SI: Minimally invasive surgery of the abdominal wall
Más datosAbdominal wall reconstruction techniques have evolved significantly over the last fifty years and continue to do so at an increasing pace. Beginning with open incisional hernia repair with bilateral rectus myofascial release, multiple techniques to offset tension at the midline by exploring options of layered myofascial release have been described. This article reviews the history, technique, advancements, and future of myofascial release in abdominal wall reconstruction leading from the open Rives-Stoppa repair to the robotic-assisted iteration of the transversus abdominis release.
Las técnicas para la reconstrucción de la pared abdominal han evolucionado significativamente durante los últimos 50 años y siguen avanzando de manera acelerada. Empezando con la reparación de hernias ventrales usando la liberación de los músculos rectos, se han realizado varias otras técnicas de liberación de las capas musculares para disminuir la tensión con el cierre de la línea alba. Este articulo revisa la historia, técnicas, avances y el futuro de las liberaciones de las capas abdominales, comenzando con la técnica de Rives-Stoppa hasta llegar a la técnica TAR por vía robótica.
Abdominal wall reconstruction techniques have evolved significantly over the last fifty years and continue to do so at an increasing pace. Beginning with open incisional hernia repair with bilateral rectus myofascial release, multiple techniques to offset tension at the midline by exploring options of layered myofascial release have been described.1–3 Beyond the standard open bilateral retro-rectus repair (Rives-Stoppa repair), perhaps the currently most discussed and employed of these techniques is the transversus abdominis release (TAR).4 This article will serve to review the history, technique, advancements, and future of myofascial release in abdominal wall reconstruction leading from the open Rives-Stoppa repair to the robotic assisted iteration of the transversus abdominis release.
Open retromuscular repair techniquesAn exhaustive technical description of each of the following techniques is beyond the scope of this paper, but they will be discussed in brief here, beginning with the Rives-Stoppa repair technique which is a rectus abdominis myofascial release. The abdominal midline is entered and the hernia sac and contents are reduced. The medial edge of the rectus myofascial complex is incised and the posterior rectus fascia is dissected free from the overlying rectus muscle bilaterally. Dissection proceeds laterally to the semilunar line, where neurovascular perforating bundles should be identified and preserved. Dissection proceeds cephalad and caudad for a goal distance of 5cm above and below the hernia defect. The posterior sheath is closed, mesh is placed in the retromuscular (RM) space, and the anterior fascia, subcutaneous tissue, and skin are closed above the muscles and mesh. Benefits of this technique include release of tension at the midline to ease closure as well as providing a vascularized space for mesh placement outside of the peritoneal cavity. Recurrence rates can be as low as 5%, but wound complications do occur and have been shown to increase risk of recurrence.2
For some defects, this technique is still inadequate to achieve fascial closure. Another myofascial release technique which can be added in tandem with a Rives-Stoppa repair is the external oblique release, commonly referred to as the anterior separation of components or Ramirez repair.1 This involves dissection of the subcutaneous plane above the rectus muscle which continues lateral to the semilunar line. The external oblique aponeurosis and muscle are incised and elevated free from the underlying internal abdominal oblique muscle. This allows up to 10cm of advancement of the rectus/internal oblique/transversus abdominis compound flap per side. This space is not contiguous with the retro-rectus space and therefore does not provide extension for RM mesh overlap. Wound morbidity including surgical site infections and surgical site occurrences, specifically seroma, are known complications related to the creation of large subcutaneous flaps.
Attempts at mitigating the wound complications by avoiding the subcutaneous dissection led hernia surgeons to seek myofascial release in more posterior abdominal wall layers as an extension of the retro-rectus dissection. The original posterior component separation, in the form of internal oblique release, involves first performing a rectus abdominis myofascial release. Next, the posterior lamina of the internal oblique aponeurosis is incised, and a plane is developed between the internal oblique fascia and the transversus abdominis muscle. This allows further mobilization of the external oblique/internal oblique/rectus complex as well as a larger space for mesh overlap within the RM space. This dissection occurs along the same plane in which the abdominal wall nerves course. As such, this technique carries a potential risk of denervation and muscle atrophy.3
The next iteration of posterior component separation came in the form of TAR. Like the internal oblique release above, this technique is performed as an adjunct to a retro-rectus dissection with incision of the posterior sheath medial to the semilunar line. Instead of dissecting between the internal oblique fascia and the transversus abdominis muscle, the transversus abdominis is also incised and a plane developed between the transversus abdominis and the underlying transversalis fascia, or alternatively the peritoneum. This technique also provides the benefit of avoiding large anterior subcutaneous flaps while continuing dissection in a posterior plane contiguous with the retro-rectus plane. Compared with the internal oblique release, the TAR more easily avoids denervation injury by working one layer posterior to the abdominal wall nerves. TAR allows 8–12cm of myofascial advancement per side as well as extending the extraperitoneal RM plane for placement of wider mesh with greater overlap. A thorough understanding of abdominal wall anatomy is critical when performing TAR. A poorly placed incision through the posterior sheath could instead result in full transection of the linea semilunaris, leading to complete separation of the oblique complex from the rectus abdominis and large lateral abdominal wall bulges.
All of the above techniques were originally described and employed in an open fashion. As discussed, wound morbidity is a significant concern in open abdominal wall reconstruction. Laparoscopic approaches to hernia repair have been shown to result in equivocal recurrence rates, a lower risk of post operative wound infections, and trend toward lower risk of enterotomy as well as post operative length of stay.5,6 At the time of the initial description of TAR, the most common minimally invasive hernia repair was the laparoscopic ventral hernia repair with intraperitoneal mesh, commonly abbreviated IPOM, despite the mesh being in a sublay and not an onlay position. This involves laparoscopic lysis of adhesions, reduction of the hernia sac, and coverage of the hernia defect with an intraperitoneal mesh. This was initially performed without defect closure but later progressed to include fascial closure of the defect. The laparoscopic transabdominal preperitoneal (TAPP) approach was well described for repair of inguinal hernia, but gained little traction for ventral hernias despite offering the benefit of extraperitoneal mesh placement versus the laparoscopic IPOM. Additionally, neither IPOM nor TAPP offers the benefit of offsetting midline tension via myofascial release.
Robotic retromusclar repair techniquesIn the year 2000 the first surgical robot was approved for use for general surgery in the United States. By 2003 the first robotic ventral hernia repair was described by Ballantyne et al., and was essentially a robotic iteration of the laparoscopic IPOM.7 Initially presented at the American Hernia Society conference in 2011 and subsequently published in 2012, Abdalla, et al. described the first robotic-assisted Rives-Stoppa repair.8 This at a minimum demonstrated the safety and technical feasibility of a minimally invasive approach to RM ventral hernia repair employing myofascial release. This breakthrough unleashed a subsequent torrent of interest in robotic RM ventral hernia repair, with the natural progression being interest in expanding the technique to also include further posterior component separation in the form of TAR. On December 20, 2013 the world's first robotic transversus abdominis release was performed at Greenville Memorial Hospital in Greenville, SC, USA.
The initially described «double dock» technique involves intraperitoneal docking of the surgical robot in a far lateral position. The hernia sac and contents are dissected from the abdominal wall and reduced. Beginning at the midline, the RM space contralateral to the docked side is developed with subsequent progression to TAR dissection. The dissected space is measured intracorporeally with a ruler. This measurement is doubled (with the assumption that the contralateral dissection will be roughly the same size) and mesh is selected and cut to these dimensions. The mesh is rolled along its vertical axis and secured loosely as a roll with a single suture. The rolled mesh is then secured and fixated to the abdominal wall lateral to the planned placement of the contralateral robotic trocars. The robot is then undocked, redocked on the contralateral side with trocars placed under the muscles but above the previously dissected RM flap. Mirrored dissection of the initially docked side in both the retro-rectus and TAR planes ensues. The initially docked trocars are repositioned into the RM space from the peritoneal cavity and the posterior sheath is closed in a running fashion with self-fixating slowly absorbing suture (2-0 barbed polydioxanone), thereby closing the visceral sac. The mesh is unrolled across the posterior sheath and again fixated to the abdominal wall lateral to the trocars with suture. The anterior fascial defect is then closed with a slowly absorbing self-fixating suture in a running fashion (#1 barbed polydioxanone).
Modifications to this technique include the RM “single dock”. This entails a similar setup as the double dock, but rather than beginning contralateral rectus sheath dissection from the midline, the ipsilateral rectus sheath is entered at its lateral aspect, just medial to the semilunar line. Dissection then proceeds lateral to medial until the posterior sheath inserts anteriorly into the linea alba. This necessitates incising the posterior sheath just proximal to its insertion into the linea alba to enter the preperitoneal space. The preperitoneal space underneath the linea alba is dissected, including dissection and reduction of the hernia sac and contents. Once across the midline the contralateral RM space is incised and dissection proceeds from medial to lateral. A contralateral TAR can be pursued from a single dock approach, but ipsilateral TAR is not feasible without contralateral trocar placement. Once bilateral RM dissection is complete the sequence differs from the double dock approach in that the anterior fascia is closed first, followed by mesh placement and fixation, and finally posterior sheath closure at the ipsilateral entry point and at the midline portion of the defect (if the posterior sheath was violated). RM space measurement, mesh deployment, and fixation are otherwise similar to the double dock approach.
A variation of the single dock approach can be employed for high epigastric or low suprapubic defects which can render lateral dock approaches technically challenging. For hernias in these locations, the robot can be docked in the opposite abdominal domain and oriented vertically to directly face the defect (docked across the lower abdomen facing upwards for epigastric defects and across the upper abdomen facing downwards for suprapubic defects). The initial posterior sheath incision is then made in a transverse fashion and dissection proceeds vertically through the abdominal wall from semilunar line to semilunar line, dividing bilateral posterior sheaths below their insertion into the linea alba with development of the preperitoneal plane between them. The hernia sac and contents are dissected and reduced when encountered. If tension release or space for mesh overlap is deemed inadequate, bilateral TAR can be performed from this approach with the understanding that the vertically oriented trajectory may slightly limit the extent of dissection when compared with a lateral double dock approach. Closure proceeds similarly to lateral single dock technique with anterior fascial closure, mesh deployment and fixation, and finally posterior sheath closure.
Robotic retromuscular repair outcomesAs previously mentioned, TAR, and particularly robotic TAR is not without technical complications. As such, efforts have been made to establish a “critical view of safety” for robotic TAR.9 This description is imperative to understand for any surgeon intending to pursue TAR (open or robotic). It is particularly helpful in clarifying the relationship of the anatomical layers of the abdominal wall to help avoid catastrophic disconnection of the abdominal wall at the linea semilunaris. It also helps avoid potential abdominal wall wound complications from failing to identify and preserve the perforating neurovascular bundles medial to the semilunar line which provide perfusion to the abdominal wall.
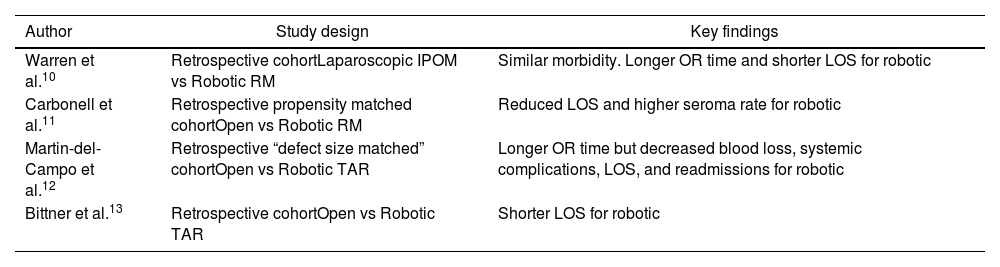
Outcomes from robotic RM repair are well reported. When compared with laparoscopic IPOM, robotic RM repair has been shown to have comparable perioperative morbidity with shorter length of stay despite longer operative times.10 Robotic RM repair also provides the added benefit of offset of tension via myofascial release and avoids intraperitoneal placement of mesh and the potential complications associated with mesh in this location. A comparative analysis of robotic versus open RM repair utilizing propensity score matched groups from the prospectively maintained Abdominal Core Health Quality Collaborative (ACHQC) database demonstrated significantly reduced length of stay for the robotic RM group.11 When specifically comparing robotic versus open TAR, robotic TAR has been demonstrated to provide reduced blood loss, reduced systemic post operative complications, reduced post operative length of stay, and reduced readmissions despite longer operative times.12,13
Despite the favorable outcomes demonstrated by the above-described robotic RM techniques, the world of advanced abdominal wall reconstruction remains ever vigilant for ways to push boundaries and improve care even further. Originally described as a minimally invasive option for treatment of large inguinoscrotal hernias via a totally extraperitoneal (TEP) approach, the extended view totally extraperitoneal (eTEP) approach was modified for ventral hernias and formally described in 2018.14 This technique is described in detail by its original innovator Dr. Jorge Daes in a respective article in this publication, but will be briefly discussed here. The eTEP repair involves trocar placement into the rectus sheath without advancement into the peritoneal cavity. The retromuscular sheath is insufflated and developed. Midline crossover under the linea alba via the preperitoneal space is pursued, with subsequent entry into the contralateral rectus sheath and development of the contralateral retromuscular space. This can be performed laparoscopically or robotically, and dissection can proceed laterally for the entire operation, or vertically from a “top down” or “bottom up” approach. The latter two iterations require an initial laparoscopic crossover dissection so that the robot may be docked in a vertically oriented fashion. Addition of a unilateral or bilateral TAR is feasible with eTEP as well. These approaches offer the same benefits of as the previously described intraperitoneal docked RM repairs in the form of tension offset via myofascial release and extraperitoneal placement of mesh, with the added benefit of avoiding entry into the peritoneal cavity. This potentially facilitates easier and safer dissection in unviolated extraperitoneal planes, particularly for the re-operative patient with a hostile intraperitoneal cavity. Early outcomes from eTEP repairs demonstrate safety, efficacy, reproducibility, and an average length of stay of less than one day, which makes it a very feasible outpatient surgery option.15 A summary of key findings from these studies can be found in Table 1.
Key findings from robotic RM comparative analyses.
| Author | Study design | Key findings |
|---|---|---|
| Warren et al.10 | Retrospective cohortLaparoscopic IPOM vs Robotic RM | Similar morbidity. Longer OR time and shorter LOS for robotic |
| Carbonell et al.11 | Retrospective propensity matched cohortOpen vs Robotic RM | Reduced LOS and higher seroma rate for robotic |
| Martin-del-Campo et al.12 | Retrospective “defect size matched” cohortOpen vs Robotic TAR | Longer OR time but decreased blood loss, systemic complications, LOS, and readmissions for robotic |
| Bittner et al.13 | Retrospective cohortOpen vs Robotic TAR | Shorter LOS for robotic |
IPOM, intraperitoneal onlay of mesh; RM, retromuscular; OR, operating room; LOS, length of stay.
In conclusion, abdominal wall reconstruction has progressively evolved since the inception of open RM ventral hernia repair and continues to evolve at a rapid pace. Robotic ventral hernia repair appears to be only gaining in popularity and utilization. With outcomes data demonstrating superior complication rates and decreased post operative length of stay for robotic over open approaches, robotic advanced myofascial release techniques like TAR are here to stay. As the technique for robotic RM repair with or without TAR continues to evolve it is imperative that surgeons intent on adding it to their surgical armamentarium familiarize themselves with the anatomy, procedural flow, and recognition/avoidance of potential pitfalls. A familiarity with the progressive history of RM hernia repair can provide a thorough knowledge base for hernia surgeons to critically analyze the “why” of an approach rather than simply the “what”, which can improve patient selection, surgeon skill acquisition, and operative outcomes.
FundingAM Carbonell has received honoraria from Intuitive Surgical, Medtronic, and Deep Blue Medical Advances.
MW Love has nothing to disclose.
Conflict of interestsThe authors declare they have no conflict of interest.