Sclerosing mucoepidermoid carcinoma with eosinophilia (SMECE) is a very uncommon thyroid tumour. A search of the literature in the Pubmed database (1991–December 2013) using the key words SMECE and thyroid sclerosing mucoepidermoid carcinoma, and also including non-indexed articles, identified only 40 patients.
We present the case of a 52-year-old woman with a history of hypothyroidism secondary to Hashimoto's thyroiditis and multinodular goitre for the past 10 years. The patient had been closely monitored, with a thyroid profile that showed no alterations and thyroid peroxidase antibody 635IU/mL (normal <35).
Fine-needle aspiration (FNA) of a dominant nodule on ultrasound revealed abundant lymphocytes and plasma cells, few follicular cells and colloid remains. Due to the progressive increase of the goitre, which caused an oppressive feeling in the cervical area, we decided on surgery. During surgery, there were macroscopic bilateral nodules that were not suspicious of malignancy, but there were no lymphadenopathies, and a total thyroidectomy was performed.
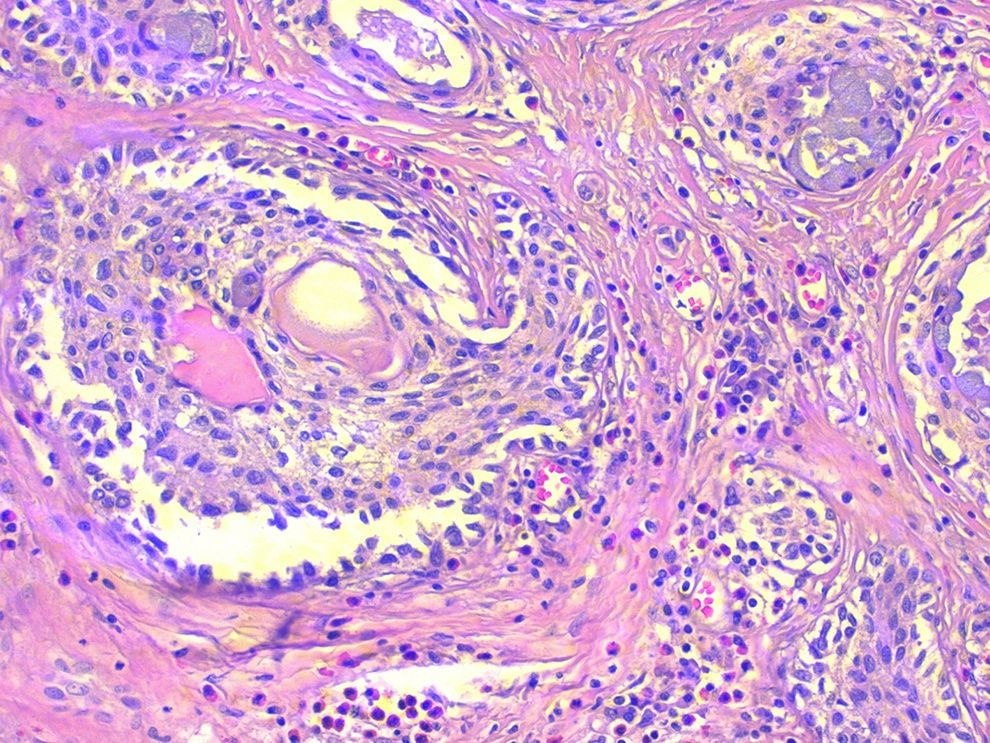
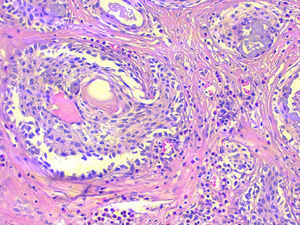
The deferred histopathologic study revealed the presence of nodular Hashimoto's thyroiditis and a 46mm nodule in the left thyroid lobe, with an expansive growth pattern that was diagnosed as sclerosing mucoepidermoid carcinoma with eosinophilia in Hashimoto's thyroiditis (Fig. 1). The immunohistochemistry study was positive with antibodies for pan-cytokeratins (cytokeratins 5, 6, 8, 17 and 19), cytokeratin clone CK-34βE12, p63 and thyroid transcription factor 1 (TTF-1). No positivity was observed for thyroglobulin, calcitonin, carcinoembryonic antigen or thyroid cancer marker antibody.
Sclerosing mucoepidermoid carcinoma with eosinophilia in Hashimoto's thyroiditis; microscope image showing the scale-like pattern of the tumour nests, presence of mucous cells, very collagenised fibrous stroma and the lymphocytic infiltrate with numerous eosinophils (haematoxylin-eosin 200×).
Currently, the patient has shown no evidence of disease recurrence 34 months after surgery.
The first published cases of SMECE date from 1991, but this pathology was not accepted by the WHO as a primary thyroid tumour entity until 2004.1 It was initially described by Chan2 as a low-grade neoplasm with an indolent course, usually in the clinical context of Hashimoto's thyroiditis, although in 5 out of 8 patients in the series there were diverse degrees of extension to the parathyroid, muscle, trachea and oesophagus, and one of these 5 patients had lymph node involvement. Later studies have confirmed that SMECE can frequently present not only extension to adjacent parathyroid tissue3–5 but also lymph node involvment3–5 (in 35% of the reviewed cases) and, less often, distant metastases (bone, hepatic and pulmonary).3–5 Its low frequency of appearance, frequent invasive behaviour and limited evidence about adequate treatment make this entity especially interesting.
The origin of SMECE is controversial. Although the origin has been proposed from the metaplastic thyroid follicles,2 the most widely accepted hypothesis indicates that they arise from vestiges of the ultimobranchial body, known as solid cell nests,6 which were first described by Getzowa in 1907. It is thought that the main cells of these nests could be pluripotent and could contribute to the histogenesis of some thyroid tumours.6 The expression of p63 in both SMECE as well as in the solid cell nests and their absence in the follicular epithelial cells of the thyroid have been proposed as evidence of their biological relationship.7
Histologically, SMECE is characterised by the presence of tumour cells with epidermoid (squamous) and glandular (mucinous) differentiation in a stroma with marked sclerosis that is accompanied by eosinophils, lymphocytes and plasma cells, frequently in the context of lymphocytic thyroiditis.1 According to the classification of the WHO, it should be differentiated from mucoepidermoid carcinoma of the thyroid described by Rhatigan et al.8 in 1977, in which glandular and squamous differentiation is also observed, but without the remaining characteristic findings of SMECE.9
Immunohistochemistry is very helpful in the differential diagnosis. SMECE always shows cytokeratin expression and absence of thyroglobulin and calcitonin expression.2 Given the heterogeneity of the results obtained, neither TTF-1 nor carcinoembryonic antigen seem to be useful markers for the differential diagnosis.
The first description about the use of fine-needle aspiration in patients later diagnosed with SMECE was by Bondeson in 1996.10 Based on a few published cases, cytology has not been observed to be useful in the preoperative diagnosis of this type of tumour.
Several treatments have been proposed for this disease,2–5 although there is no scientific evidence. The most frequently used type of surgery, both in lesions confined to the thyroid as well as in cases of extrathyroidal invasion, is total thyroidectomy (65% of the cases reviewed), which can be associated with some type of lymphadenectomy. In very few cases, more radical surgical procedures have been done, with excision of the affected extrathyroidal tissue, laryngectomy, oesophagectomy, etc.
Adjuvant therapies have been used, including external radiotherapy,2,5 chemotherapy3–5 and radioactive iodine ablation,4,5 which have provided heterogeneous results, so we currently cannot determine which patients could benefit from adjuvant therapy. We also do not have sufficient information about hormone suppression after thyroidectomy.
In short, we still do not understand in detail the biology of this disease. It is necessary to increase our knowledge about diagnostic techniques in order to improve preoperative diagnosis and early surgical treatment.
Part of the information found in this manuscript was previously presented in poster format at the 18th National Conference on Surgery in Madrid, 2011.
Please cite this article as: Orbeal R, Jimeno J, Monroy G, Badia F, Parés D. Carcinoma mucoepidermoide esclerosante con eosinofilia de tiroides. Cir Esp. 2015;93:e137–e138.