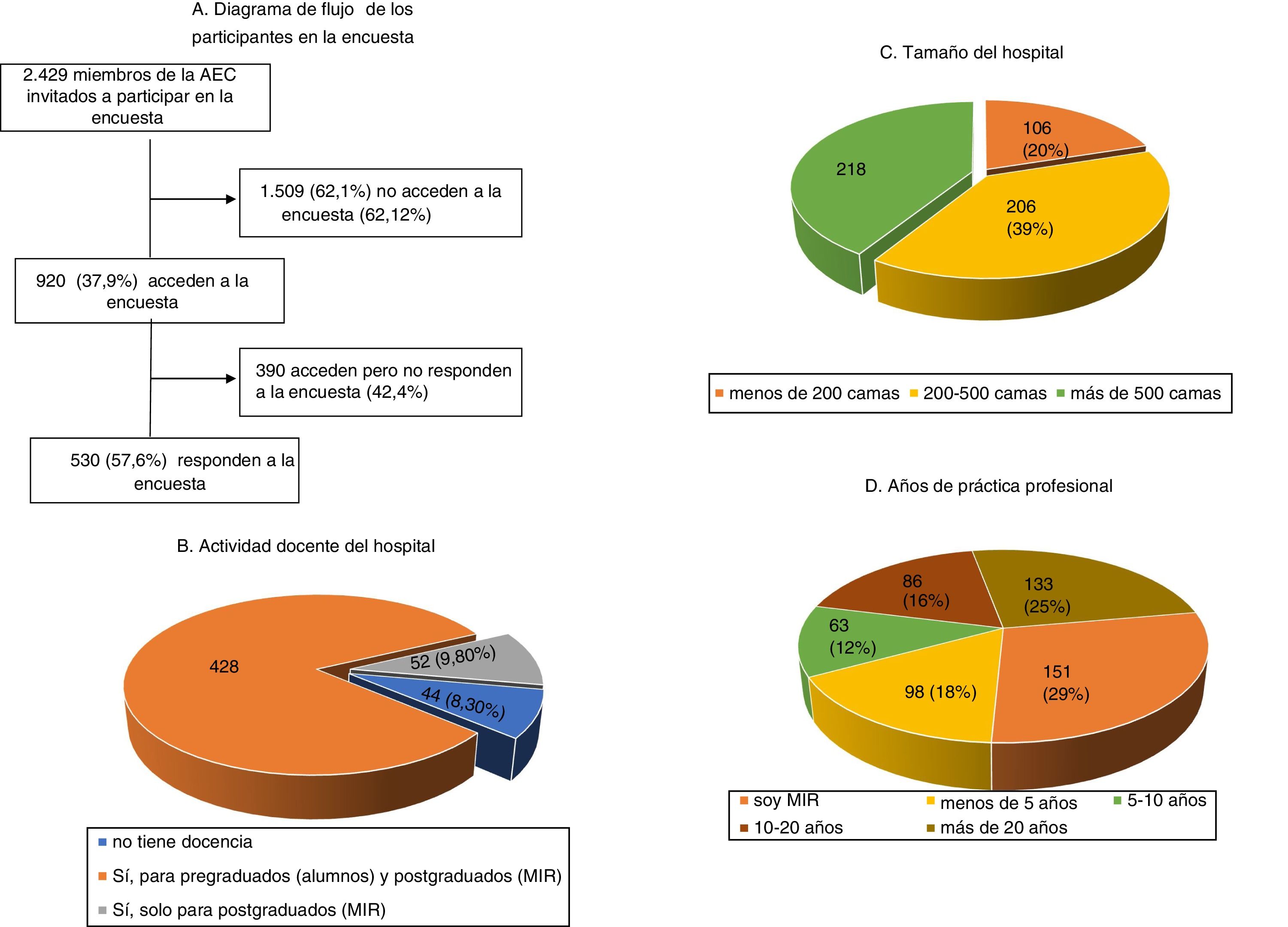
Results530 surgeons, 21.8% of the 2429 invited by electronic mail to participate, completed the survey. Most of the answering clinicians work on in big teaching hospitals, and 28.5% are residents. VTE represents a serious problem for 28% of participants. Although 81% consider that their knowledge on the prevention of postoperative VTE is adequate, a similar percentage recognizes the need for further education. The vast majority (98.7%) use low molecular weight heparins, which are considered the most effective and safe modality, followed by mechanical methods. The Caprini risk assessment score is used by 81% of surgeons, who usually start pharmacological prophylaxis preoperatively. However, there are remarkable differences in the dosing of heparins, timing of initiation, and duration, especially in non-oncologic surgical patients.
ConclusionsMost Spanish surgeons are interested in the prevention of postoperative VTE. Overall, the level of knowledge on thromboprophylaxis is adequate. However, our results indicate that there is a need for better education on relevant practical aspects of prophylaxis that could be achieved by incorporating recommendations from recent guidelines to local hospital-based protocols.
El venous thromboembolism (VTE) representa una complicación postoperatoria grave, pero evitable con una profilaxis adecuada. Las encuestas aportan información útil acerca de las actitudes y preferencias respecto a la prevención del VTE.
MétodosEstudio transversal descriptivo, basado en una encuesta, remitida a los cirujanos generales miembros de la Asociación Española de Cirujanos (AEC), y que incluye 31 preguntas acerca del VTE postoperatorio y su prevención, así como 3 casos clínicos.
ResultadosLa encuesta fue contestada por 530 cirujanos, lo que representa el 21,8% de los 2.429 miembros invitados a participar por correo electrónico. La mayoría de los cirujanos participantes trabajan en hospitales docentes grandes, siendo el 28,5% médicos residentes. Para el 28% el VTE representa un problema importante. Aunque el 81% considera que tiene un conocimiento adecuado sobre la prevención del VTE postoperatorio, un porcentaje similar reconoce necesitar más formación. La mayoría (98,7%) utiliza las heparinas de bajo peso molecular, consideradas la modalidad más eficaz y segura, seguida de los métodos mecánicos. El método de estratificación de riesgo más utilizado es el de Caprini (81%). La mayoría comienza la profilaxis farmacológica preoperatoriamente, pero existe bastante variación en las dosis utilizadas, así como en las pautas de inicio y duración, sobre todo en cirugía no oncológica.
ConclusionesExiste interés y, en general, un adecuado conocimiento acerca de la prevención del VTE entre los cirujanos españoles. Sin embargo, creemos necesaria mayor formación sobre aspectos prácticos de la profilaxis, adaptando las recomendaciones de las guías recientes a protocolos locales.
Venous thromboembolism (VTE) is a frequent and potentially serious problem in surgery and is considered a main cause of avoidable mortality. A study conducted in Sweden reported that 29% of patients who died during the postoperative period had pulmonary embolism at autopsy.1 Another study carried out in the United States showed that the probability of dying after oncological surgery increases 5-fold if a postoperative VTE develops.2 In more than 700 patients from an international registry who suffered symptomatic VTE after surgery for abdominal cancer, one-third of the cases had poor progress in the first 3 months after developing this complication.3
There is abundant evidence that postoperative VTE can be effectively and safely prevented using pharmacological and mechanical methods. In the field of general surgery, there are several international4–6 and Spanish7,8 clinical practice guidelines for the prevention of this complication. However, several cohort studies have shown that thromboprophylaxis in actual clinical practice is insufficiently used and suboptimal in quality.9–11
In order to better understand the differences between guideline recommendations and what is actually done in practice, surveys –despite their biases and limitations– can provide relevant information on the approach of surgeons regarding postoperative VTE and its prevention.12–17 In Spain, the last survey published about the prevention of VTE in general and gastrointestinal surgery dates back to 1988.18
The objective of this study is to analyze the approach and preferences of Spanish general surgeons regarding postoperative VTE, utilizing a survey sent to the members of the Spanish Association of Surgeons (Asociación Española de Cirujanos, AEC), which included clinical scenarios and questions about practical aspects of thromboprophylaxis.
MethodsWe designed a descriptive cross-sectional study, based on a survey of Spanish general surgeons. On May 1, 2017, an e-mail message was sent to the 2429 active members of the AEC, requesting them to answer a questionnaire on VTE and its prevention. Surgeons were able to access the survey through a link to the AEC website (https://www.aecirujanos.es). Three reminders were sent, reiterating the invitation to participate in the survey, which remained accessible until July 31, 2017.
The questionnaire consisted of 31 multiple-choice questions, which contemplated several aspects of postoperative VTE and its prevention (see Appendix B in Additional Material). The survey also included 3 clinical cases with questions referring to the dose of anticoagulant drugs, their initiation and duration, as well as the use of mechanical methods. The analysis of the completed surveys was conducted by Im3dia Comunicación S.L., a company selected by the AEC.
Statistical analysisA descriptive analysis of the questionnaire items was carried out, calculating absolute and relative frequencies. In order to study the possible association between the degree of knowledge and the quality of thromboprophylaxis with other variables, the Pearson chi-square test was applied. A P value of 0.05 was considered statistically significant. All analyses were carried out using the SPSS version 19 program (IBM Corp. Armonk, NY, United States).
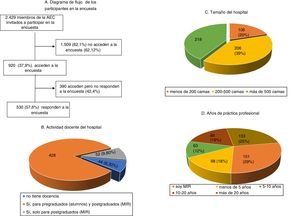
ResultsDuring the 3 months that the survey was available on the AEC website, it was visited by 920 (37.9%) of the 2429 surgeons invited to participate, ultimately being answered by more than half (Fig. 1). The characteristics of the surgeons participating in the survey, as well as their professional experience, are shown in Fig. 1. Specifically, 151 (28.5%) were resident physicians and more than half were specialists with more than 10 years of medical practice. The most frequently performed procedures are listed in Table 1.
Perception of postoperative VTE, risk factors and prevention, and most frequently practiced surgical procedures.
| Level of knowledge about prevention of VTE | |||
|---|---|---|---|
| Personal knowledge | [Adequate knowledge of the specialty | ||
| Low | 46 (8.7%) | Yes | 382 (72.9%) |
| Adequate | 429 (81.1%) | No | 148 (27.1%) |
| High | 54 (10.2%) | – | – |
| Need for training | |
|---|---|
| Yes | 420 (79.4%) |
| No | 109 (20.6%) |
| Who decides on antithrombotic prophylaxis at your hospital? | |
|---|---|
| Surgery | 487 (92.1%) |
| Anesthesia | 110 (20.8%) |
| Hematology | 88 (16.6%) |
| A specific commission | 49 (9.3%) |
| Other | 5 (0.9%) |
| In your clinical practice, what are the 3 most common surgical interventions? | |
|---|---|
| General gastrointestinal surgery | 291 (20.8%) |
| Abdominal wall surgery | 252 (18.0%) |
| Colorectal surgery | 321 (22.9%) |
| Laparoscopic surgery | 373 (26.6%) |
| Bariatric surgery | 64 (4.6%) |
| Endocrine surgery | 51 (3.6%) |
| Other | 48 (3.4%) |
| Advanced age | 54 (10.2%) |
| Laparoscopic approach | 25 (4.7%) |
| Varicose veins | 13 (2.5%) |
| Most commonly used methods of VTE prevention | |
|---|---|
| Low molecular weight heparin | 519 (98.7%) |
| Elastic stockings | 288 (54.8%) |
| Intermittent pneumatic compression | 248 (47.1% |
| Elastic wraps | 60 (11.4%) |
| Unfractionated heparin | 9 (1.7%) |
| Oral anticoagulant (like apixaban) | 6 (1.1%) |
| Other | 4 (0.8%) |
| Aspirin | 3 (0.6%) |
| In your clinical practice, what are the 3 most common surgical interventions? | |
|---|---|
| General gastrointestinal surgery | 291 (20.8%) |
| Abdominal wall surgery | 252 (18.0%) |
| Colorectal surgery | 321 (22.9%) |
| Laparoscopic surgery | 373 (26.6%) |
| Bariatric surgery | 64 (4.6%) |
| Endocrine surgery | 51 (3.6%) |
| Other | 48 (3.4%) |
VTE: venous thromboembolism.
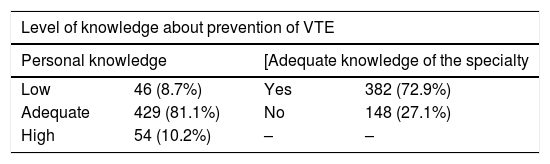
As for the level of knowledge about the prevention of postoperative VTE (Table 1), although the majority considered it adequate, 79% acknowledged that more training is needed in this regard. Meanwhile, the impact of postoperative VTE was considered a minimal, moderate or significant problem by 110 (20.8%), 271 (51.2%) and 148 (28%) participants, respectively.
Some 376 (74.5%) participants stated that they knew or used the AEC antithrombotic guidelines,7 122 (24%) the guidelines of the Spanish Society of Obesity Surgery (SECO) for bariatric surgery (www.seco.org), and 90 (18%) followed the Spanish Association of Major Ambulatory Surgery (ASECMA) guidelines for this type of surgery.8
Most of the survey participants (487 [2%]) reflected that the thromboprophylaxis regimen was decided by the surgery department, 110 (21%) by the anesthesiology department, and 49 (9.3%) by a hospital commission. 57.7% indicated that there is a defined protocol in their service for the prevention of VTE (305 [57.7%]), while 211 (39.9%) were unaware of the existence of a thrombosis commission at their hospital.
Table 1 demonstrates the main thrombotic risk factors contemplated by surgeons when determining the use of prophylaxis: 324 (81%) used the Caprini19 thrombotic risk stratification model, and 73 (18.3%) used the ASECMA guidelines for day surgery.8 The mobile app version of these guidelines were used by 202 (53.2%), while 113 (29%) used a printed document.
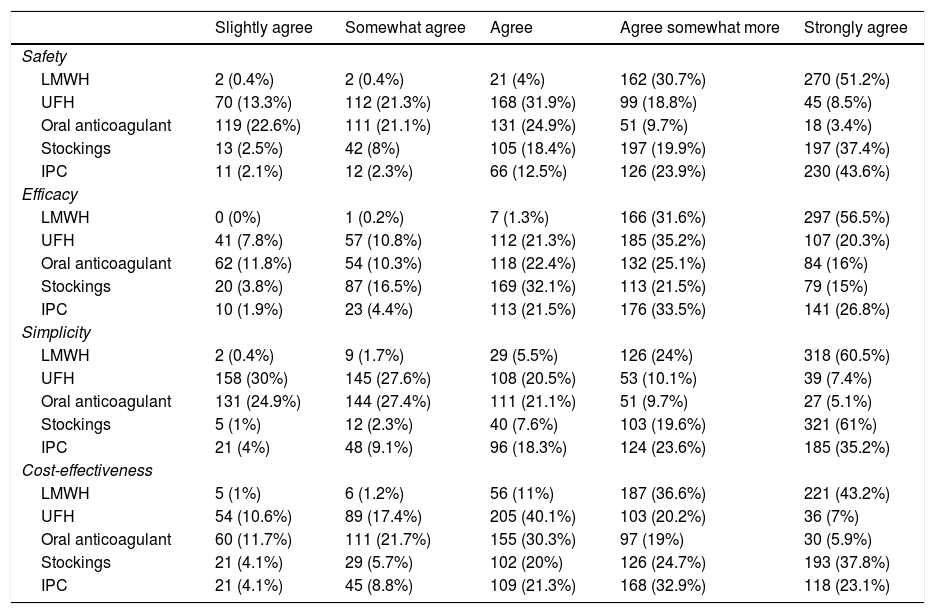
Regarding the prevention of VTE, 522 (99.1%) surgeons reported practicing it regularly. Out of the 46 (8.7%) who did not do so, 27 (58.7%) argued the fear of bleeding complications and 15 (32.6%) the low incidence of VTE. The preferred thromboprophylaxis methods are shown in Table 1, and low-molecular-weight heparin (LMWH) is used by close to 99% of surgeons. Furthermore, more than half would add mechanical methods, mainly elastic stockings. Regarding the perception of the participants about the safety, efficacy, simplicity of use and cost-effectiveness of the most frequently used methods (Table 2), the majority consider LMWH the most effective and safe option, and as simple to use as elastic stockings. The methods considered less cost-effective were intermittent pneumatic compression (IPC) and oral anticoagulants, which were used by 1% of surgeons.
Perception of participating surgeons about the different VTE prevention methods in terms of safety, efficacy, simplicity and cost-effectiveness.
| Slightly agree | Somewhat agree | Agree | Agree somewhat more | Strongly agree | |
|---|---|---|---|---|---|
| Safety | |||||
| LMWH | 2 (0.4%) | 2 (0.4%) | 21 (4%) | 162 (30.7%) | 270 (51.2%) |
| UFH | 70 (13.3%) | 112 (21.3%) | 168 (31.9%) | 99 (18.8%) | 45 (8.5%) |
| Oral anticoagulant | 119 (22.6%) | 111 (21.1%) | 131 (24.9%) | 51 (9.7%) | 18 (3.4%) |
| Stockings | 13 (2.5%) | 42 (8%) | 105 (18.4%) | 197 (19.9%) | 197 (37.4%) |
| IPC | 11 (2.1%) | 12 (2.3%) | 66 (12.5%) | 126 (23.9%) | 230 (43.6%) |
| Efficacy | |||||
| LMWH | 0 (0%) | 1 (0.2%) | 7 (1.3%) | 166 (31.6%) | 297 (56.5%) |
| UFH | 41 (7.8%) | 57 (10.8%) | 112 (21.3%) | 185 (35.2%) | 107 (20.3%) |
| Oral anticoagulant | 62 (11.8%) | 54 (10.3%) | 118 (22.4%) | 132 (25.1%) | 84 (16%) |
| Stockings | 20 (3.8%) | 87 (16.5%) | 169 (32.1%) | 113 (21.5%) | 79 (15%) |
| IPC | 10 (1.9%) | 23 (4.4%) | 113 (21.5%) | 176 (33.5%) | 141 (26.8%) |
| Simplicity | |||||
| LMWH | 2 (0.4%) | 9 (1.7%) | 29 (5.5%) | 126 (24%) | 318 (60.5%) |
| UFH | 158 (30%) | 145 (27.6%) | 108 (20.5%) | 53 (10.1%) | 39 (7.4%) |
| Oral anticoagulant | 131 (24.9%) | 144 (27.4%) | 111 (21.1%) | 51 (9.7%) | 27 (5.1%) |
| Stockings | 5 (1%) | 12 (2.3%) | 40 (7.6%) | 103 (19.6%) | 321 (61%) |
| IPC | 21 (4%) | 48 (9.1%) | 96 (18.3%) | 124 (23.6%) | 185 (35.2%) |
| Cost-effectiveness | |||||
| LMWH | 5 (1%) | 6 (1.2%) | 56 (11%) | 187 (36.6%) | 221 (43.2%) |
| UFH | 54 (10.6%) | 89 (17.4%) | 205 (40.1%) | 103 (20.2%) | 36 (7%) |
| Oral anticoagulant | 60 (11.7%) | 111 (21.7%) | 155 (30.3%) | 97 (19%) | 30 (5.9%) |
| Stockings | 21 (4.1%) | 29 (5.7%) | 102 (20%) | 126 (24.7%) | 193 (37.8%) |
| IPC | 21 (4.1%) | 45 (8.8%) | 109 (21.3%) | 168 (32.9%) | 118 (23.1%) |
IPC: intermittent pneumatic compression; LMWH: low-molecular-weight heparin; UFH: unfractionated heparin; VTE: venous thromboembolism.
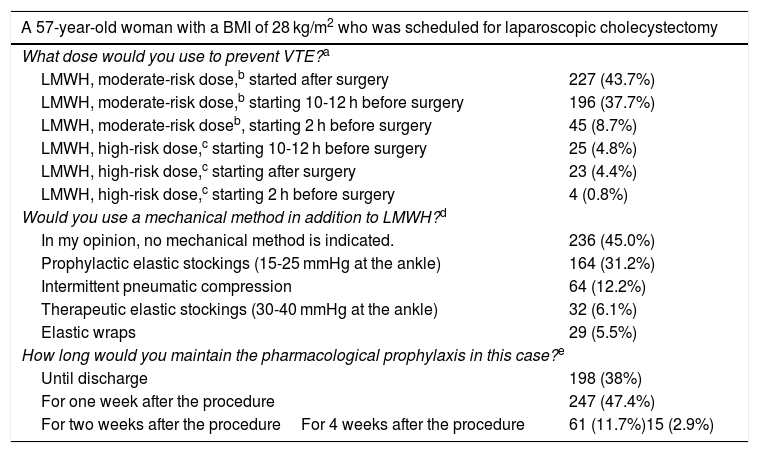
In the case of a 57-year-old overweight patient scheduled for laparoscopic cholecystectomy for cholelithiasis (Table 3), more than half of the participants would start LMWH prophylaxis preoperatively, and most at a dose for moderate risk. In this patient, half of those surveyed reported using mechanical methods in combination with LMWH. Regarding the duration of prophylaxis, more than one-third would maintain it until discharge, which occurred on the first postoperative day, while 47% would maintain it for one week, especially younger surgeons, such as MIR (51%) and specialists with less than 10 years of experience (56%) (P = .003).
Preferred dosage for the prevention of VTE in a patient requiring cholecystectomy for symptomatic cholelithiasis.
| A 57-year-old woman with a BMI of 28 kg/m2 who was scheduled for laparoscopic cholecystectomy | |
|---|---|
| What dose would you use to prevent VTE?a | |
| LMWH, moderate-risk dose,b started after surgery | 227 (43.7%) |
| LMWH, moderate-risk dose,b starting 10-12 h before surgery | 196 (37.7%) |
| LMWH, moderate-risk doseb, starting 2 h before surgery | 45 (8.7%) |
| LMWH, high-risk dose,c starting 10-12 h before surgery | 25 (4.8%) |
| LMWH, high-risk dose,c starting after surgery | 23 (4.4%) |
| LMWH, high-risk dose,c starting 2 h before surgery | 4 (0.8%) |
| Would you use a mechanical method in addition to LMWH?d | |
| In my opinion, no mechanical method is indicated. | 236 (45.0%) |
| Prophylactic elastic stockings (15-25 mmHg at the ankle) | 164 (31.2%) |
| Intermittent pneumatic compression | 64 (12.2%) |
| Therapeutic elastic stockings (30-40 mmHg at the ankle) | 32 (6.1%) |
| Elastic wraps | 29 (5.5%) |
| How long would you maintain the pharmacological prophylaxis in this case?e | |
| Until discharge | 198 (38%) |
| For one week after the procedure | 247 (47.4%) |
| For two weeks after the procedureFor 4 weeks after the procedure | 61 (11.7%)15 (2.9%) |
LMWH: low-molecular-weight heparin; BMI: body mass index; VTE: venous thromboembolism.
The guidelines recommend initiating high-risk doses 10-12 h before surgery for most LMWH4,7,8. In the case of bemiparin, it can be initiated 6-8 h after surgery. No high-risk doses are recommended 2−4 h before surgery.
Moderate-risk dose: enoxaparin 20 mg (2000 IU), bemiparin 2500 IU, and the remainder LMWH < 3500 IU.
The guidelines recommend maintaining pharmacological prophylaxis at least one week after the procedure in this type of surgery.7,8.
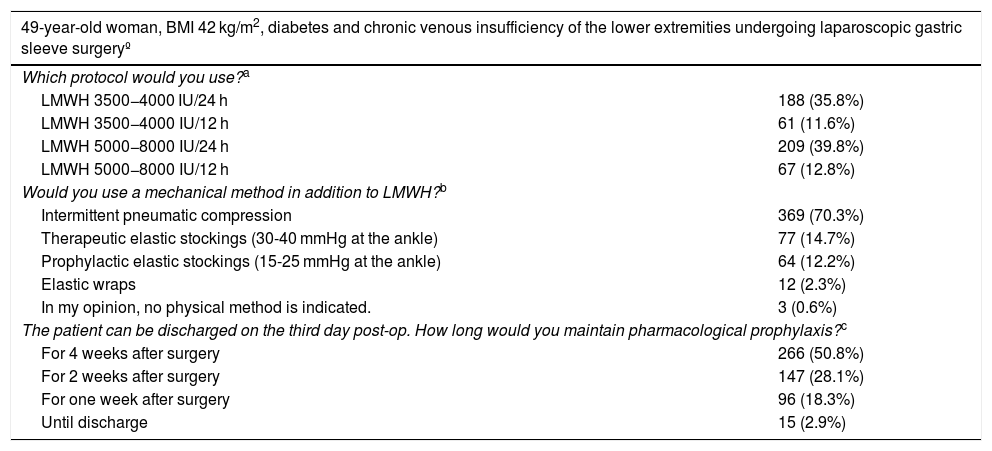
When the patient was a morbidly obese, middle-aged woman undergoing bariatric surgery (Table 4), one-third would administer LMWH at high-risk doses (3500-4000 IU/twice per day), while about 40% of surgeons would resort to higher doses of LMWH (5000-8000 IU). Most surgeons state that they would add mechanical methods, mainly IPC. Regarding the duration of prophylaxis, more than half would maintain it for 4 weeks. There were no significant differences in terms of the options stated or the experience of the surgeons.
Preferred measures for the prevention of VTE in a patient undergoing bariatric surgery.
| 49-year-old woman, BMI 42 kg/m2, diabetes and chronic venous insufficiency of the lower extremities undergoing laparoscopic gastric sleeve surgeryº | |
|---|---|
| Which protocol would you use?a | |
| LMWH 3500−4000 IU/24 h | 188 (35.8%) |
| LMWH 3500−4000 IU/12 h | 61 (11.6%) |
| LMWH 5000−8000 IU/24 h | 209 (39.8%) |
| LMWH 5000−8000 IU/12 h | 67 (12.8%) |
| Would you use a mechanical method in addition to LMWH?b | |
| Intermittent pneumatic compression | 369 (70.3%) |
| Therapeutic elastic stockings (30-40 mmHg at the ankle) | 77 (14.7%) |
| Prophylactic elastic stockings (15-25 mmHg at the ankle) | 64 (12.2%) |
| Elastic wraps | 12 (2.3%) |
| In my opinion, no physical method is indicated. | 3 (0.6%) |
| The patient can be discharged on the third day post-op. How long would you maintain pharmacological prophylaxis?c | |
| For 4 weeks after surgery | 266 (50.8%) |
| For 2 weeks after surgery | 147 (28.1%) |
| For one week after surgery | 96 (18.3%) |
| Until discharge | 15 (2.9%) |
LMWH: low-molecular-weight heparin; BMI: body mass index; VTE: venous thromboembolism.
There is no unanimity in the guidelines in terms of the dose of LMWH to use in bariatric surgery, although the latest European guidelines recommend administering 3000-4000 IU/12 h24 for a patient of these characteristics, while the guidelines of the Spanish Society of Obesity Surgery (SECO) recommend 3000 IU/12 h or 6000 IU/24 h.
Most guidelines recommend adding mechanical methods to LMWH, especially intermittent pneumatic compression, in patients undergoing bariatric surgery with high thrombotic risk24.
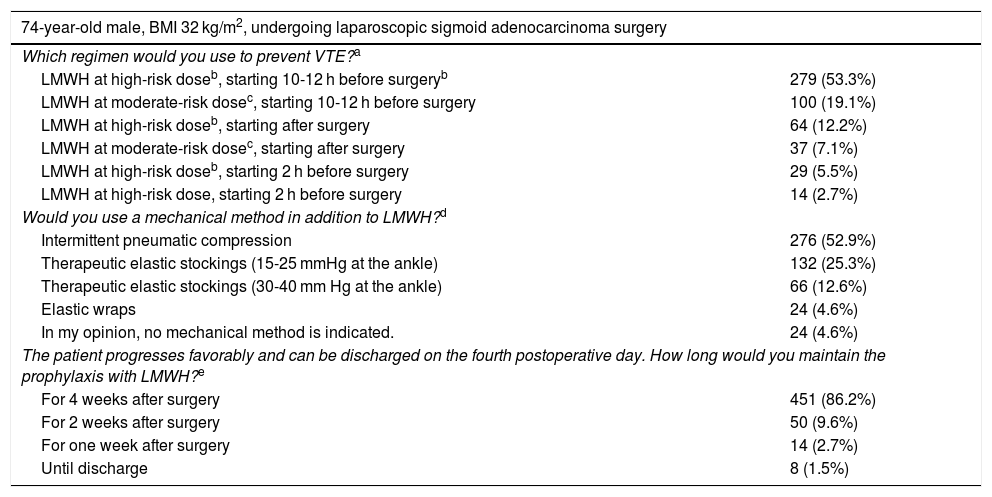
In the case of a 70-year-old obese man who underwent surgery for sigmoid carcinoma (Table 5), 71% of those surveyed would prescribe LMWH at high-risk doses. Overall, a considerable majority (81%) would initiate it preoperatively, 90% of which would start 10−12 h before the procedure. More than 90% would indicate mechanical methods, predominantly IPC (52%). Most surgeons (86%) would maintain LMWH for 4 weeks and only 14 for one week. The surgeons who opted for 4 weeks were younger (MIR or specialists with less than 10 years of experience) (P = .018), working more frequently at teaching hospitals (P = .025) or centers with more than 500 beds (P = .001).
Preferred measures for the prevention of VTE in a patient undergoing colon cancer surgery.
| 74-year-old male, BMI 32 kg/m2, undergoing laparoscopic sigmoid adenocarcinoma surgery | |
|---|---|
| Which regimen would you use to prevent VTE?a | |
| LMWH at high-risk doseb, starting 10-12 h before surgeryb | 279 (53.3%) |
| LMWH at moderate-risk dosec, starting 10-12 h before surgery | 100 (19.1%) |
| LMWH at high-risk doseb, starting after surgery | 64 (12.2%) |
| LMWH at moderate-risk dosec, starting after surgery | 37 (7.1%) |
| LMWH at high-risk doseb, starting 2 h before surgery | 29 (5.5%) |
| LMWH at high-risk dose, starting 2 h before surgery | 14 (2.7%) |
| Would you use a mechanical method in addition to LMWH?d | |
| Intermittent pneumatic compression | 276 (52.9%) |
| Therapeutic elastic stockings (15-25 mmHg at the ankle) | 132 (25.3%) |
| Therapeutic elastic stockings (30-40 mm Hg at the ankle) | 66 (12.6%) |
| Elastic wraps | 24 (4.6%) |
| In my opinion, no mechanical method is indicated. | 24 (4.6%) |
| The patient progresses favorably and can be discharged on the fourth postoperative day. How long would you maintain the prophylaxis with LMWH?e | |
| For 4 weeks after surgery | 451 (86.2%) |
| For 2 weeks after surgery | 50 (9.6%) |
| For one week after surgery | 14 (2.7%) |
| Until discharge | 8 (1.5%) |
IPC: intermittent pneumatic compression; LMWH: low-molecular-weight heparin; BMI: body mass index; VTE: venous thromboembolism.
The guidelines suggest adding mechanical methods to pharmacological prophylaxis in oncological high-risk cases, especially IPC5,6. Prophylactic stockings should exert a pressure of 15-25 mmHg at the ankle. Therapeutic stockings (30-40 mmHg) are not recommended for the primary prevention of VTE due to the risk of ischemia7.
In open or laparoscopic surgery for abdominal cancer, most guidelines recommend prolonging the prophylaxis 4 weeks after the procedure.5,6.
The results of this study show that most surgeons who answered the survey consider that postoperative VTE is a moderate or significant problem, and almost all of them report using thromboprophylaxis, mainly LMWH. Among the minority who do not routinely use prophylaxis, the main reasons are fear of bleeding complications and considering the incidence of VTE to be low. These results are similar to those of other surveys conducted in the United States.16,20
Although 80% of that surveyed feel that they have an adequate level of knowledge about thromboprophylaxis, a similar percentage admits that they need more training. It should be noted that most of the participants are residents or specialists with less than 10 years of experience, working at medium-sized and large teaching hospitals. The decision about VTE prophylaxis is mainly made by surgeons and anesthetists, and more than 40% of the services do not have a specific protocol in this regard. Only 24% are aware of the existence of a Thrombosis Committee at their hospital. This would explain, in part, the variability observed in antithrombotic prophylaxis measures.
With regard to the most frequently assessed thrombotic risk factors when indicating VTE prophylaxis, it is striking that advanced age is only mentioned by 10% and immobilization by 18%, while in other surveys these percentages reach 25% and 44% in general surgery13 or 46% and 68% in cervical surgery,20 respectively. These differences could be reduced by incorporating validated risk stratification models.19
The analysis of the perceived characteristics of thromboprophylaxis modalities seems to explain why LMWH is preferred, as it is considered the most efficient, safest, most cost-effective, and easiest to use. On this point, most surveys and cohort studies have shown similar results, especially in Europe,10,11,21 Canada,17 and Israel.22 On the other hand, mechanical methods are more popular in the United States.13,14,16,17,23 It is striking that half of the surgeons in our study reported using stockings and IPC with certain frequency since their use has been very scarce in cohort studies. In the study by Vallano et al,11 although 10% of the patients had contraindications for the use of anticoagulants, only 2.5% were treated with elastic stockings. Something similar happened in the ENDORSE study, in which only 1% of surgical patients in Spain were prescribed these methods.9 These discrepancies are possibly due to the fact that many surgeons who would opt for mechanical methods cannot do so in their actual practice, probably because they are not available. It is worrying that, among the surgeons who opted for elastic stockings in the cases considered, up to 50% opted for the therapeutic compression profile (30-40 mmHg at the ankle), a pressure higher than that recommended for the primary prophylaxis of VTE (15-25 mmHg). This profile could cause ischemic complications in patients with peripheral artery disease.
The majority of the participants opted for preoperative initiation of LMWH, as in other similar studies,17,21,22 which is recommended for most LMWH in general surgery, except in the case of bemiparin, which can be started 6 h after. Although moderate-risk doses can be administered 2-4 h before the procedure, high-risk doses should be administered 10-12 h before in order to reduce the risk of bleeding, as most survey respondents in our study and other studies report doing.21,22
Regarding the duration of prophylaxis with LMWH, most guidelines recommend 7–10 days after general non-cancer surgery and day surgery.7,8 However, in the case of an obese patient who underwent laparoscopic cholecystectomy, we are concerned that more than one-third of surgeons only maintained treatment until discharge, meaning for only one day. In contrast, in bariatric surgery, half would extend prophylaxis for 4 weeks (as in a recent French survey15), although 28% would extend it for 2 weeks, which is recommended in European guidelines.24 In an older patient treated laparoscopically for colon cancer, more than 80% opt for the 4-week dosage, recommended by the most recent guidelines for abdominal and pelvic cancer.5,6
The differences observed between clinical practice, based on the preferences expressed in the survey, and the recommendations of the available clinical practice guidelines (especially LMWH doses, initiation and duration) can be attributed to different causes, such as non-compliance with these guidelines, which also do not coincide in some recommendations, as is the case with bariatric surgery. More information is also needed on the pressure profile of elastic stockings, which has not been well defined in many guidelines. In our opinion, local multidisciplinary protocols that adapt the recommendations to the needs of each hospital would reduce the variability observed in this survey and improve the quality of thromboprophylaxis.
Our analysis has limitations that are inherent to any survey, such as the possible selection bias of the participants, meaning that surgeons most interested in the problem would respond. On the other hand, the response rate obtained, close to 58% of those who accessed the survey and 22% of the total, is acceptable and exceeds the rates of other similar surveys sent individually to surgeons, ranging between 11% and 21%;13,20,25,26 meanwhile, surveys sent to departments achieve higher percentages, between 45% and 80%.17,21,22 In our opinion, it is more realistic to obtain the individual opinion of the clinicians than that of the service, which is usually expressed by the department heads.
We understand that the results of this survey provide up-to-date information on the situation of VTE prophylaxis in general surgery in our country, and that their analysis will allow us to adopt measures to correct the areas in which there is greater deviation between the recommendations and current practice.
To conclude, this survey reflects the approach and preferences of a significant number of Spanish surgeons, and the young age of the participants stands out. In general terms, it is perceived that most survey respondents consider VTE a relevant problem, and the majority implement measures for its prevention. In our study, however, we have detected certain instances in which the preferences of many participants did not follow the recommendations of the main guidelines, especially for practical aspects such as initiation, dose and duration of LMWH prophylaxis, and specifically in non-oncological and bariatric surgery. Although the respondents would indicate the mechanical methods more frequently than expected, there is a lack of knowledge regarding the appropriate pressure profile of elastic stockings. In view of these results, we feel that it is necessary to have thromboprophylaxis protocols at each hospital, with a consensus of other services, that incorporate and adapt the recommendations of the main clinical practice guidelines.
FundingThis study has been partially financed by the Sanofi company, which covered the expenses for the creation of the electronic version of the survey and the analysis of the responses obtained, without the aforementioned company having participated in or influenced the composition of the manuscript or the interpretation of the results.
Conflict of interestsNone.
We would like to thank the Asociación Española de Cirujanos for their invaluable collaboration in carrying out this study, as well as the Sanofi company for the financial support to defray the costs of the study. We also want to express our gratitude to all the surgeons who took the time to respond to the survey.
Please cite this article as: Arcelus Martínez JI, Leiva Jiménez B, Ruiz Barrera L, Expósito Ruiz M, Muñoz Pérez N, Villar del Moral J, et al. Profilaxis del tromboembolismo venoso en cirugía general en España. Análisis de una encuesta nacional. Cir Esp. 2020. https://doi.org/10.1016/j.ciresp.2020.04.020