Controversy persists as regards the indications and results of surgery in the treatment of patients with stage pIIIA-N2 non-small cell lung cancer (NSCLC). The objective of this study was to analyze the overall survival of a multicenter series of these patients and the role of adjuvant treatment, looking for factors that may define subgroups of patients with an increased benefit from this treatment.
MethodsA retrospective study was conducted on 287 patients, with stage pIIIA-N2 NSCLC subjected to complete resection, taken from a multi-institutional database of 2994 prospectively collected consecutive patients who underwent surgery for lung cancer. Adjuvant treatment was administered in 238 cases (82.9%). Analyses were made of the age, gender, histological type, administration of induction and adjuvant chemotherapy and/or radiation therapy treatments.
ResultsThe 5-year survival was 24%, with a median survival of 22 months. Survival was 26.5% among patients receiving with adjuvant treatment, versus 10.7% for those without it (P=.069). Age modified the effect of adjuvant treatment on survival (interaction P=.049). In patients under 70 years of age with squamous cell carcinoma, adjuvant treatment reduced the mortality rate by 37% (hazard ratio: 0.63; 95% CI; 0.42–0.95; P=.036).
ConclusionsCompletely resected patients with stage pIIIA-N2 NSCLC receiving adjuvant treatment reached higher survival rates than those who did not. Maximum benefit was achieved by the subgroup of patients under 70 years of age with squamous cell carcinoma.
Las indicaciones y resultados de la cirugía en el tratamiento de pacientes con carcinoma broncogénico no microcítico (CBNM) en estadio IIIA-N2 continúa siendo objeto de debate. Este estudio analiza la supervivencia global y el papel del tratamiento adyuvante en una serie multicéntrica de pacientes en estadio IIIA-N2 completamente resecados, buscando factores que puedan definir subgrupos de pacientes en quienes esta adyuvancia resulte más beneficiosa.
Material y métodosEstudio retrospectivo de una serie multicéntrica de pacientes con CBNM resecado en estadio IIIA por N2 tomados de una base de datos con 2.994 pacientes intervenidos por CBNM recogidos prospectivamente. Se estudia la supervivencia y la influencia de las variables edad, género, tipo histológico y administración de tratamiento neoadyuvante y coadyuvante con quimioterapia o radioterapia.
ResultadosLa supervivencia global de la serie a 5 años fue del 24% con una supervivencia media de 22 meses. En pacientes con tratamiento adyuvante fue de 26,5%, contra un 10,7% en aquellos sin adyuvancia (p=0,069). La edad modificó el efecto de la adyuvancia (interacción p=0,049). En pacientes por debajo de 70 años con carcinoma epidermoide, el tratamiento adyuvante redujo la tasa de mortalidad un 37% (hazard ratio: 0,63; p=0,036).
ConclusionesEl tratamiento adyuvante aumentó la supervivencia en los pacientes con CBNM resecado en estadio IIIA-N2. El beneficio fue mayor en pacientes de menos de 70 años con carcinoma epidermoide.
Up to 80% of lung neoplasms are non-small-cell lung cancer (NSCLC). Approximately 15% are found in stage IIIA-N2 at diagnosis,1 meaning that they have ipsilateral mediastinal lymph node involvement. However, it is a very heterogenous group of patients whose treatment is still the object of debate.2–4 It is still not clear which patients will benefit from surgical resection. Many studies have tried to identify prognostic factors in this stage. What seems to be becoming more and more evident is that it is a disease that requires multidisciplinary treatment in most cases.5 The objective of this study is to analyze overall survival in a multicenter study of patients with NSCLC and resected N2 mediastinal disease, and to determine the role of adjuvant treatment while analyzing whether there is a subgroup of patients in whom adjuvant therapy was significantly more beneficial.
Materials and MethodsThis is an observational, retrospective, multicenter study of patients with NSCLC in surgical-pathological stage IIIA-N2 that had been completely resected. The cases came from a database of 2994 patients who underwent surgery for lung cancer at hospitals belonging to the Bronchogenic Carcinoma Workgroup of the Spanish Society of Pulmonology and Thoracic Surgery (in Spanish, GCCB-SEPAR) between October 1993 and September 1997.6 These patients represent approximately 50% of the cases with these characteristics in Spain during this time period. Although the hospitals were of different types, the surgical and oncologic aspects were homogeneous.7 The data collection process underwent a strict quality control to ensure the reliability and homogeneity of the determinations.8 The study was approved by the Ethics Committee of the coordinating hospital center. Staging was done in accordance with the TNM classification for NSCLC published in 1997.1 Induction chemotherapy was indicated in patients with histologic confirmation of N2 mediastinal lymph node involvement. In all the cases analyzed, the resection was complete, meaning that there were tumor-free resection margins, no extracapsular extension of the lymph nodes, no involvement of the most cranial or most caudal lymph node stations analyzed, and cytology confirmation negative for malignancy in the cases with associated pleural effusion.9 For our study, we selected the 319 cases with N2 post-op pathology diagnosis, in other words, patients with resected N2 disease. We excluded the 32 patients who died during the post-op period (either in hospital or within the first 30 days after resection). The remaining 287 patients made up the final sample of our study. The patients were followed up in the outpatient setting for more than 5 years with thoracoabdominal computed tomography (CT) at least every 6 months. The following variables were analyzed: sex, age (in 2 age groups: younger than 70, or 70 and older), histology, resection type and adjuvant treatment (chemotherapy or radiotherapy and time of administration). The qualitative variables are shown in their distribution of frequencies, and the quantitative variables as mean with standard deviation or as mean and interquartile range. For the survival analysis, the Kaplan–Meier method was used. For the analysis of the possible impact on survival of the different variables, the Cox proportional hazards model was used. The hazard ratios (HR) are presented with a 95% confidence interval (95% CI). The statistical study was performed with the SPSS 15.0 program (IBM Systems, Chicago, United States).
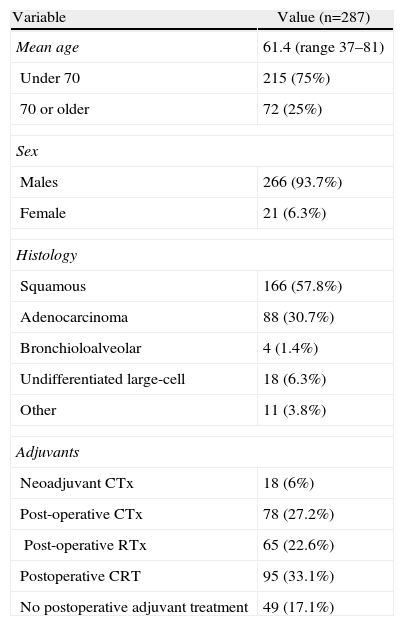
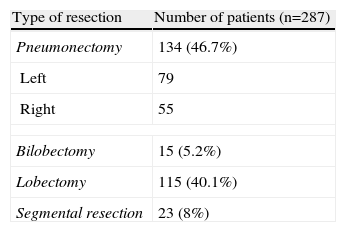
ResultsThe total number of patients who met the inclusion criteria was 287. The average follow-up period was 92 months (range 66–113), meaning that the patients were followed for more than 5 years, which was one of the initial conditions of the study. The basic characteristics of the patients are detailed in Table 1. The types of surgical resection performed are detailed in Table 2. In all cases, systematic mediastinal lymph node dissection, systematic lymph node sampling or lobe-specific lymph node samplings were performed.
Descriptive Data of the Series.
| Variable | Value (n=287) |
| Mean age | 61.4 (range 37–81) |
| Under 70 | 215 (75%) |
| 70 or older | 72 (25%) |
| Sex | |
| Males | 266 (93.7%) |
| Female | 21 (6.3%) |
| Histology | |
| Squamous | 166 (57.8%) |
| Adenocarcinoma | 88 (30.7%) |
| Bronchioloalveolar | 4 (1.4%) |
| Undifferentiated large-cell | 18 (6.3%) |
| Other | 11 (3.8%) |
| Adjuvants | |
| Neoadjuvant CTx | 18 (6%) |
| Post-operative CTx | 78 (27.2%) |
| Post-operative RTx | 65 (22.6%) |
| Postoperative CRT | 95 (33.1%) |
| No postoperative adjuvant treatment | 49 (17.1%) |
CRT: chemoradiotherapy; CTx: chemotherapy; RTx: radiotherapy.
The actuarial survival estimated by applying the Kaplan–Meier method for the entire series of 287 patients at 12, 24, 36, 48 and 60 months was 71%, 48%, 36%, 30%, and 24%, respectively.
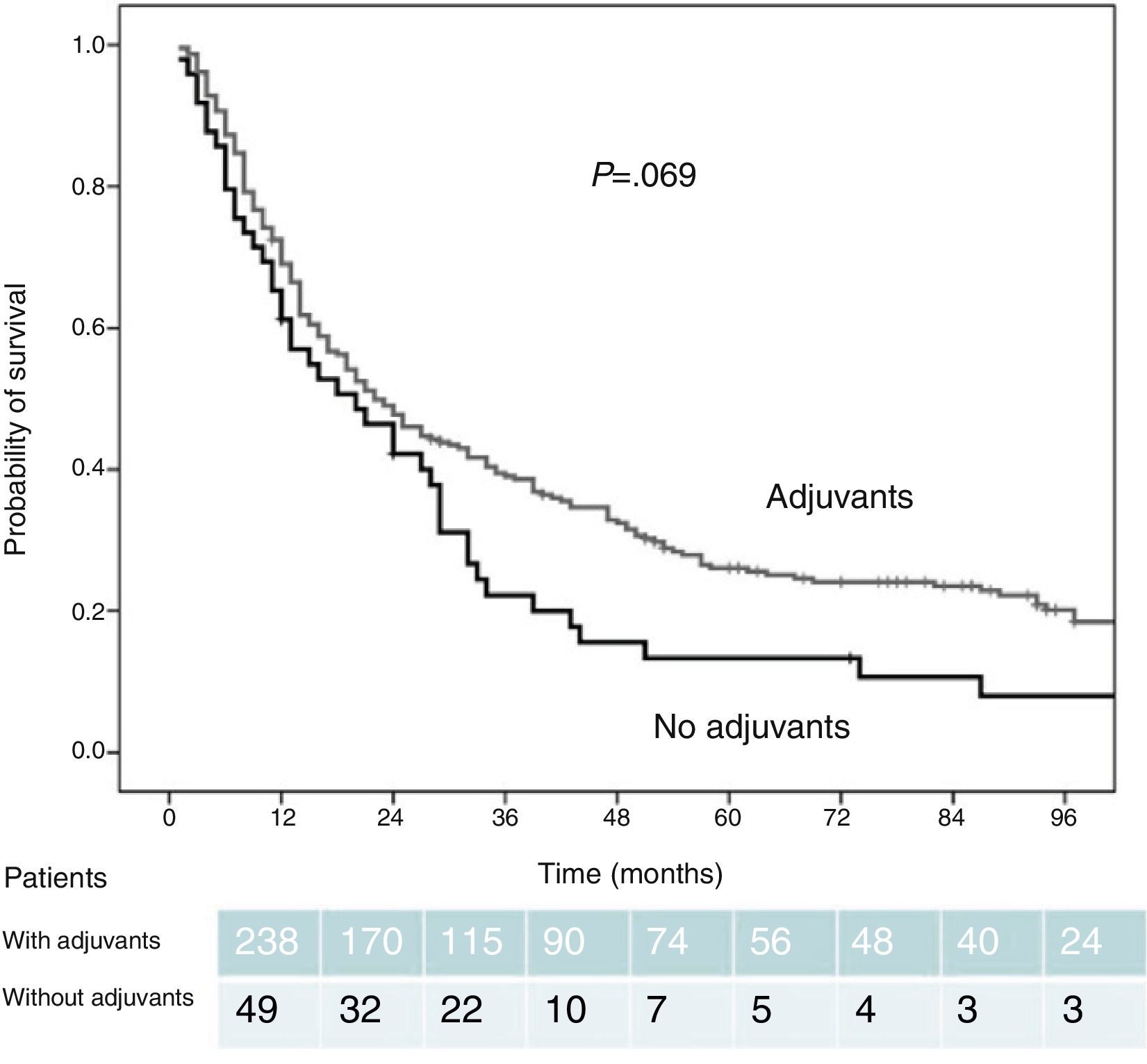
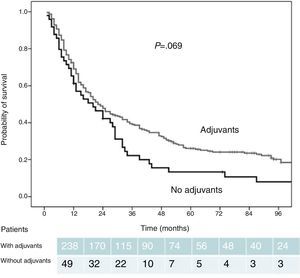
Among the 238 patients who were administered postoperative adjuvant treatment (83% of the total series), the actuarial survival estimated at 12, 24, 36, 48, and 60 months was 72%, 49%, 39%, 32%, and 26%, respectively. The survival rates were greater than those of the patients who did not receive adjuvant therapy (61%, 42%, 22%, 13%, and 11%). These differences did not reach statistical significance, although they did show a clear trend (P=.069) (Fig. 1).
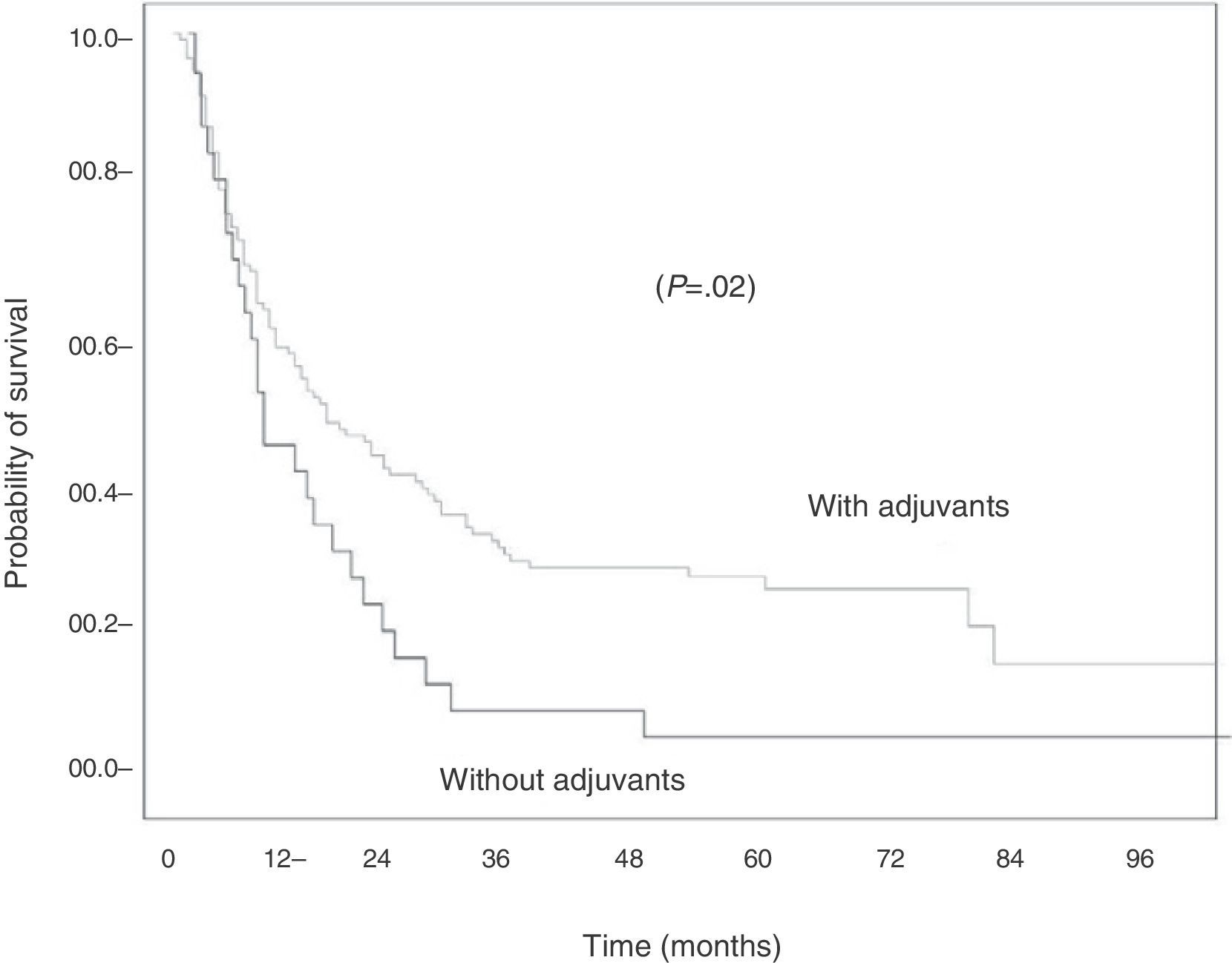
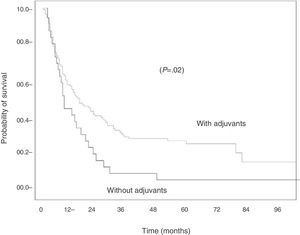
When histology was analyzed, the difference in survival in favor of the patients who received adjuvant treatment was significant when the tumor was squamous-cell carcinoma. Thus, among the 113 cases of epidermoid carcinoma, survival at 12, 24, 36, 48 and 60 months was 67%, 47%, 37%, 29%, and 24%, respectively, for those who received adjuvant therapy, and 52%, 31%, 10%, 7%, and 7%, respectively, for those who did not (P=.02) (Fig. 2).
In patients with NSCLC with non-squamous histology, these differences disappear (75%, 59%, 29%, 23%, and 18% vs 74%, 49%, 40%, 35%, and 28%–with and without adjuvant treatment, respectively).
There were no differences in survival with respect to sex. However, when classified by age with the arbitrary cut-off of 70, we found that the effect of adjuvant therapy seems to be influenced by this variable.
Thus, in patients under the age of 70, the actuarial survival at 12, 24, 36, 48 and 60 months was 50%, 35%, 25%, 21%, and 19%, which ascends to 77%, 55%, 44%, 36%, and 30%, respectively, in patients who received adjuvant treatment. In contrast, the patients aged 70 or older who did not receive adjuvancy presented higher survival rates, although this finding was not significant.
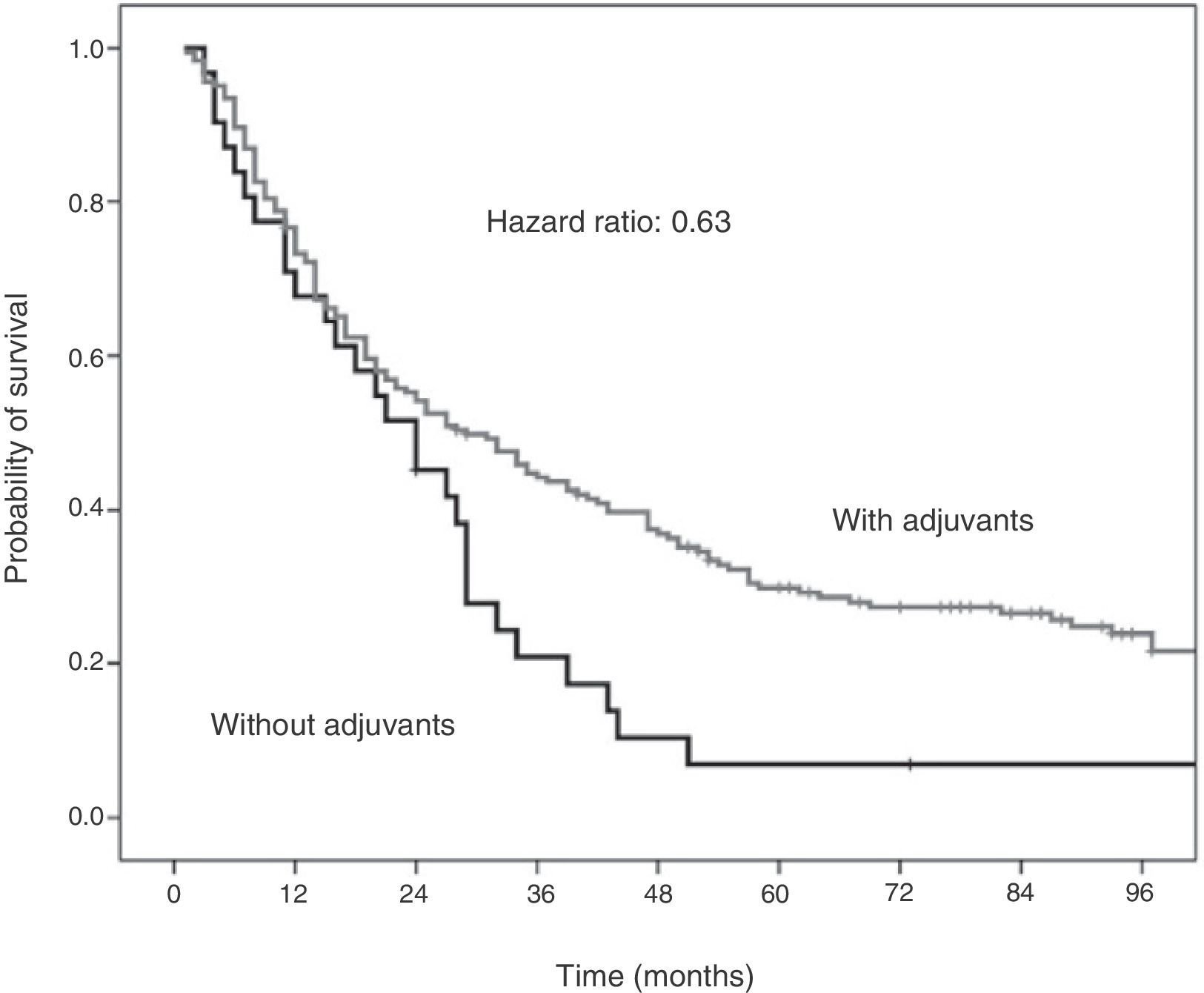
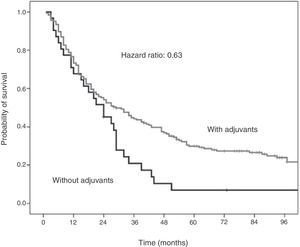
The Cox proportional hazards analysis demonstrates that, in patients under the age of 70 with epidermoid NSCLC, adjuvant treatment reduces the mortality rate by 37% (HR: 0.63; 95% CI: 0.42–0.95). This result is statistically significant (P=.036) (Fig. 3).
DiscussionThe overall 5-year survival for patients with NSCLC in stage IIIA due to ipsilateral mediastinal lymph node involvement (N2) ranges from 15% to 30%. These rates have hardly changed in the last 20 years.4,10,11
A recent European survey of thoracic surgeons demonstrated the heterogeneity of NSCLC in stage IIIA-N2. While 50% of survey participants were in favor of neoadjuvant therapy and a subsequent proposal of surgical resection, almost one-third opted directly for definitive chemo-radiotherapy treatment. And a significant group of those surveyed would proceed directly to surgical resection. The most recent edition of the clinical practice guidelines published by the American College of Chest Physicians (ACCP) indicates that “In patients with discrete N2 involvement by NSCLC identified preoperatively (IIIA), primary surgical resection followed by adjuvant therapy is not recommended (except as part of a clinical trial),” and that the standard treatment is chemo-radiotherapy.12 In addition, the only two randomized clinical trials published to date, the European Organization for Research and Treatment of Cancer-Lung Cancer Group (EORTC) and the Radiation Therapy Oncology Group (RTOG), do not support surgical treatment in N2.13,14 Nevertheless, it is becoming clearer that there are patient subgroups with favorable factors (patient-related as well as tumor-related) that can benefit from multidisciplinary treatment including surgery in N2.15,16 The clinical guidelines of the ACCP classifies N2 disease into 4 groups: 1) incidental N2, which is detected in the histopathology study after surgical resection; 2) incidental N2, found intraoperatively; 3) pre-operative N2 with histologic confirmation; and 4) multiple/bulky N2.12
This classification is frequently reduced to patients with pre-operatively discovered N2 and those with “incidental N2”, or those cases that are found in the pathology studies after resection.17,18 But it seems that heterogeneity also has an influence, to the point that the type of lymph node tumor involvement (extracapsular or not) or the number of affected lymph nodes or regions play an important role in the prognosis of these patients.19,20 One cannot forget that the surgery variable in this type of studies, meaning the type of lymph node dissection used, also defines the precision of patient staging.21,22
Most consensuses propose adjuvant treatment with chemotherapy or radiotherapy in patients with resected NSCLC in stage IIIA-N2.23–26
Adjuvant radiotherapy has been shown to reduce both locoregional and distant recurrences, together with an overall increase in survival.25,27 This benefit is clearer when the involvement is single station.28
With regards to adjuvant chemotherapy after complete surgical resection, some randomized studies have demonstrated that platin-based treatment increases survival.29 Douillard and Rosell30 found in the ANITA study an increase in 5-year survival, 19% in the group without treatment and 40% in the group with adjuvant therapy. These high rates, compared with our study, may be related with a lower percentage of pneumonectomies in the ANITA study (38% vs 47%). In addition, they established the cut-off age for the comparative study at 55 instead of 70, which was the cut-off age in our article. The meta-analysis performed by the LACE workgroup shows a reduction in the risk of mortality between 17% and 20% with adjuvant chemotherapy treatment based on cisplatin.31
Even though adjuvant chemotherapy in resected N2 patients is accepted, it is not clear whether the combination with radiotherapy can improve results in terms of survival and recurrence-free interval.32–35
The most recent meta-analysis analyzing the role of adjuvant chemotherapy in completely resected stage N2 CPNM demonstrates an increase of 4% in the 5-year survival (HR 0.86; 95% CI: 0.81–0.92).
Even with all the limitations of a retrospective study, our series shows that the patients who received adjuvant therapy after surgical resection presented longer survival rates than those who did not, although the difference was not significant. It demonstrates that the role of adjuvant therapy was significant among patients under the age of 70 with epidermoid carcinomas, with a reduction of 37% in the 5-year mortality rate (P=.036), adjusting the remaining variables with the Cox proportional hazard model.
In spite of the fact that it is a series with a rather significant number of patients for a national study (n=283), it presents the limitations of the studies in patients with CPNM in stage IIIA due to N2, which is its own heterogeneity. Thus, the causes for which the group without treatment did not receive adjuvant therapy are not homogenous. Likewise, the series has a high percentage of pneumonectomies, which itself is a factor that could bias the results.
In conclusion, in our series of patients who had complete stage N2 CPNM resection, adjuvant treatment was associated with increased overall survival, which was significant only in patients under the age of 70 who presented epidermoid carcinoma.
The confirmation of these data in a prospective study might question the indication for adjuvant treatment in patients over the age of 70, and especially those with non-epidermoid histology, in whom the benefits of this therapy are less clear.
Conflict of InterestsThe authors have no conflict of interests to declare.
José Luis Duque (Hospital Universitario, Valladolid); Angel López Encuentra, (Hospital Universitario 12 de Octubre, Madrid); Ramón Rami Porta, (Hospital Mutua de Terrassa, Barcelona).
Julio Astudillo (Hospital Germans Trias i Pujol, Barcelona); Josep Maria Gimferrer (Hospital Clinic, Barcelona); Antonio Cantó (Hospital Clínico, Valencia); Juan Casanova (Hospital de Cruces, Bilbao); Jorge Cerezal (Hospital Universitario, Valladolid); Antonio Fernández de Rota (Hospital Carlos Haya, Málaga); Federico González Aragoneses (Hospital Gregorio Marañón, Madrid); Jorge Freixinet (Hospital Nuestra Señora del Pino, Las Palmas); Nicolás Llobregat (Hospital Universitario del Aire, Madrid); Nuria Mañes (Fundación Jiménez Díaz, Madrid); Miguel Mateu (Hospital Mutua de Terrassa, Barcelona); José Luis Martín de Nicolás (Hospital Universitario 12 de Octubre, Madrid); Nuria Novoa (Complejo Hospitalario, Salamanca); Jesús Rodríguez (Complejo Hospitalario, Oviedo); Antonio José Torres García (Hospital Universitario San Carlos, Madrid); Mercedes de la Torre (Hospital Juan Canalejo, La Coruña); Abel Sánchez-Palencia (Hospital Virgen de las Nieves, Granada); Andrés Varela Ugarte (Clínica Puerta de Hierro, Madrid); Yat Wah Pun (Hospital de la Princesa, Madrid).
The names of the members of the Bronchogenic Carcinoma Cooperative Group of the Spanish Society of Pneumology and Thoracic Surgery (CBAG-SEPAR) listed in Addendums 1 and 2.
Please cite this article as: Gómez AM, Jarabo JR, Fernandez C, Calatayud J, Fernández E, Torres AJ, et al. Carcinoma broncogénico no microcítico resecado, estadio pIIIA-N2. ¿En qué pacientes la adyuvancia ofrece mayor beneficio? Cir Esp. 2014;92:277–282.